Evaluation of Anti-Inflammatory, Antidiabetic, Antioxidant, and Anticholinergic Activities, as Well as Chemical Composition and Polyphenolic Compounds in Novel SCOBY-Fermented Juices
Abstract
1. Introduction
2. Results and Discussion
2.1. Basic Chemical Composition
2.1.1. pH and Total Soluble Solids
2.1.2. Organic Acids and Reducing Sugars
2.2. Identification and Quantification of Polyphenolic Compounds
2.3. Determination of Biological Activities
2.3.1. Antioxidant Capacity
2.3.2. Antidiabetic Activity
2.3.3. Anticholergenic Activity
2.3.4. Anti-Inflammatory Activity
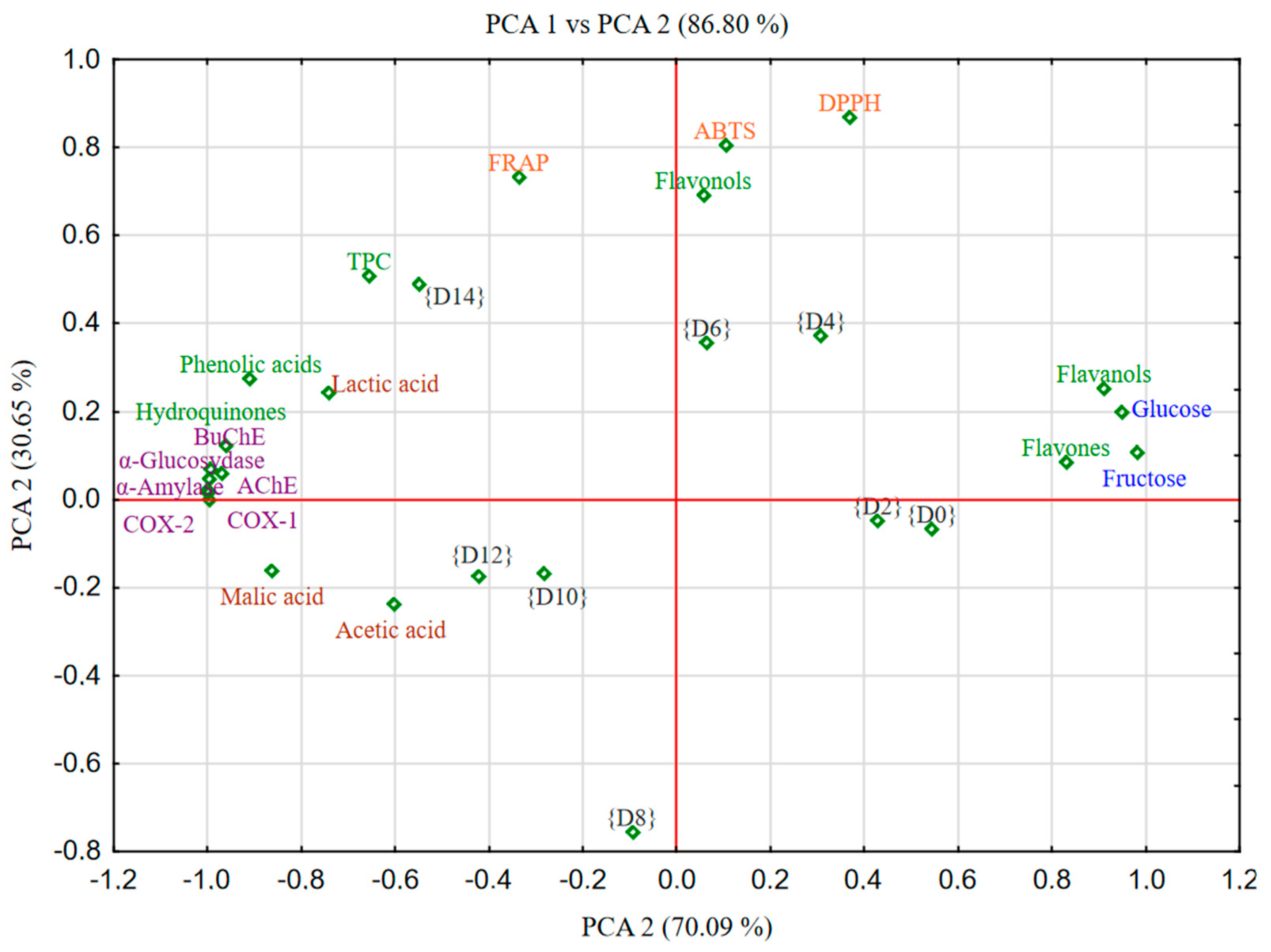
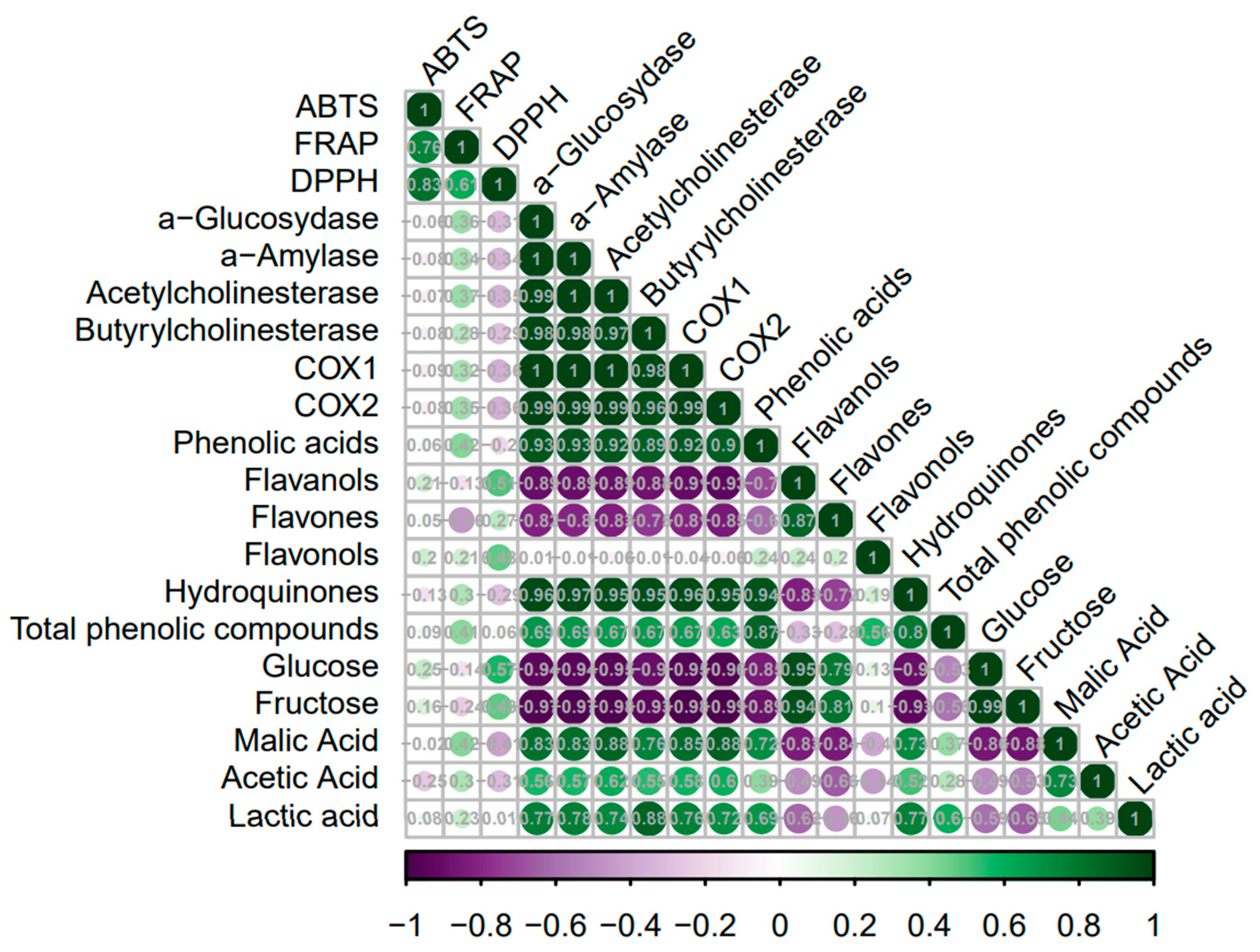
2.4. Chemometric Analysis
3. Materials and Methods
3.1. Raw Material and Sample Processing
3.2. Physicochemical Analyses
3.3. Determination of Sugars and Organic Acids
3.4. UPLC-PDA-Q/Tof-MS Analysis of Polyphenolic Compounds
3.5. Determination of Biological Activities: Antioxidant, Anti-Inflammatory, Antidiabetic, and Anticholinergic
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DPPH | 2,2-Diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl |
| FRAP | Ferric reducing antioxidant power assay |
| AChE | Acetylcholinesterase |
| BuChE | Butyrylcholinesterase |
| LAB | Lactic Acid Bacteria |
| AAB | Acetic Acid Bacteria |
References
- Nowak, D.; Gośliński, M.; Kłębukowska, L. Antioxidant and Antimicrobial Properties of Selected Fruit Juices. Plant Foods Hum. Nutr. 2022, 77, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Lewko, K. Leczenie Dobrą Dietą, 1st ed.; Wydawnictwo SBM: Warszawa, Poland, 2017; pp. 152–153. ISBN 978-83-8348-082-4. (In Polish) [Google Scholar]
- Kolniak-Ostek, J.; Kłopotowska, D.; Rutkowski, K.P.; Skorupińska, A.; Kruczyńska, D.E. Bioactive Compounds and Health-Promoting Properties of Pear (Pyrus communis L.) Fruits. Molecules 2020, 25, 4444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, X.; Wang, Y.; Rao, L.; Zhao, L. Health effects of fruit juices and beverages with varying degrees of processing. Food Sci. Hum. Wellness 2024, 13, 2456–2479. [Google Scholar] [CrossRef]
- Pabich, M.; Materska, M. Biological Effect of Soy Isoflavones in the Prevention of Civilization Diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]
- Kitajewska, W.; Szeląg, W.; Kopański, Z.; Maslyak, Z.; Sklyarov, I. Choroby cywilizacyjne i ich prewencja. J. Clin. Healthc. 2014, 1, 3–7. Available online: http://jchc.eu/numery/2014_1/201411.pdf (accessed on 28 February 2025). (In Polish).
