Therapeutic Potential of Ficus benjamina: Phytochemical Identification and Investigation of Antimicrobial, Anticancer, Pro-Wound-Healing, and Anti-Inflammatory Properties
Abstract
:1. Introduction
2. Results
2.1. Identification of Phytochemicals
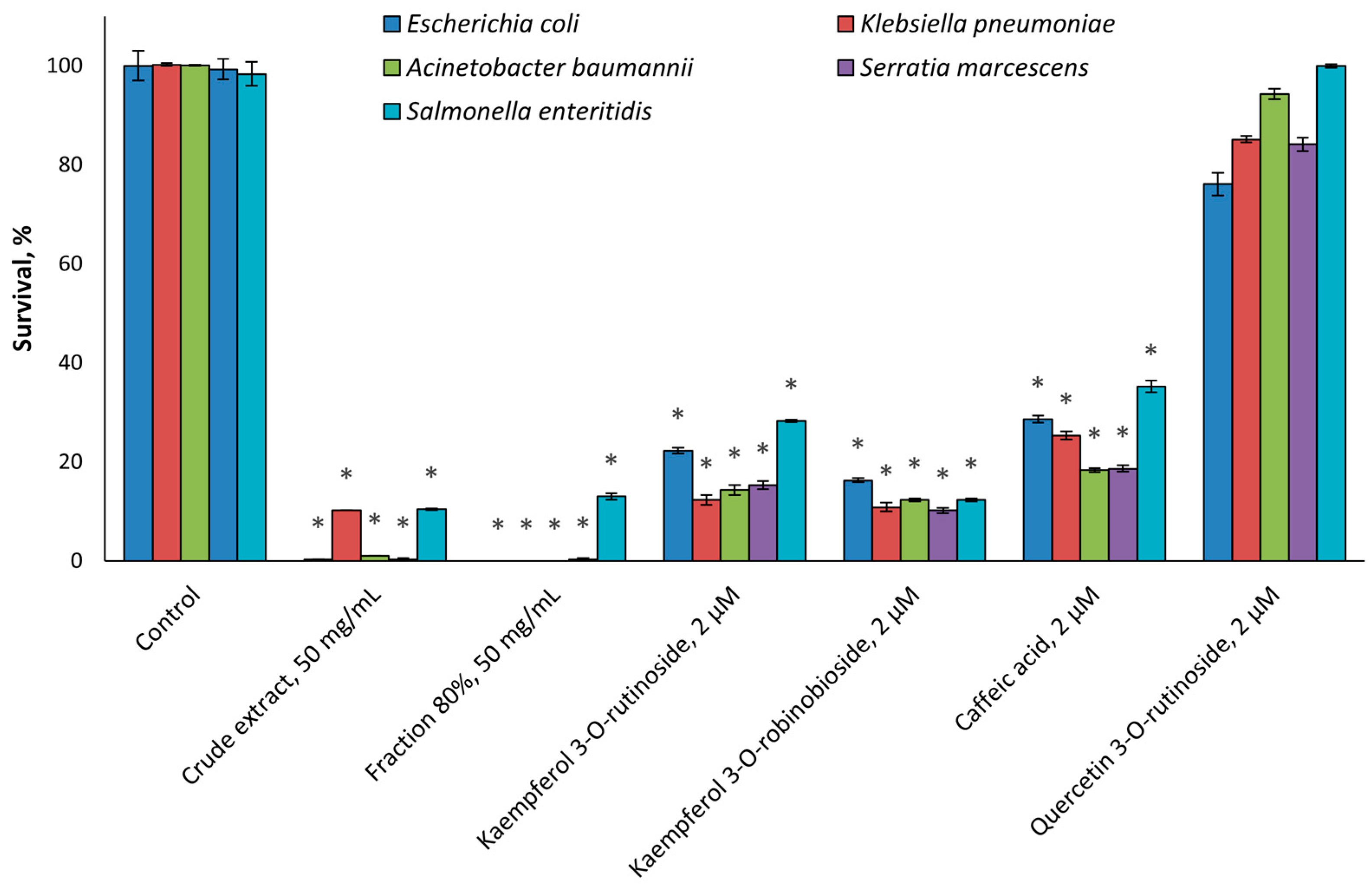
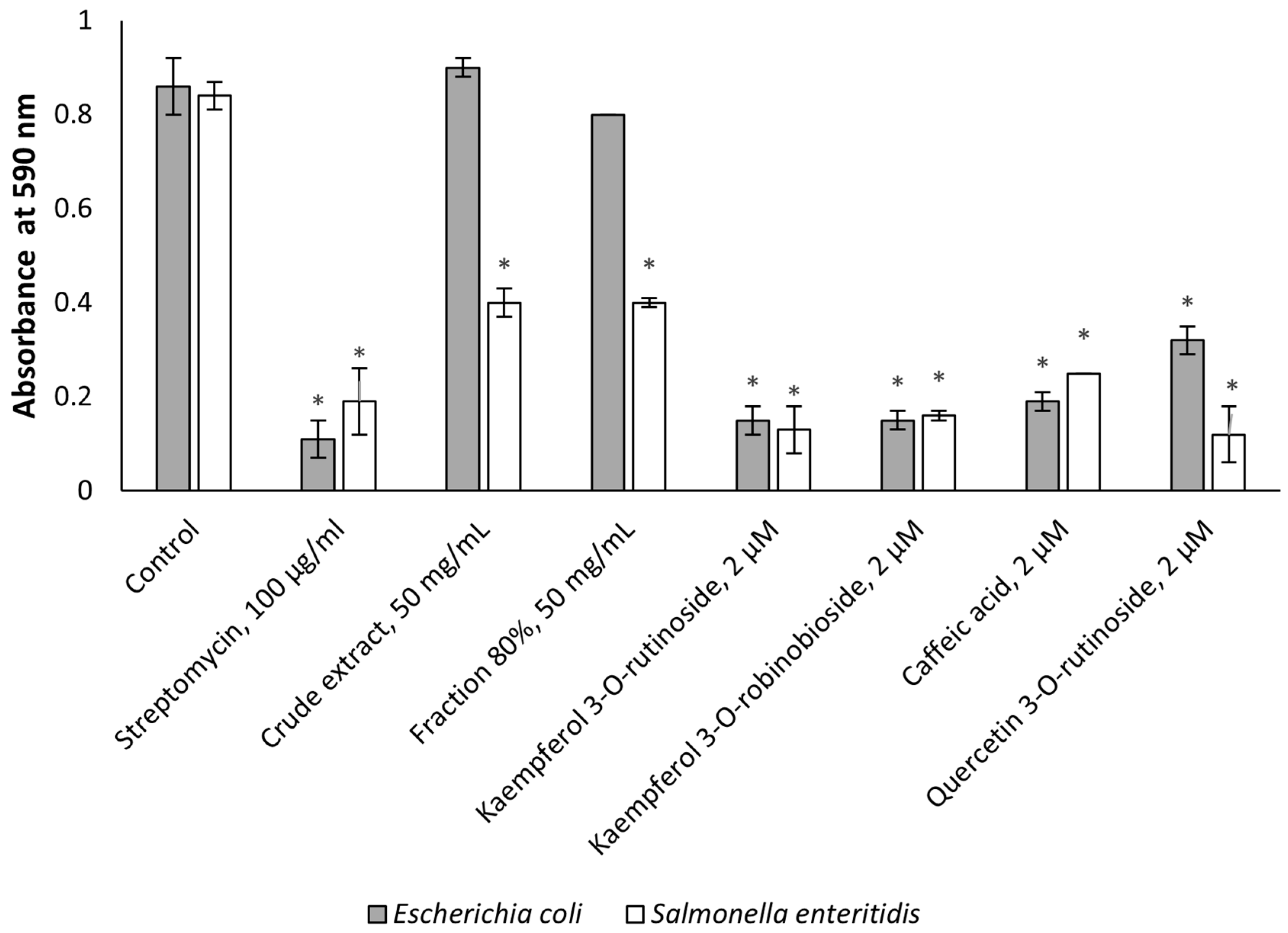
2.2. Antimicrobial Activity
2.3. Effect on Cancer Cell Line Proliferation
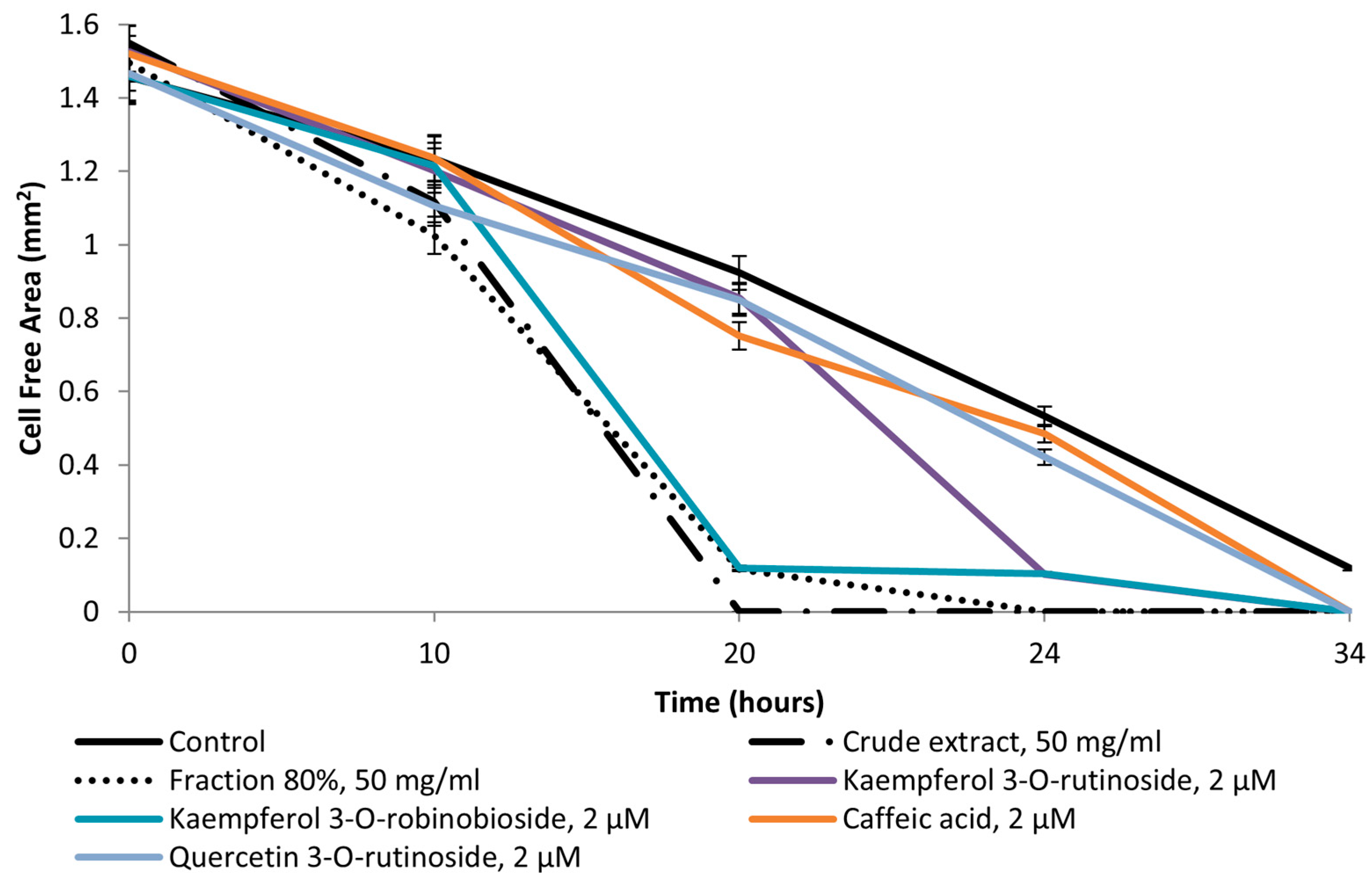
2.4. Pro-WH Activity
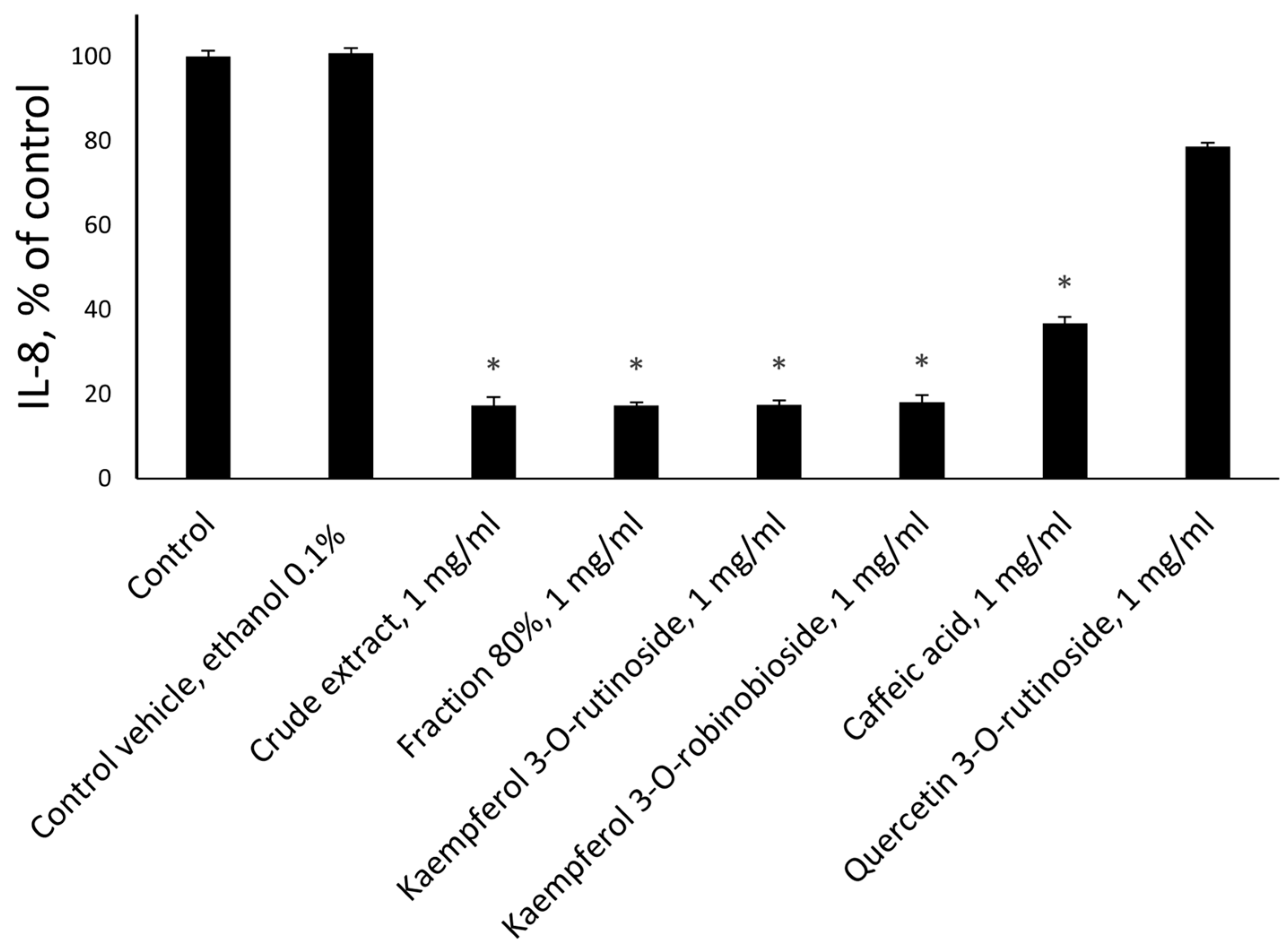
2.5. Anti-Inflammatory Activity
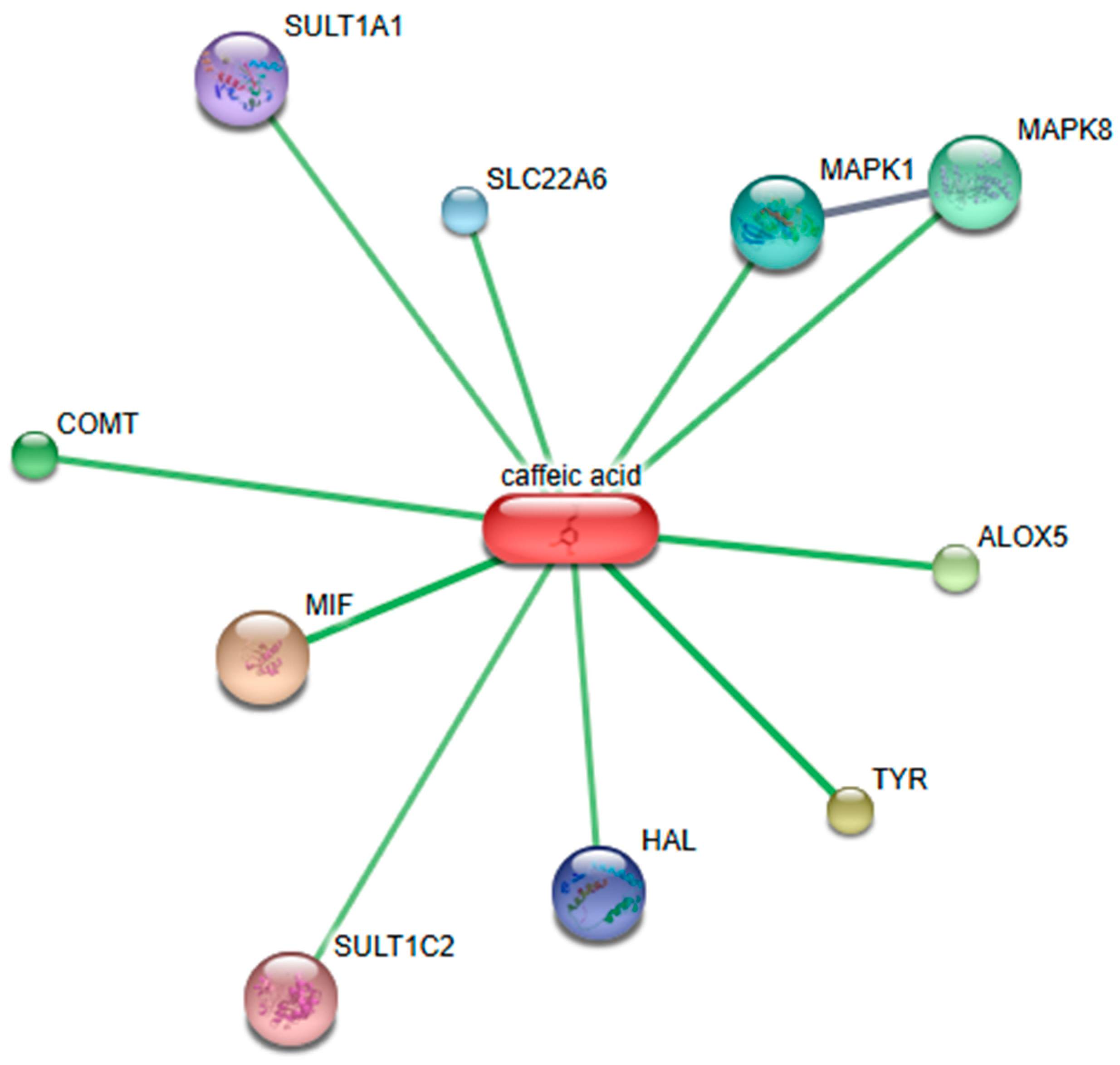
2.6. Bioinformatic Analysis of Identified Phytochemicals
3. Discussion
4. Materials and Methods
4.1. Preparation of Plant Material
4.2. Compounds
4.3. Phytochemical Analysis
4.4. Bacterial Strains
4.5. Investigation of Antimicrobial Activities
4.6. Investigation of Effect on Cancer Cell Line Proliferation
4.7. Investigation of Pro-WH Activity
4.8. Investigation of Pro-Inflammatory Cytokine Secretion
4.9. Bioinformatic Analysis
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yarmolinsky, L.; Zaccai, M.; Ben-Shabat, S.; Mills, D.; Huleihel, M. Antiviral Activity of Ethanol Extracts of Ficus Binjamina and Lilium Candidum in Vitro. N. Biotechnol. 2009, 26, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Mousa, O.; Vuorela, P.; Kiviranta, J.; Wahab, S.A.; Hiltunen, R.; Vuorela, H. Bioactivity of Certain Egyptian Ficus Species. J. Ethnopharmacol. 