- Chwała, W.; Jankowicz-Szymańska, A. Man in Health and Disease. Prevention and Management of Civilizational Diseases: International Scientific Conference, May 24, 2024, Tarnów, Poland. Health Promot. Phys. Act. 2024, 27, 35–67. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, J.; Lu, J.; Chen, D.; Song, S.; Wang, H.; Sun, M.; Feng, T. Revealing the influence of microbiota on the flavor of kombucha during natural fermentation process by metagenomic and GC-MS analysis. Food Res. Int. 2023, 169, 112909. [Google Scholar] [CrossRef]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Pawluś, P.; Kolniak-Ostek, J. Innovative Analogs of Unpasteurized Kombucha Beverages: Comparative Analysis of Mint/Nettle Kombuchas, Considering Their Health-Promoting Effect, Polyphenolic Compounds and Chemical Composition. Int. J. Mol. Sci. 2024, 25, 7572. [Google Scholar] [CrossRef]
- Nieumywakin, I. Grzyb Herbaciany: Naturalny Uzdrowiciel; Hartigrama: Warszawa, Poland, 2018. (In Polish) [Google Scholar]
- Kitwetcharoen, H.; Phung, L.T.; Klanrit, P.; Thanonkeo, S.; Tippayawat, P.; Yamada, M.; Thanonkeo, P. Kombucha Healthy Drink—Recent Advances in Production, Chemical Composition and Health Benefits. Fermentation 2023, 9, 48. [Google Scholar] [CrossRef]
- de Oliveira, P.V.; da Silva Júnior, A.H.; de Oliveira, C.R.S.; Assumpção, C.F.; Ogeda, C.H. Kombucha benefits, risks and regulatory frameworks: A review. Food Chem. Adv. 2023, 2, 100288. [Google Scholar] [CrossRef]
- Sanwal, N.; Gupta, A.; Bareen, M.A.; Sharma, N.; Sahu, J.K. Kombucha fermentation: Recent trends in process dynamics, functional bioactivities, toxicity management, and potential applications. Food Chem. Adv. 2023, 3, 100421. [Google Scholar] [CrossRef]
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A review of substrates, regulations, composition, and biological properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef]
- Morales, D.; Gutiérrez-Pensado, R.; Bravo, F.I.; Muguerza, B. Novel kombucha beverages with antioxidant activity based on fruits as alternative substrates. LWT-Food Sci. Technol. 2023, 189, 115482. [Google Scholar] [CrossRef]
- Chong, A.Q.; Chin, N.L.; Talib, R.A.; Basha, R.K. Modelling pH Dynamics, SCOBY Biomass Formation, and Acetic Acid Production of Kombucha Fermentation Using Black, Green, and Oolong Teas. Processes 2024, 12, 1301. [Google Scholar] [CrossRef]
- Fonteles, T.V.; dos Santos, A.Y.S.; Linhares, M.D.F.D.; Miguel, T.B.A.R.; Miguel, E.d.C.; Rodrigues, S. Metabolic responses of kombucha consortium fermentation upon ultrasound-processing. Food Chem. Adv. 2024, 4, 100646. [Google Scholar] [CrossRef]
- Tomar, O. Determination of some quality properties and antimicrobial activities of kombucha tea prepared with different berries. Turk. J. Agric. For. 2023, 47, 252–262. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Gutowska, I.; Antoniewicz, J.; Janda, K. Evaluation of Fluoride and Selected Chemical Parameters in Kombucha Derived from White, Green, Black and Red Tea. Biol. Trace Elem. Res. 2021, 199, 3547–3552. [Google Scholar] [CrossRef]
- Nurikasari, M.; Puspitasari, Y.; Siwi, R.P.Y. Characterization and analysis kombucha tea antioxidant activity based on long fermentation as a beverage functional. J. Glob. Res. Public Health 2017, 2, 48–52. [Google Scholar] [CrossRef]
- Zubaidah, E.; Yurista, S.; Rahmadani, N.R. Characteristic of physical, chemical, and microbiological kombucha from various varieties of apples. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012040. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef]
- de Oliveira, P.M.; Santos, L.P.; Coelho, L.F.; Neto, P.M.A.; Sass, D.C.; Contiero, J. Production of L (+) Lactic Acid by Lactobacillus casei Ke11: Fed Batch Fermentation Strategies. Fermentation 2021, 7, 151. [Google Scholar] [CrossRef]
- Sheeladevi, A.; Ramanathan, N. Lactic Acid Production Using Lactic Acid Bacteria under Optimized Conditions. Int. J. Pharm. Biol. Arch. 2011, 2, 1686–1691. [Google Scholar]
- Costa, S.; Summa, D.; Semeraro, B.; Zappaterra, F.; Rugiero, I.; Tamburini, E. Fermentation as a Strategy for Bio-Transforming Waste into Resources: Lactic Acid Production from Agri-Food Residues. Fermentation 2020, 7, 3. [Google Scholar] [CrossRef]
- Oyewole, O.A.; Yakubu, J.G.; Kalu, J.; Abdulfatah, M.T.; Abioye, O.P.; Adeniyi, O.D.; Egwim, E.C. Microbial conversion of agro-wastes for lactic acid production. Sci. Afr. 2023, 22, e01915. [Google Scholar] [CrossRef]
- de Avila, L.D.; Daudt, C.E. Indução da fermentação maloláctica em vinho Gewürztraminer. Cienc. Rural. 1997, 27, 331–336. [Google Scholar] [CrossRef]
- Binati, R.L. Avaliação da fermentação maloláctica em vinhos de altitude com bactérias ácido-lácticas autóctones selecionadas. 2015. Available online: https://repositorio.ufsc.br/bitstream/handle/123456789/135391/334491.pdf?sequence=1&isAllowed=y (accessed on 5 March 2025).
- Guillamón, J.M.; Mas, A. Acetic Acid Bacteria. In Biology of Microorganisms on Grapes, in Must and Wine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 31–46. [Google Scholar] [CrossRef]
- De Roos, J.; Verce, M.; Aerts, M.; Vandamme, P.; De Vuyst, L. Temporal and Spatial Distribution of the Acetic Acid Bacterium Communities throughout the Wooden Casks Used for the Fermentation and Maturation of Lambic Beer Underlines Their Functional Role. Appl. Environ. Microbiol. 2018, 84, e02846-17. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Ying, W.; Wang, S.; Zhou, Y.; Wu, W.; Lei, Y. Addition of Lactic Acid Bacteria Modulates Microbial Community and Promotes the Flavor Profiles of Kombucha. Food Biosci. 2024, 60, 104340. [Google Scholar] [CrossRef]
- Naik, V.; Kerkar, S. Fermentation assisted functional foods. Futur. Trends Biotechnol. 2024, 3, 11–32. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Fermented Foods: Fermented Vegetables and Other Products. Encycl. Food Health 2016, 2, 668–674. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmiański, J.; Wojdyło, A. Effect of l-ascorbic acid addition on quality, polyphenolic compounds and antioxidant capacity of cloudy apple juices. Eur. Food Res. Technol. 2013, 236, 777–798. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Identification and quantification of polyphenolic compounds in ten pear cultivars by UPLC-PDA-Q/TOF-MS. J. Food Compos. Anal. 2016, 49, 65–77. [Google Scholar] [CrossRef]
- Chandran, A.K.; Stach, M.; Kucharska, A.Z.; Sokół-Łętowska, A.; Szumny, A.; Moreira, H.; Szyjka, A.; Barg, E.; Kolniak-Ostek, J. Comparison of polyphenol and volatile compounds and in vitro antioxidant, anti-inflammatory, antidiabetic, anti-ageing, and anticancer activities of dry tea leaves. LWT-Food Sci. Technol. 2025, 222, 117632. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hur, S.; Lim, J.; Jin, K.; Yang, T.-H.; Keehm, I.-S.; Kim, S.W.; Kim, T.; Kim, D. Enhancement of the phenolic compounds and antioxidant activities of Kombucha prepared using specific bacterial and yeast. Food Biosci. 2023, 56, 103431. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Saimaiti, A.; Luo, M.; Huang, S.-Y.; Xiong, R.-G.; Shang, A.; Gan, R.-Y.; Li, H.-B. Fermentation with Tea Residues Enhances Antioxidant Activities and Polyphenol Contents in Kombucha Beverages. Antioxidants 2022, 11, 155. [Google Scholar] [CrossRef]
- Liang, Z.; Huang, Y.; Zhang, P.; Fang, Z. Impact of fermentation on the structure and antioxidant activity of selective phenolic compounds. Food Biosci. 2023, 56, 103147. [Google Scholar] [CrossRef]
- Sharma, R.; Diwan, B.; Singh, B.P.; Kulshrestha, S. Probiotic fermentation of polyphenols: Potential sources of novel functional foods. Food Prod. Process. Nutr. 2022, 4, 21. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; He, W.; Song, X.; Peng, Y.; Hu, X.; Bian, S.; Li, Y.; Nie, S.; Yin, J.; et al. Exploring the Biogenic Transformation Mechanism of Polyphenols by Lactobacillus plantarum NCU137 Fermentation and Its Enhancement of Antioxidant Properties in Wolfberry Juice. J. Agric. Food Chem. 2024, 72, 12752–12761. [Google Scholar] [CrossRef]
- Su, J.; Fu, X.; Zhang, R.; Li, X.; Li, Y.; Chu, X. Exploring the Effects of Solid-State Fermentation on Polyphenols in Acanthopanax senticosus Based on Response Surface Methodology and Nontargeted Metabolomics Techniques. J. Food Biochem. 2023, 2023, 1–21. [Google Scholar] [CrossRef]
- Battikh, H.; Bakhrouf, A.; Ammar, E. Antimicrobial effect of Kombucha analogues. LWT-Food Sci. Technol. 2012, 47, 71–77. [Google Scholar] [CrossRef]
- Sudarshan, K.; Aidhen, I.S. Convenient Synthesis of 3-Glycosylated Isocoumarins. Eur. J. Org. Chem. 2016, 2017, 34–38. [Google Scholar] [CrossRef]
- Angeli, L.; Populin, F.; Morozova, K.; Ding, Y.; Asma, U.; Bolchini, S.; Cebulj, A.; Busatto, N.; Costa, F.; Ferrentino, G.; et al. Comparative analysis of antioxidant activity and capacity in apple varieties: Insights from stopped flow DPPH• kinetics, mass spectrometry and electrochemistry. Food Biosci. 2024, 58, 103729. [Google Scholar] [CrossRef]
- Kartelias, I.G.; Karantonis, H.C.; Giaouris, E.; Panagiotakopoulos, I.; Nasopoulou, C. Kombucha Fermentation of Olympus Mountain Tea (Sideritis scardica) Sweetened with Thyme Honey: Physicochemical Analysis and Evaluation of Functional Properties. Foods 2023, 12, 3496. [Google Scholar] [CrossRef] [PubMed]
- Kartelias, I.G.; Panagiotakopoulos, I.; Nasopoulou, C.; Karantonis, H.C. Evaluating the Effect of Adding Selected Herbs, Spices, and Fruits to Fermented Olympus Mountain Tea (Sideritis scardica) Kombucha Sweetened with Thyme Honey: Assessment of Physicochemical and Functional Properties. Beverages 2024, 10, 9. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Balasuriya, N.; Wang, Y. Prevention of Type 2 Diabetes by Polyphenols of Fruits. In Nutritional Antioxidant Therapies: Treatments and Perspectives; Springer: Berlin/Heidelberg, Germany, 2017; pp. 447–466. [Google Scholar] [CrossRef]
- Zin, C.A.J.C.M.; Mohamed, W.M.I.W.; Khan, N.A.K.; Ishak, W.R.W. Effects of Fruit and Vegetable Polyphenols on the Glycemic Control and Metabolic Parameters in Type 2 Diabetes Mellitus: A Review. Prev. Nutr. Food Sci. 2022, 27, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Repub. Iran 2017, 31, 886–892. [Google Scholar] [CrossRef]
- Visvanathan, R.; Williamson, G. Effect of citrus fruit and juice consumption on risk of developing type 2 diabetes: Evidence on polyphenols from epidemiological and intervention studies. Trends Food Sci. Technol. 2021, 115, 133–146. [Google Scholar] [CrossRef]