1994, 41, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rasool, N.; Rizwan, K.; Zubair, M.; Riaz, M.; Zia-Ul-Haq, M.; Rana, U.A.; Nafady, A.; Jaafar, H.Z. Chemical Composition and Biological Studies of Ficus benjamina. Chem. Cent. J. 2014, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, L.; Huleihel, M.; Zaccai, M.; Ben-Shabat, S. Potent Antiviral Flavone Glycosides from Ficus benjamina Leaves. Fitoterapia 2012, 83, 362–367. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress in Infection and Consequent Disease. Oxid. Med. Cell. Longev. 2017, 2017, 3496043. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative Stress and Its Role in Cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef]
- Menéndez-Velázquez, A.; García-Delgado, A.B. A Novel Photopharmacological Tool: Dual-Step Luminescence for Biological Tissue Penetration of Light and the Selective Activation of Photodrugs. Int. J. Mol. Sci. 2023, 24, 9404. [Google Scholar] [CrossRef]
- Cantón, R.; Gijón, D.; Ruiz-Garbajosa, P. Antimicrobial Resistance in ICUs: An Update in the Light of the COVID-19 Pandemic. Curr. Opin. Crit. Care 2020, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Huang, M.; Melander, C.; Kjellerup, B. V Dispersal and Inhibition of Biofilms Associated with Infections. J. Appl. Microbiol. 2020, 128, 1279–1288. [Google Scholar] [CrossRef]