- Chen, Y.; Qie, X.; Quan, W.; Zeng, M.; Qin, F.; Chen, J.; Adhikari, B.; He, Z. Omnifarious fruit polyphenols: An omnipotent strategy to prevent and intervene diabetes and related complication? Crit. Rev. Food Sci. Nutr. 2021, 63, 4288–4324. [Google Scholar] [CrossRef]
- Wang, B.; Liu, K.; Mi, M.; Wang, J. Effect of Fruit Juice on Glucose Control and Insulin Sensitivity in Adults: A Meta-Analysis of 12 Randomized Controlled Trials. PLoS ONE 2014, 9, e95323. [Google Scholar] [CrossRef]
- Murphy, M.M.; Barrett, E.C.; Barraj, L.M. 100% Fruit juice and measures of glycemic control and insulin sensitivity: A meta-analysis of randomized controlled trials. FASEB J. 2017, 6, e59. [Google Scholar] [CrossRef]
- Mendelson, C.; Sparkes, S.; Merenstein, D.J.; Christensen, C.; Sharma, V.; Desale, S.; Auchtung, J.M.; Kok, C.R.; Hallen-Adams, H.E.; Hutkins, R. Kombucha tea as an anti-hyperglycemic agent in humans with diabetes—A randomized controlled pilot investigation. Front. Nutr. 2023, 10, 1190248. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, J.F.; Ruiz, L.F.; Uekane, T.M.; Silva, K.A.; Lima, A.R. Kombucha e Seu Potencial Efeito Antidiabético: Revisão; Agron Food Academy: São Carlos, Brazil, 2022. [Google Scholar] [CrossRef]
- Amalia, R.I.; Fahrudin, F.I.; Alvi, A.S.; Intipunya, P. Myths and Potential Benefits of Kombucha as a Functional Food: A Review. Int. J. Res. Innov. Appl. Sci. 2024, IX, 484–491. [Google Scholar] [CrossRef]
- Filho, A.B.B.; Barros, L.A.d.O.; Ribeiro, K.R.d.C.; da Silva, C.A.; Koike, B.D.V. Efficiency of daily kombucha consumption in reducing glycemic levels and hypercholesterolemia. Braz. J. Health Rev. 2024, 7, e68785. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Wang, J.; Geng, W. Kombucha Reduces Hyperglycemia in Type 2 Diabetes of Mice by Regulating Gut Microbiota and Its Metabolites. Foods 2022, 11, 754. [Google Scholar] [CrossRef]
- de Bortoli Beal, S.; Silva, C.A.; do Amaral, A.P.L.; Florz, J.A.K.; Potuk, C.F.W.; Adami, E.R. Utilização da kombucha em pacientes diabéticos: Uma revisão de literatura. Rev. Gestao E Secr. 2024, 15, e4501. [Google Scholar] [CrossRef]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef]
- Wahyanto, K.N.; Agustini, R. Total Flavonoid Content and In Vitro Anti-Inflammatory Potentials of Kombucha with Enrichment of Butterfly Pea (Clitoria ternatea) Flower Extract. J. Pijar Mipa 2024, 19, 254–259. [Google Scholar] [CrossRef]

| Reducing Sugars | Organic Acids | |||||||
|---|---|---|---|---|---|---|---|---|
| pH | Total Soluble Solids | Fructose | Glucose | Malic Acid | Acetic Acid | Lactic Acid | ||
| Fresh apple juice | 3.22 ± 0.01 de | 12.70 ± 0.00 a | 1.19 ± 0.01 a | 0.36 ± 0.01 a | 0.71 ± 0.01 b | 0.10 ± 0.01 e | — | |
| Apple juice | Day 0 | 3.19 ± 0.00 e | 11.75 ± 0.07 bc | 0.92 ± 0.02 c | 0.26 ± 0.02 bc | 0.57 ± 0.02 c | 0.07 ± 0.00 ef | — |
| Day 2 | 3.19 ± 0.06 e | 12.00 ± 0.14 c | 0.90 ± 0.01 c | 0.29 ± 0.01 b | 0.60 ± 0.03 c | 0.08 ± 0.01 e | — | |
| Day 4 | 3.14 ± 0.00 f | 11.40 ± 0.00 c | 0.73 ± 0.03 d | 0.28 ± 0.02 b | 0.58 ± 0.03 c | 0.05 ± 0.00 f | — | |
| Day 6 | 3.14 ± 0.00 f | 11.30 ± 0.00 cd | 0.71 ± 0.02 d | 0.36 ± 0.03 a | 0.69 ± 0.02 b | 0.09 ± 0.01 e | — | |
| Day 8 | 3.14 ± 0.00 f | 7.50 ± 0.00 f | 0.47 ± 0.02 f | 0.35 ± 0.02 a | 0.75 ± 0.03 b | 0.13 ± 0.00 d | — | |
| Day 10 | 3.14 ± 0.05 f | 6.60 ± 0.00 g | 0.16 ± 0.01 h | 0.14 ± 0.01 d | 0.55 ± 0.00c | 0.09 ± 0.01 e | 0.01 ± 0.00 a | |
| Day 12 | 3.10 ± 0.00 g | 7.20 ± 0.00 f | 0.17 ± 0.01 h | 0.15 ± 0.00 d | 0.88 ± 0.02 a | 0.11 ± 0.01 de | 0.03 ± 0.00 a | |
| Day 14 | 3.10 ± 0.00 g | 5.70 ± 0.00 i | 0.11 ± 0.00 i | 0.09 ± 0.00 e | 0.88 ± 0.02 a | 0.11 ± 0.01 de | 0.03 ± 0.00 a | |
| Fresh pear juice | 4.40 ± 0.03 a | 12.35 ± 0.10 b | 0.96 ± 0.01 ab | 0.18 ± 0.01 cd | 0.15 ± 0.01 e | 0.33 ± 0.01 b | — | |
| Pear juice | Day 0 | 4.12 ± 0.00 b | 11.60 ± 0.00 c | 1.02 ± 0.03 b | 0.23 ± 0.02 c | 0.18 ± 0.00 e | 0.35 ± 0.02 b | — |
| Day 2 | 4.07 ± 0.03 b | 12.30 ± 0.00 b | 0.97 ± 0.02 b | 0.24 ± 0.02 c | 0.19 ± 0.01 e | 0.37 ± 0.02 ab | — | |
| Day 4 | 3.71 ± 0.00 bc | 11.00 ± 0.00 d | 0.68 ± 0.01 d | 0.15 ± 0.01 d | 0.21 ± 0.02 e | 0.29 ± 0.00 c | — | |
| Day 6 | 3.58 ± 0.00 c | 8.95 ± 0.07 e | 0.59 ± 0.02 e | 0.15 ± 0.01 d | 0.32 ± 0.01 d | 0.40 ± 0.02 a | — | |
| Day 8 | 3.51 ± 0.00 c | 6.70 ± 0.00 g | 0.27 ± 0.01 g | 0.03 ± 0.00 f | 0.31 ± 0.02 d | 0.38 ± 0.01 a | — | |
| Day 10 | 3.48 ± 0.00 c | 6.10 ± 0.00 h | 0.13 ± 0.00 h | --- | 0.31 ± 0.01 d | 0.39 ± 0.02 a | — | |
| Day 12 | 3.35 ± 0.00 d | 6.30 ± 0.00 gh | 0.06 ± 0.01 i | --- | 0.32 ± 0.00 d | 0.40 ± 0.01 a | 0.03 ± 0.00 a | |
| Day 14 | 3.32 ± 0.00 d | 5.60 ± 0.00 i | 0.02 ± 0.00 j | --- | 0.30 ± 0.02 d | 0.39 ± 0.00 a | 0.03 ± 0.00 a | |
| Rt (min) | [M–H]− (m/z) 1 | MS/MS Fragments (m/z) 1 | Tentative Identification | Kombucha Starter | Apple Juice | Pear Juice |
|---|---|---|---|---|---|---|
| Phenolic acids | ||||||
| 0.66 | 341.0574 | 191.0167 | Caffeoylhexose | - | √ | - |
| 0.77 | 517.0757 | 387.5185/307.4093/163.9868 | Coumaric acid derivative | - | √ | - |
| 0.88 | 341.1230 | 191.8541/173.0211 | Caffeoylhexose | √ | - | - |
| 0.94 | 341.0571 | 174.9891 | Caffeoylhexose | - | √ | - |
| 1.10 | 335.1482 | 179.0955 | Caffeoylshikimic acid | √ | - | - |
| 1.28 | 341.0566 | 190.9808 | Caffeoylhexose | √ | √ | √ |
| 1.42 | 341.0550 | 173.0659 | Coffeoylhexose | - | - | √ |
| 1.44 | 609.1277 | 291.0152/173.1445 | Gallocatechin dimer | √ | - | - |
| 1.46 | 331.0330 | 168.9945 | Galloylhexose | √ | - | - |
| 1.59 | 169.0122 | 125.0368/107.0263/97.1233 | Gallic acid 2 | √ | - | - |
| 1.67 | 191.0185 | 173.9566 | Quinic acid 2 | - | - | √ |
| 1.69 | 331.0330 | 168.9945 | Galloylhexose | √ | - | - |
| 1.78 | 343.0782 | 191.0030 | Theogallin 2 | √ | - | - |
| 1.84 | 687.1256 | 343.0778/191.1543 | Theogallin dimer | √ | - | - |
| 1.85 | 341.0603 | Caffeoylhexose | - | √ | - | |
| 2.06 | 341.0465 | 173.0659 | Coffeoylhexose | - | - | √ |
| 2.43 | 341.1326 | 191.1225 | Coffeoylhexose | √ | - | - |
| 2.47 | 341.1152 | 191.0152/163.1143 | Coffeoylhexose | √ | - | - |
| 2.59 | 365.0780 | 229.0397 | Caffeoyl N-tryptophan | - | - | √ |
| 2.67 | 359.0391 | 197.0391 | Syryngic acid galactoside | - | - | √ |
| 2.78 | 163.0436 | p-Coumaric acid 2 | - | - | √ | |
| 2.90 | 353.0316 | 191.0148 | 3-Caffeoylquinic acid 2 | - | - | √ |
| 2.91 | 635.0922 | 483.1077/465.1228/313.0702 | Trigalloylhexose | √ | - | - |
| 3.03 | 353.0136 | 191.0553. 179.2167 | cis-3-Caffeoylquinic acid | - | - | √ |
| 3.14 | 325.0641 | 307.1234 | p-Coumaric acid 4-O-glucoside | √ | - | - |
| 3.16 | 337.0658 | 173.0507 | cis-4-p-Coumaroylquinic acid | - | - | √ |
| 3.34 | 483.0769 | 331.0214/168.2301 | 1,6-Digalloyl glucose | √ | - | - |
| 3.46 | 353.0822 | 191.0544 | 5-Caffeoylquinic acid 2 | - | - | √ |
| 3.53 | 355.1933 | 193.0247 | Ferulic acid hexoside | - | - | √ |
| 3.75 | 359.0921 | 197.0046. 153.9228 | Syryngic acid glucoside | - | - | √ |
| 3.81 | 385.0677 | 223.1583 | Sinapic acid hexoside | - | - | √ |
| 3.95 | 337.0536 | 191.0531, 163.0162 | trans-5-p-Coumaroyloquinic acid | √ | √ | - |
| 3.96 | 483.0803 | 331.0214/169.9105 | 1,6-Digalloyl glucose | √ | - | - |
| 4.12 | 353.0346 | 191.0164 | 4-Caffeoylquinic acid 2 | - | √ | √ |
| 4.23 | 353.0339 | 191.0165 | cis-4-Caffeoylquinic acid | - | √ | - |
| 4.32 | 511.0567 | 365.0225/265.2115/63.0394 | Caffeoyl-N-tryptophanrhamnoside | √ | - | - |
| 4.33 | 295.0978 | 179.0328 | Caffeoyl-l-malic acid | - | - | √ |
| 4.46 | 325.0833 | 191.0125. 163.0313 | p-Coumaroylhexose | - | - | √ |
| 4.71 | 511.0813 | 341.0654/179.0573 | Cafeoyl galloyl hexoside | √ | - | - |
| 4.78 | 337.0924 | 191.0236 | 5-p-Coumaroyloquinic acid | √ | - | - |
| 5.07 | 353.0282 | 191.0159 | 1-Caffeoylquinic acid | - | √ | √ |
| 5.23 | 337.0408 | 191.0079 | cis-5-p-Coumaroyloquinic acid | √ | √ | - |
| 5.54 | 337.1267 | 173.0612 | 3-p-Coumaroylquinic acid | √ | - | √ |
| 7.90 | 515.1266 | 353.0355. 191.0623 | di-O-Caffeoylquinic acid | - | - | √ |
| Flavanols and procyanidins | ||||||
| 2.19 | 609.2322 | 305.0269 | Gallocatechin dimer | √ | - | - |
| 2.61 | 305.0691 | 219.4306/191.0652 | (+)-Gallocatechin 2 | √ | - | - |
| 3.21 | 577.1326 | 289.1136 | B-type Procyanidin dimer | - | - | √ |
| 3.27 | 557.1322 | 289.1022 | B-type Procyanidin dimer | - | - | √ |
| 3.59 | 761.1357 | 609.1226/591.2341 | Theasinensin-gallate | √ | - | - |
| 3.65 | 305.1302 | 125.1401 | (−)-Epigallocatechin 2 | √ | - | - |
| 3.75 | 577.0678 | 289.0214 | B-type Procyanidin dimer | - | √ | - |
| 3.93 | 1153.2258 | 865.1234. 577.1993. 287.0715 | B-type Procyanidin tetramer | - | - | √ |
| 4.00 | 577.1326 | 289.0043 | B-type Procyanidin dimer | - | - | √ |
| 4.11 | 915.1637 | 457.1220/169.5023 | (−)-Epigallocatechin gallate 2 | √ | - | - |
| 4.23 | 577.1277 | 289.1456 | B-type Procyanidin dimer | - | - | √ |
| 4.26 | 1153.2217 | 865.1937. 577.1322. 389.1134 | B-type Procyanidin tetramer | - | - | √ |
| 4.30 | 865.1090 | 575.0361; 289.0272 | B-type Procyanidin trimer | √ | √ | - |
| 4.45 | 1154.1523 | 865.1246; 575.0493; 289.0390 | B-type Procyanidin tetramer | - | √ | √ |
| 4.46 | 577.1509 | 289.1345 | B-type Procyanidin dimer | √ | - | - |
| 4.56 | 1153.2258 | 865.1234. 577.1993. 287.0715 | B-type Procyanidin tetramer | - | - | √ |
| 4.72 | 865.1023 | 577.0698; 289.0229 | B-type Procyanidin trimer | - | √ | - |
| 4.73 | 577.0820 | 289.0180 | B-type procyanidin dimer | - | - | √ |
| 4.88 | 1153.1634 | 865.1252; 287.0016 | A-type Procyanidin tetramer | - | √ | - |
| 4.90 | 865.2100 | 577.1254. 289.1344 | B-type Procyanidin trimer | - | - | √ |
| 4.95 | 1153.1844 | 577.0530; 289.0229 | B-type Procyanidin tetramer | - | √ | - |
| 5.13 | 289.0236 | 245.0487 | (+)-Catechin 2 | √ | √ | √ |
| 5.15 | 457.0715 | 305.1266/275.0324 | Gallocatechin gallate | √ | - | - |
| 5.28 | 863.1920 | 577.1320. 289.0818 | A-type Procyanidin trimer | - | - | √ |
| 5.45 | 720.6527 | 577.1333. 389.0677 | Double-charged pentameric procyanidin | - | - | √ |
| 5.48 | 865.1158 | 287.0067 | A-type Procyanidin trimer | - | √ | - |
| 5.76 | 1008.2216 | 864.1896. 287.0190 | Double-charged heptameric procyanidin | - | - | √ |
| 5.78 | 1153.1429 | 865.1124; 577.0516; 289.0265 | B-type Procyanidin tetramer | - | √ | - |
| 5.90 | 557.1646 | 289.0289 | B-type Procyanidin dimer | - | √ | - |
| 6.00 | 865.1998 | 577.1558. 287.1135 | B-type Procyanidin trimer | - | - | √ |
| 6.38 | 577.1345 | 289.0246 | B-type Procyanidin dimer | √ | - | - |
| 6.70 | 441.0845 | 289.0263 | (+)-Epicatechin gallate | √ | - | - |
| 6.81 | 289.0241 | 245.0475 | (−)-Epicatechin 2 | √ | √ | √ |
| 6.96 | 597.1932 | 291.1346 | A-type procyanidin dimer 2 | √ | - | - |
| 7.57 | 723.2398 | 289.1250 | Catechin dimer + deoxyhexose | √ | - | - |
| Purine Alkaloids | ||||||
| 0.80 | 173.0745 | 156.0213/82.1153 | Theanine 2 | √ | - | - |
| 2.55 | 179.0652 | 136.1205 | Theobromine 2 | √ | - | - |
| Theaflavins | ||||||
| 5.74 | 563.0724 | 443.0628/315.0266 | Theaflavin unknown isomer | √ | - | - |