- Donlan, R.M. Role of Biofilms in Antimicrobial Resistance. ASAIO J. 2000, 46, S47–S52. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.; Burmester, J.K. Gene Therapy for Cancer Treatment: Past, Present and Future. Clin. Med. Res. 2006, 4, 218–227. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Bassal, M.; Mertens, A.C.; Taylor, L.; Neglia, J.P.; Greffe, B.S.; Hammond, S.; Ronckers, C.M.; Friedman, D.L.; Stovall, M.; Yasui, Y.Y.; et al. Risk of Selected Subsequent Carcinomas in Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2006, 24, 476–483. [Google Scholar] [CrossRef]
- Al-Kawlani, B.; Murrieta-Coxca, J.M.; Chaiwangyen, W.; Fröhlich, K.; Fritzsche, A.; Winkler, S.; Markert, U.R.; Morales-Prieto, D.M. Doxorubicin Induces Cytotoxicity and MiR-132 Expression in Granulosa Cells. Reprod. Toxicol. 2020, 96, 95–101. [Google Scholar] [CrossRef]
- Roti Roti, E.C.; Salih, S.M. Dexrazoxane Ameliorates Doxorubicin-Induced Injury in Mouse Ovarian Cells. Biol. Reprod. 2012, 86, 96. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- da Cruz, R.C.; Agertt, V.; Boligon, A.A.; Janovik, V.; Anraku de Campos, M.M.; Guillaume, D.; Athayde, M.L. In Vitro Antimycobacterial Activity and HPLC-DAD Screening of Phenolics from Ficus benjamina L. and Ficus luschnathiana (Miq.) Miq. Leaves. Nat. Prod. Res. 2012, 26, 2251–2254. [Google Scholar] [CrossRef] [PubMed]