| 5.79 | 563.0724 | 443.0628/315.0266 | Theaflavin unknown isomer | √ | - | - |
| Flavonols | ||||||
| 5.20 | 593.1326 | 285.0312 | Kaempferol 3-O-p-coumaroylhexoside | √ | - | - |
| 6.01 | 771.0100 | 301.0104 | Quercetin 3-O-glucosylrutinoside | √ | - | - |
| 6.14 | 593.1500 | 285.1526 | Kaempferol 3-O-p-coumaroylhexoside | √ | - | - |
| 6.21 | 771.2050 | 301.0523 | Quercetin 3-O-glucosylrutinoside | √ | - | - |
| 6.30 | 755.2150 | 301.0098 | Quercetin 3-O-dirhamnosylhexoside | √ | - | - |
| 6.36 | 739.1573 | 593.1212; 285.7339 | Kaempferol hexoside-dideoxyhexoside | - | - | √ |
| 6.45 | 755.2154 | 285.1326 | Kaempferol 3-O-glucosylrutinoside | √ | - | - |
| 6.50 | 609.0786 | 301.0698 | Quercetin 3-O-rutinoside | - | - | √ |
| 6.59 | 463.0210 | 301.0354 | Quercetin 3-O-galactoside 2 | √ | - | √ |
| 6.71 | 463.0331 | 301.0355 | Quercetin 3-O-glucoside 2 | √ | √ | √ |
| 6.85 | 623.1700 | 315.0551 | Isorhamnetin 3-O-rhamnosyl-hexoside | - | - | √ |
| 6.86 | 755.2154 | 285.0314 | Kaempferol 3-O-glucosylrutinoside | √ | - | - |
| 6.98 | 463.0329 | 301.1265 | Quercetin-3-O-hexoside | - | √ | - |
| 7.14 | 433.0100 | 301.0014 | Quercetin-3-O-xyloside | - | √ | - |
| 7.20 | 593.1542 | 285.0321 | Kaempferol 3-O-p-coumaroylhexoside | √ | - | - |
| 7.35 | 505.0515 | 463.0900. 301.0354 | Quercetin-acylated-hexoside | - | - | √ |
| 7.47 | 623.0863 | 315.0551 | Isorhamnetin 3-O-rhamnosyl-hexoside | - | - | √ |
| 7.48 | 433.0256 | 301.9845 | Quercetin-3-O-arabinoside | - | √ | - |
| 7.61 | 477.1220 | 315.0551 | Isorhamnetin 3-O-galactoside | - | - | √ |
| 7.66 | 447.0241 | 301.1111 | Quercetin-3-O-rhamnoside 2 | - | √ | - |
| 7.74 | 447.0816 | 285.0312 | Kaempferol 3-O-glucoside 2 | √ | - | √ |
| 8.11 | 433.1159 | 301.1111 | Quercetin-3-O-pentoside | - | √ | - |
| 8.16 | 477.1022 | 315.0551 | Isorhamnetin 3-O-glucoside | - | - | √ |
| 8.4 | 489.1042 | 285.0312 | Kaempferol 3-O-6-acetylated-glucoside | - | - | √ |
| 8.68 | 1079.3120 | 539.1263/301.1121 | Quercetin 3-O-acylglycoside | √ | - | - |
| 8.89 | 1079.2259 | 539.1259/301.1120 | Quercetin 3-O-acylglycoside | √ | - | - |
| 8.96 | 917.2359 | 609.0244/301.0112 | Quercetin 3-O-p-coumaroyl-glucosyl-rhamnosyl-galactoside | √ | - | - |
| 9.02 | 1049.2788 | 301.0133 | Quercetin 3-O-acylglycoside | √ | - | - |
| 9.16 | 1063.2851 | 285.0321 | Kaempferol 3-O-acylglycoside | √ | - | - |
| 9.25 | 885.2170 | 301.0211 | Quercetin 3-O-p-coumaroyl-dirhamnosyl-hexoside | √ | - | - |
| 9.28 | 519.1166 | 315.0457 | Isorhamnetin-acylated-hexoside | - | - | √ |
| 9.32 | 1063.2983 | 285.1332 | Kaempferol 3-O-acylglycoside | √ | - | - |
| 9.40 | 1049.2754 | 301.0133 | Quercetin 3-O-acylglycoside | √ | - | - |
| 9.44 | 1049.2854 | 301.0133 | Quercetin 3-O-acylglycoside | √ | - | - |
| 9.53 | 901.2348 | 301.1254 | Quercetin 3-O-p-coumaroyl-glucosyl-rutinoside | √ | - | - |
| 9.64 | 1063.2983 | 301.0211 | Quercetin 3-O-acylglycoside | √ | - | - |
| 9.77 | 1033.2848 | 285.1532 | Kaempferol 3-O-acylglycoside | √ | - | - |
| 9.85 | 917.2359 | 609.0244/301.0112 | Quercetin 3-O-p-coumaroyl-glucosyl-rhamnosyl-galactoside | √ | - | - |
| 9.93 | 1047.2903 | 285.1322 | Kaempferol 3-O-p-coumaroyl-rhamnosyl-dihexoside | √ | - | - |
| Flavones | ||||||
| 5.86 | 401.1399 | 269.1803 | Apigenin pentoside | - | - | √ |
| 7.00 | 533.1366 | 433.1183/291.1219 | Apigenin-6,8-C-dipentoside | √ | - | - |
| Hydroquinones | ||||||
| 1.85 | 211.0855 | Arbutin 2 | - | - | √ | |
| Dihydrochalcones | ||||||
| 7.83 | 567.0808 | 273.8322 | Phloretin 2′-O-xyloglucoside 2 | - | √ | - |
| 8.58 | 435.0708 | 273.0303 | Phloretin 2′-O-glucoside 2 | - | √ | - |
| Kombucha Starter | |
|---|---|
| Compound | mg/100 mL |
| Phenolic acids | |
| Caffeoylhexose | 0.01 ± 0.00 |
| Caffeoylshikimic acid | 0.04 ± 0.00 |
| Caffeoylhexose | 0.01 ± 0.00 |
| Gallocatechin dimer | 0.01 ± 0.00 |
| Galloylhexose | 0.02 ± 0.00 |
| Gallic acid 1 | 0.77 ± 0.01 |
| Galloylhexose | 0.08 ± 0.00 |
| Theogallin | 0.28 ± 0.01 |
| Theogallin dimer | 0.09 ± 0.00 |
| Caffeoylhexose | 0.01 ± 0.00 |
| Caffeoylhexose | 0.01 ± 0.00 |
| Trigalloylhexose | 0.20 ± 0.01 |
| p-Coumaric acid 4-O-glucoside | 0.11 ± 0.01 |
| 1,6-Digalloyl glucose | 0.03 ± 0.00 |
| trans-5-p-Coumaroylquinic acid | 0.18 ± 0.01 |
| 1,6-Digalloyl glucose | 0.02 ± 0.00 |
| Caffeoyl-N-tryptophanrhamnoside | 0.05 ± 0.00 |
| Cafeoyl galloyl hexoside | 0.04 ± 0.00 |
| 5-p-Coumaroylquinic acid | 0.12 ± 0.01 |
| cis-5-p-Coumaroylquinic acid | 0.04 ± 0.00 |
| 3-p-Coumaroylquinic acid | 0.03 ± 0.00 |
| Sum | 2.13 ± 0.05 |
| Flavan-3-ols and Proanthocyanidins | |
| Gallocatechin dimer | 0.02 ± 0.00 |
| (+)-Gallocatechin | 0.02 ± 0.00 |
| Theasinensin-gallate | 0.11 ± 0.01 |
| (−)-Epigallocatechin | 5.66 ± 0.02 |
| (−)-Epigallocatechin gallate | 49.21 ± 0.16 |
| B-type procyanidin trimer | 0.25 ± 0.01 |
| B-type procyanidin dimer | 0.14 ± 0.00 |
| (+)-Catechin 1 | 0.17 ± 0.01 |
| Gallocatechin gallate | 2.50 ± 0.01 |
| B-type procyanidin dimer | 0.11 ± 0.00 |
| (+)-Epicatechin gallate | 47.22 ± 0.09 |
| (−)-Epicatechin 1 | 5.79 ± 0.01 |
| A-type procyanidin dimer | 0.01 ± 0.00 |
| Catechin dimer + deoxyhexose | 0.04 ± 0.00 |
| Sum | 111.26 ± 0.59 |
| Purine Alkaloids | |
| Theanine 1 | 0.12 ± 0.01 |
| Theobromine 1 | 1.15 ± 0.01 |
| Sum | 1.27 ± 0.05 |
| Theaflavins | |
| Theaflavin unknown isomer | 0.22 ± 0.00 |
| Theaflavin unknown isomer | 0.25 ± 0.01 |
| Sum | 0.47 ± 0.06 |
| Flavonols | |
| Kaempferol 3-O-p-coumaroylhexoside | 0.04 ± 0.00 |
| Quercetin 3-O-glucosylrutinoside | 0.09 ± 0.00 |
| Kaempferol 3-O-p-coumaroylhexoside | 0.08 ± 0.00 |
| Quercetin 3-O-glucosylrutinoside | 0.26 ± 0.01 |
| Quercetin 3-O-dirhamnosylhexoside | 0.02 ± 0.00 |
| Kaempferol 3-O-glucosylrutinoside | 0.38 ± 0.00 |
| Quercetin 3-O-galactoside 1 | 0.04 ± 0.00 |
| Quercetin 3-O-glucoside 1 | 0.03 ± 0.00 |
| Kaempferol 3-O-glucosylrutinoside | 0.49 ± 0.02 |
| Kaempferol 3-O-p-coumaroylhexoside | 0.11 ± 0.00 |
| Kaempferol 3-O-glucoside 1 | 0.03 ± 0.00 |
| Quercetin 3-O-acylglycoside | 0.08 ± 0.00 |
| Quercetin 3-O-acylglycoside | 0.03 ± 0.00 |
| Quercetin 3-O-p-coumaroyl-glucosyl-rhamnosyl-galactoside | 0.01 ± 0.00 |
| Quercetin 3-O-acylglycoside | 0.05 ± 0.00 |
| Kaempferol 3-O-acylglycoside | 0.11 ± 0.01 |
| Quercetin 3-O-p-coumaroyl-dirhamnosyl-hexoside | 0.01 ± 0.00 |
| Kaempferol 3-O-acylglycoside | 0.02 ± 0.00 |
| Quercetin 3-O-acylglycoside | 0.02 ± 0.00 |
| Quercetin 3-O-acylglycoside | 0.03 ± 0.00 |
| Quercetin 3-O-p-coumaroyl-glucosyl-rutinoside | 0.02 ± 0.00 |
| Quercetin 3-O-acylglycoside | 0.01 ± 0.00 |
| Kaempferol 3-O-acylglycoside | 0.01 ± 0.00 |
| Quercetin 3-O-p-coumaroyl-glucosy-lrhamnosyl-galactoside | 0.02 ± 0.00 |
| Kaempferol 3-O-p-coumaroyl-rhamnosyl-dihexoside | 0.02 ± 0.00 |
| Sum | 2.02 ± 0.05 |
| Flavones | |
| Apigenin-6,8-C-dipentoside | 0.01 ± 0.00 |
| TOTAL | 117.16 ± 1.26 |
| Apple Juice | |||
|---|---|---|---|
| Compound | Fresh Juice | Day 0 | Day 14 |
| Phenolic acids | |||
| Caffeoylhexose | 3.2 ± 0.1 a | 3.2 ± 0.1 a | 1.5 ± 0.1 c |
| Coumaric acid derivative | 36.5 ± 0.8 a | 36.4 ± 0.5 a | 31.2 ± 1.0 b |
| Caffeoylhexose | 6.7 ± 0.3 ab | 6.6 ± 0.2 ab | 6.3 ± 0.1 b |
| Caffeoylhexose | 4.6 ± 0.1 d | 4.4 ± 0.1 d | 22.1 ± 0.5 a |
| Caffeoylhexose | 37.0 ± 1.2 e | 37.6 ± 0.8 e | 71.8 ± 1.2 a |
| trans-5-p-Coumaroyloquinic acid | 4.9 ± 0.0 a | 4.9 ± 0.1 a | 3.6 ± 0.0 c |
| 4-Caffeoylquinic acid | 281.6 ± 3.6 a | 299.2 ± 2.6 a | 295.4 ± 3.2 a |
| cis-4-Caffeoylquinic acid | 10.1 ± 0.5 a | 9.3 ± 0.2 ab | 8.8 ± 0.3 b |
| 1-Caffeoylquinic acid | 9.5 ± 0.3 b | 9.3 ± 0.2 b | 8.8 ± 0.1 c |
| cis-5-p-Coumaroyloquinic acid | 21.3 ± 0.7 a | 20.6 ± 0.5 b | 20.8 ± 0.4 ab |
| Sum | 415.4 ± 6.9 b | 431.4 ± 8.5 b | 470.2 ± 4.8 a |
| Flavanols and procyanidins | |||
| B-type Procyanidin dimer | 47.5 ± 2.3 a | 45.8 ± 0.6 a | 30.5 ± 0.3 b |
| B-type Procyanidin trimer | 55.1 ± 1.5 a | 52.4 ± 0.3 a | 41.6 ± 0.2 b |
| B-type Procyanidin tetramer | 45.2 ± 0.9 a | 44.7 ± 0.2 a | 36.9 ± 0.3 c |
| B-type Procyanidin trimer | 123.2 ± 2.2 a | 118.5 ± 0.5 a | 93.6 ± 0.6 b |
| A-type Procyanidin tetramer | 345.9 ± 2.6 a | 343.00 ± 2.5 a | 106.9 ± 1.5 h |
| B-type Procyanidin tetramer | 33.6 ± 0.3 b | 31.4 ± 0.2 c | 36.1 ± 0.2 a |
| (+)-Catechin | 37.9 ± 0.5 f | 37.3 ± 0.5 f | 91.1 ± 0.8 a |
| A-type Procyanidin trimer | 280.9 ± 1.5 a | 279.5 ± 1.0 a | 178.6 ± 1.1 d |
| B-type Procyanidin tetramer | 42.6 ± 0.6 a | 41.7 ± 0.3 a | 32.9 ± 0.2 c |
| B-type Procyanidin dimer | 46.9 ± 0.3 b | 46.5 ± 0.3 b | 44.9 ± 0.3 b |
| (−)-Epicatechin | 48.5 ± 0.5 b | 45.1 ± 0.4 c | 54.0 ± 0.3 a |
| Sum | 1107.3 ± 10.2 a | 1085.9 ± 9.8 b | 747.0 ± 8.7 e |
| Flavonols | |||
| Quercetin-3-O-galactoside | 1.6 ± 0.1 a | 1.4 ± 0.1 a | 0.9 ± 0.0 b |
| Quercetin-3-O-glucoside | 2.5 ± 0.0 a | 2.4 ± 0.1 a | 2.0 ± 0.1 b |
| Quercetin-3-O-xyloside | 1.2 ± 0.1 a | 0.9 ± 0.0 a | 0.7 ± 0.0 b |
| Quercetin-3-O-arabinoside | 2.1 ± 0.1 a | 2.0 ± 0.1 a | 1.5 ± 0.2 c |
| Quercetin-3-O-rhamnoside | 6.5 ± 0.2 a | 6.5 ± 0.2 a | 6.5 ± 0.2 a |
| Quercetin-3-O-xyloside | 4.0 ± 0.1 a | 3.9 ± 0.1 a | 3.7 ± 0.1 b |
| Sum | 17.9 ± 0.5 a | 17.1 ± 0.9b | 15.3 ± 0.6 e |
| Dihydrochalcones | |||
| Phloretin 2′-O-xyloglucoside | 4.1 ± 0.1 a | 3.9 ± 0.1 a | 3.8 ± 0.2 a |
| Phloretin 2′-O-glucoside | 24.6 ± 0.4 d | 23.9 ± 0.5 d | 27.4 ± 0.7 b |
| Sum | 28.7 ± 0.7 c | 27.9 ± 0.5 d | 31.1 ± 0.8 b |
| TOTAL | 1569.3 ± 9.9 a | 1562.3 ± 10.1 a | 1263.6 ± 11.0 c |
| Pear Juice | |||
|---|---|---|---|
| Compound | Fresh Juice | Day 0 | Day 14 |
| Phenolic acids | |||
| Coffeoylhexose | 5.7 ± 0.1 c | 5.7 ± 0.1 c | 9.2 ± 0.3 a |
| Coffeoylhexose | 5.4 ± 0.1 e | 5.3 ± 0.0 e | 10.5 ± 0.2 a |
| Quinic acid | 3.0 ± 0.2 e | 3.0 ± 0.1 e | 7.3 ± 0.2 a |
| Coffeoylhexose | 2.3 ± 0.1 d | 2.2 ± 0.0 d | 4.0 ± 0.1 a |
| Caffeoyl N-tryptophan | 7.9 ± 0.3 b | 7.8 ± 0.4 b | 9.5 ± 0.3 a |
| Syryngic acid galactoside | 0.5 ± 0.0 d | 0.5 ± 0.0 d | 1.6 ± 0.1 a |
| p-Coumaric acid | 0.2 ± 0.0 c | 0.2 ± 0.0 c | 1.6 ± 0.2 a |
| 3-Caffeoylquinic acid | 0.9 ± 0.0 c | 0.8 ± 0.0 d | 2.8 ± 0.1 a |
| cis-3-Caffeoylquinic acid | 1.3 ± 0.1 b | 1.2 ± 0.0 b | 2.8 ± 0.2 a |
| cis-4-p-Coumaroylquinic acid | 0.4 ± 0.0 cd | 0.3 ± 0.0 d | 1.7 ± 0.1 a |
| 5-Caffeoylquinic acid | 0.3 ± 0.0 cd | 0.3 ± 0.0 cd | 0.7 ± 0.0 b |
| Ferulic acid hexoside | 1.3 ± 0.1 a | 1.2 ± 0.0 a | 0.2 ± 0.0 c |
| Syryngic acid glucoside | 0.9 ± 0.0 cd | 0.9 ± 0.0 cd | 2.1 ± 0.2 a |
| Sinapic acid hexoside | 0.9 ± 0.1 d | 0.8 ± 0.0 d | 3.1 ± 0.2 a |
| 4-Caffeoylquinic acid | 142.3 ± 3.2 cd | 140.0 ± 5.2 d | 168.8 ± 4.9 a |
| Caffeoyl-l-malic acid | 8.4 ± 0.3 a | 8.3 ± 0.5 a | 3.6 ± 0.2 c |
| p-Coumaroylhexose | 1.8 ± 0.2 a | 1.8 ± 0.2 a | 0.0 ± 0.0 d |
| 1-Caffeoylquinic acid | 3.0 ± 0.1 e | 2.9 ± 0.1 e | 7.2 ± 0.2 a |
| 3-p-Coumaroylquinic acid | 0.4 ± 0.0 c | 0.4 ± 0.1 c | 1.1 ± 0.1 a |
| di-O-Caffeoylquinic acid | 1.1 ± 0.1 b | 1.0 ± 0.0 b | 1.5 ± 0.1 a |
| Sum | 188.0 ± 4.8 d | 184.6 ± 5.6 d | 239.2 ± 3.2 a |
| Flavanols and procyanidins | |||
| B-type Procyanidin dimer | 15.5 ± 1.0 a | 15.2 ± 1.2 a | 6.3 ± 0.5 d |
| B-type Procyanidin dimer | 13.4 ± 0.6 a | 13.3 ± 0.4 a | 10.6 ± 0.4 c |
| B-type Procyanidin tetramer | 11.9 ± 0.3 a | 11.7 ± 0.2 a | 4.8 ± 0.0 e |