- Polatoglu, K. “Chemotypes”—A Fact That Should Not Be Ignored in Natural Product Studies. Nat. Prod. J. 2013, 3, 10–14. [Google Scholar] [CrossRef]
- Dai, J.; Shen, D.; Yoshida, W.Y.; Parrish, S.M.; Williams, P.G. Isoflavonoids from Ficus benjamina and Their Inhibitory Activity on BACE1. Planta Med. 2012, 78, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.-M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Apostolidis, E.; Labbe, R.G.; Shetty, K. Inhibition of Staphylococcus Aureus by Phenolic Phytochemicals of Selected Clonal Herbs Species of Lamiaceae Family and Likely Mode of Action through Proline Oxidation. Food Biotechnol. 2007, 21, 71–89. [Google Scholar] [CrossRef]
- Dos Santos, J.F.S.; Tintino, S.R.; de Freitas, T.S.; Campina, F.F.; de A Menezes, I.R.; Siqueira-Júnior, J.P.; Coutinho, H.D.M.; Cunha, F.A.B. In Vitro e in Silico Evaluation of the Inhibition of Staphylococcus Aureus Efflux Pumps by Caffeic and Gallic Acid. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 22–28. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial Effect of Caffeic Acid Phenethyl Ester on Cariogenic Bacteria and Streptococcus Mutans Biofilms. Antimicrob. Agents Chemother. 2020, 64, e00251-20. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Nakonechny, F.; Budovsky, A.; Zeigerman, H.; Khalfin, B.; Sharon, E.; Yarmolinsky, L.; Ben-Shabat, S.; Nisnevitch, M. Antimicrobial and Antiviral Compounds of Phlomis Viscosa Poiret. Biomedicines 2023, 11, 441. [Google Scholar] [CrossRef]
- Mendiondo, M.E.; Juáreza, B.E.; Zampini, C.; Isla, M.I.; Ordoñez, R. Bioactivities of Chuquiraga Straminea Sandwith. Nat. Prod. Commun. 2011, 6, 965–968. [Google Scholar] [CrossRef]
- Boff, J.S.; Reis, A.C.; Patricia, D.S.G.; Pretto, V.E.; Garlet, C.G.; Melo, A.A.; Bernardi, O. The Effect of Synergistic Compounds on the Susceptibility of Euschistus Heros (Hemiptera: Pentatomidae) and Chrysodeixis Includens (Lepidoptera: Noctuidae) to Pyrethroids. Environ. Entomol. 2022, 51, 421–429. [Google Scholar] [CrossRef]
- Boff, J.S.; Reis, A.C.; de Oliveira, J.L.; Gross, R.B.; Fraceto, L.F.; Melo, A.A.; Bernardi, O. Development and Biological Evaluation of Nanoencapsulated-Based Pyrethroids with Synergists for Resistance Management of Two Soybean Pests: Insights for New Insecticide Formulations. Pest Manag. Sci. 2023, 79, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.T.B.; Araújo-Filho, H.G.; Barreto, A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S.; Barreto, R.S.S. Wound Healing Properties of Flavonoids: A Systematic Review Highlighting the Mechanisms of Action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory Mechanism against Oxidative Stress of Caffeic Acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Shady, N.H.; Mostafa, N.M.; Fayez, S.; Abdel-Rahman, I.M.; Maher, S.A.; Zayed, A.; Saber, E.A.; Khowdiary, M.M.; Elrehany, M.A.; Alzubaidi, M.A.; et