| B-type Procyanidin dimer | 6.9 ± 0.3 a | 6.8 ± 0.3 a | 6.5 ± 0.2 a |
| B-type Procyanidin dimer | 42.3 ± 1.9 ab | 40.6 ± 2.8 b | 36.8 ± 0.3 c |
| B-type Procyanidin tetramer | 26.1 ± 1.0 a | 24.0 ± 1.1 b | 0.0 ± 0.0 d |
| B-type Procyanidin tetramer | 10.9 ± 0.5 a | 10.1 ± 0.5 a | 10.1 ± 0.3 a |
| B-type Procyanidin tetramer | 12.5 ± 0.4 a | 11.0 ± 0.9 b | 5.0 ± 0.1 f |
| B-type procyanidin dimer | 11.9 ± 0.5 f | 10.4 ± 0.2f | 44.1 ± 0.3 a |
| B-type Procyanidin trimer | 6.6 ± 0.2 a | 6.4 ± 0.2 a | 5.4 ± 0.2 b |
| (+)-Catechin | 10.5 ± 0.2 e | 9.9 ± 0.7 e | 17.9 ± 0.2 a |
| A-type Procyanidin trimer | 84.0 ± 1.9 a | 81.1 ± 2.7 a | 9.6 ± 0.1 g |
| Double-charged pentameric procyanidin | 1.9 ± 0.1 a | 1.4 ± 0.3 b | 0.6 ± 0.0 c |
| Double-charged heptameric procyanidin | 4.6 ± 0.2 ab | 3.8 ± 0.1 b | 1.9 ± 0.1 d |
| B-type Procyanidin trimer | 3.2 ± 0.1 b | 2.7 ± 0.1 c | 2.1 ± 0.1 d |
| (−)-Epicatechin | 2.5 ± 0.1 a | 0.0 ± 0.0 d | 2.5 ± 0.1 a |
| Sum | 264.7 ± 5.3 a | 248.3 ± 4.8 b | 164.2 ± 1.8 e |
| Flavonols | |||
| Kaempferol hexoside-dideoxyhexoside | 0.6 ± 0.0 a | 0.5 ± 0.0 a | 0.3 ± 0.0 b |
| Quercetin 3-O-rutinoside | 0.8 ± 0.0 cd | 0.7 ± 0.0 d | 2.0 ± 0.1 a |
| Quercetin 3-O-galactoside | 1.3 ± 0.1 a | 1.1 ± 0.1 a | 0.4 ± 0.0 d |
| Quercetin 3-O-glucoside | 0.6 ± 0.0 a | 0.4 ± 0.0 a | 0.3 ± 0.0 b |
| Isorhamnetin 3-O-rhamnosyl-hexoside | 1.5 ± 0.1 b | 1.3 ± 0.1 c | 2.9 ± 0.2 a |
| Quercetin-acylated-hexoside | 2.1 ± 0.1 a | 1.9 ± 0.1 b | 2.4 ± 0.2 a |
| Isorhamnetin 3-O-rhamnosyl-hexoside | 2.1 ± 0.0 a | 1.8 ± 0.1 b | 2.1 ± 0.1 a |
| Isorhamnetin 3-O-galactoside | 1.5 ± 0.0 a | 1.5 ± 0.1 a | 0.3 ± 0.0 c |
| Kaempferol 3-O-glucoside | 1.8 ± 0.1 b | 1.8 ± 0.0 b | 0.4 ± 0.0 e |
| Isorhamnetin 3-O-glucoside | 0.3 ± 0.0 b | 0.1 ± 0.0 a | 0.0 ± 0.0 c |
| Kaempferol 3-O-6-acetylated-glucoside | 1.1 ± 0.1 b | 0.9 ± 0.0 bc | 1.5 ± 0.1 a |
| Isorhamnetin-acylated-hexoside | 3.6 ± 0.1 b | 3.3 ± 0.1 c | 3.9 ± 0.1 a |
| Sum | 17.3 ± 0.9 a | 15.1 ± 0.5 c | 16.5 ± 0.2 b |
| Flavones | |||
| Apigenin pentoside | 4.1 ± 0.1 a | 4.1 ± 0.2 a | 0.9 ± 0.0 e |
| Hydroquinones | |||
| Arbutin | 40.9 ± 1.8 e | 40.8 ± 2.8 e | 155.9 ± 2.9 a |
| TOTAL | 515.0 ± 5.2 b | 492.9 ± 8.9 b | 576.7 ± 10.5 a |
| Days | DPPH | ABTS | FRAP | α-Glucosidase | α-Amylase | AChE | BuChE | COX1 | COX2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| μM Tx/100 mL | [% of Inhibition] | |||||||||
| Fresh apple juice | 590.99 ± 0.5 b | 1295.81 ± 1.2 d | 1097.24 ± 0.9 l | 10.87 ± 0.6 c | 5.69 ± 0.2 c | 10.70 ± 0.3 d | 10.27 ± 0.0 d | 88.60 ± 0.5 b | 36.12 ± 0.1 c | |
| Apple juice | Day 0 | 596.27 ± 0.4 b | 1297.7 ± 0.6 d | 1275.83 ± 0.5 h | 10.88 ± 0.5 c | 5.69 ± 0.1 c | 10.76 ± 0.1 d | 10.28 ± 0.1 d | 88.63 ± 0.7 b | 36.15 ± 0.2 c |
| Day 2 | 596.07 ± 0.2 b | 1351.18 ± 0.4 c | 1595.24 ± 0.8 c | |||||||
| Day 4 | 604.33 ± 0.0 ab | 1530.51 ± 0.7 b | 1633.42 ± 0.4 b | |||||||
| Day 6 | 613.18 ± 0.6 a | 1646.28 ± 0.1 a | 2138.81 ± 0.2 a | |||||||
| Day 8 | 238.27 ± 0.7 d | 978.70 ± 0.2 g | 1510.67 ± 0.1 d | |||||||
| Day 10 | 245.04 ± 0.2 c | 823.29 ± 0.9 i | 1388.73 ± 0.4 f | |||||||
| Day 12 | 246.53 ± 0.8 c | 669.13 ± 1.0 j | 1300.47 ± 0.1 g | |||||||
| Day 14 | 240.63 ± 0.1 bd | 675.09 ± 0.8 j | 990.5 ± 0.9 m | 60.82 ± 0.8 a | 57.15 ± 0.6 a | 18.56 ± 0.2 b | 19.30 ± 0.4 b | 93.91 ± 1.0 a | 42.48 ± 0.2 b | |
| Fresh pear juice | 211.11 ± 1.4 f | 598.03 ± 0.8 k | 836.13 ± 1.5 p | 5.60 ± 0.3 d | 2.31 ± 0.3 d | 11.30 ± 0.1 c | 13.90 ± 0.4 c | 75.36 ± 0.2 d | 36.41 ± 0.2 c | |
| Pear juice | Day 0 | 215.86 ± 0.7 f | 811.96 ± 0.3 ii | 873.90 ± 1.0 o | 5.66 ± 0.1 d | 2.38 ± 0.2 d | 11.31 ± 0.2 c | 14.06 ± 0.2 c | 75.58 ± 0.6 d | 36.68 ± 0.3 c |
| Day 2 | 222.54 ± 0.5 e | 824.54 ± 0.1 i | 1129.27 ± 0.9 k | |||||||
| Day 4 | 224.59 ± 0.4 e | 1184.45 ± 0.7 f | 1205.22 ± 1.1 i | |||||||
| Day 6 | 226.87 ± 0.6 e | 1264.98 ± 0.9 e | 1642.86 ± 0.9 b | |||||||
| Day 8 | 197.46 ± 0.9 h | 677.31 ± 0.2 j | 941.23 ± 0.6 n | |||||||
| Day 10 | 206.11 ± 0.1 g | 677.73 ± 0.4 j | 1151.44 ± 0.8 j | |||||||
| Day 12 | 212.40 ± 0.9 f | 896.90 ± 0.6 h | 1126.80 ± 0.7 k | |||||||
| Day 14 | 219.00 ± 0.6 ef | 993.17 ± 0.9 g | 1435.13 ± 0.6 e | 42.30 ± 0.3 b | 35.82 ± 0.5 b | 19.39 ± 0.3 a | 25.10 ± 0.4 a | 81.73 ± 0.8 c | 61.48 ± 0.5 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grondalska, J.; Kolniak-Ostek, J. Evaluation of Anti-Inflammatory, Antidiabetic, Antioxidant, and Anticholinergic Activities, as Well as Chemical Composition and Polyphenolic Compounds in Novel SCOBY-Fermented Juices. Molecules 2025, 30, 1940. https://doi.org/10.3390/molecules30091940
Grondalska J, Kolniak-Ostek J. Evaluation of Anti-Inflammatory, Antidiabetic, Antioxidant, and Anticholinergic Activities, as Well as Chemical Composition and Polyphenolic Compounds in Novel SCOBY-Fermented Juices. Molecules. 2025; 30(9):1940. https://doi.org/10.3390/molecules30091940
Chicago/Turabian StyleGrondalska, Joanna, and Joanna Kolniak-Ostek. 2025. "Evaluation of Anti-Inflammatory, Antidiabetic, Antioxidant, and Anticholinergic Activities, as Well as Chemical Composition and Polyphenolic Compounds in Novel SCOBY-Fermented Juices" Molecules 30, no. 9: 1940. https://doi.org/10.3390/molecules30091940
APA StyleGrondalska, J., & Kolniak-Ostek, J. (2025). Evaluation of Anti-Inflammatory, Antidiabetic, Antioxidant, and Anticholinergic Activities, as Well as Chemical Composition and Polyphenolic Compounds in Novel SCOBY-Fermented Juices. Molecules, 30(9), 1940. https://doi.org/10.3390/molecules30091940