al. Mechanistic Wound Healing and Antioxidant Potential of Moringa Oleifera Seeds Extract Supported by Metabolic Profiling, In Silico Network Design, Molecular Docking, and In Vivo Studies. Antioxidants 2022, 11, 1743. [Google Scholar] [CrossRef]
- Petpiroon, N.; Suktap, C.; Pongsamart, S.; Chanvorachote, P.; Sukrong, S. Kaempferol-3-O-Rutinoside from Afgekia Mahidoliae Promotes Keratinocyte Migration through FAK and Rac1 Activation. J. Nat. Med. 2015, 69, 340–348. [Google Scholar] [CrossRef]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Garcia-Ruiz, P.A.; Saura-Sanmartin, A.; Berna, J.; Rodríguez-López, J.N.; Garcia-Canovas, F. Action of Tyrosinase on Caffeic Acid and Its N-Nonyl Ester. Catalysis and Suicide Inactivation. Int. J. Biol. Macromol. 2018, 107, 2650–2659. [Google Scholar] [CrossRef]
- Doiron, J.A.; Leblanc, L.M.; Hébert, M.J.G.; Levesque, N.A.; Paré, A.F.; Jean-François, J.; Cormier, M.; Surette, M.E.; Touaibia, M. Structure-Activity Relationship of Caffeic Acid Phenethyl Ester Analogs as New 5-Lipoxygenase Inhibitors. Chem. Biol. Drug Des. 2017, 89, 514–528. [Google Scholar] [CrossRef]
- Yarmolinsky, L.L.; Budovsky, A.; Yarmolinsky, L.L.; Khalfin, B.; Glukhman, V.; Ben-Shabat, S. Effect of Bioactive Phytochemicals from Phlomis Viscosa Poiret on Wound Healing. Plants 2019, 8, 609. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Budovsky, A.; Ben-Shabat, S.; Khalfin, B.; Gorelick, J.; Bishitz, Y.; Miloslavski, R.; Yarmolinsky, L. Recent Updates on the Phytochemistry and Pharmacological Properties of Phlomis Viscosa Poiret. Rejuvenation Res. 2019, 22, 282–288. [Google Scholar] [CrossRef]
- Fraifeld, V.; Seidman, R.; Sagi, O.; Muradian, K.; Wolfson, M. Aurintricarboxylic Acid Decreases Proliferative Potential of SKOV3 and MCF7 Human Carcinoma Cells. Anticancer Res. 2001, 21, 1975–1978. [Google Scholar]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING V10: Protein-Protein Interaction Networks, Integrated over the Tree of Life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]


| Compound | Molecular Structure | Methods of Identification | Conc., mg/kg |
|---|---|---|---|
| Caffeic acid |  | HPLC LC-ESI-MS | 7.6 ± 0.58 |
| Quercetin 3-O-rutinoside | 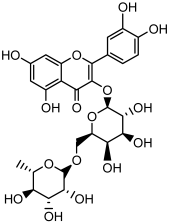 | HPLC LC-ESI-MS | 11.5 ± 0.21 |
| Kaempferol 3-O-robinobioside | 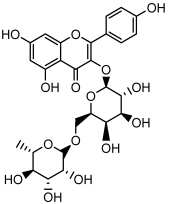 | HPLC LC-ESI-MS | 4.3 ± 0.19 |
| Kaempferol 3-O- rutinoside |  | HPLC LC-ESI-MS | 3.9 ± 0.55 |
| Cyclopropyl Methylcarbinol | 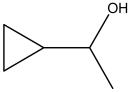 | HS-GS-MS | 7.11 ± 0.32 |
| 2-Propanol-1,1 dimethoxy acetate | 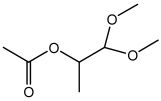 | HS-GS-MS | 46.91 ± 0.18 |
| Acetic acid,1- methyl-ethyl ester | 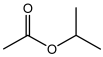 | HS-GS-MS | 26.33 ± 0.97 |
| Gluoctanic acid lactone | 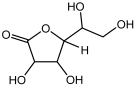 | HS-GS-MS | 286.63 ± 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahan, A.; Yarmolinsky, L.; Budovsky, A.; Khalfin, B.; Ben-Shabat, S. Therapeutic Potential of Ficus benjamina: Phytochemical Identification and Investigation of Antimicrobial, Anticancer, Pro-Wound-Healing, and Anti-Inflammatory Properties. Molecules 2025, 30, 1961. https://doi.org/10.3390/molecules30091961
Dahan A, Yarmolinsky L, Budovsky A, Khalfin B, Ben-Shabat S. Therapeutic Potential of Ficus benjamina: Phytochemical Identification and Investigation of Antimicrobial, Anticancer, Pro-Wound-Healing, and Anti-Inflammatory Properties. Molecules. 2025; 30(9):1961. https://doi.org/10.3390/molecules30091961
Chicago/Turabian StyleDahan, Arik, Ludmila Yarmolinsky, Arie Budovsky, Boris Khalfin, and Shimon Ben-Shabat. 2025. "Therapeutic Potential of Ficus benjamina: Phytochemical Identification and Investigation of Antimicrobial, Anticancer, Pro-Wound-Healing, and Anti-Inflammatory Properties" Molecules 30, no. 9: 1961. https://doi.org/10.3390/molecules30091961
APA StyleDahan, A., Yarmolinsky, L., Budovsky, A., Khalfin, B., & Ben-Shabat, S. (2025). Therapeutic Potential of Ficus benjamina: Phytochemical Identification and Investigation of Antimicrobial, Anticancer, Pro-Wound-Healing, and Anti-Inflammatory Properties. Molecules, 30(9), 1961. https://doi.org/10.3390/molecules30091961









