Abstract
Trimethylsilyl enol ethers of 1,3-diketones are generated “in situ” or obtained in high isolated yield by the reaction of 1,3-diketones with trimethylcyanosilane in various solvents such as cyclohexane, hexane, benzene, methylene chloride, chloroform, carbon tetrachloride, and acetonitrile.
Introduction
Silyl enol ethers of 1,3-diketones are effective silylating agents and useful precursors for the preparation of Diels-Alder dienes, 1,3-bis(silyloxy)buta-1,3-dienes. They have found extensive application in organic synthesis. The preparation of the silyl enol ethers of 1,3-diketones resulted in only fair yields by using reagents such as trimethylchlorosilane/strong bases [1], bis(trimethylsilyl)formamide [2], bis(trimethylsilyl)acetamide [3], triethylsilyl-thiobenzene [4], trimethylsilyl trifluoromethanesulfonate [5], and ethyl trimethylsilylacetate/tetra-n-butylamonium fluoride [6], employed previously for the silylation of carbonyl compounds. A few methods for the preparation of trimethylsilyl enol ethers of 1,3-diketones have been developed by using hexamethyldisilazane [7], hexamethyldisilazane/imidazole [8], 2-oxo-3-trimethylsilyltetrahydro-1,3-oxazole [9], and (trimethylsilyl)-diethylamine /methyl iodide [10].
Trimethylcyanosilane (Me3SiCN) has been used widely in organic synthesis [11,12]. It has been used to protect a variety of alcohols, phenols, carboxylic acids, amines and thiols [13]. Previously we have shown that α-cyano enols were conveniently converted to trimethylsilyl enol ethers by using Me3SiCN [14]. We now wish to report a method for the silylation of 1,3-diketones to their trimethylsilyl enol ethers by using Me3SiCN.
Results and Discussion
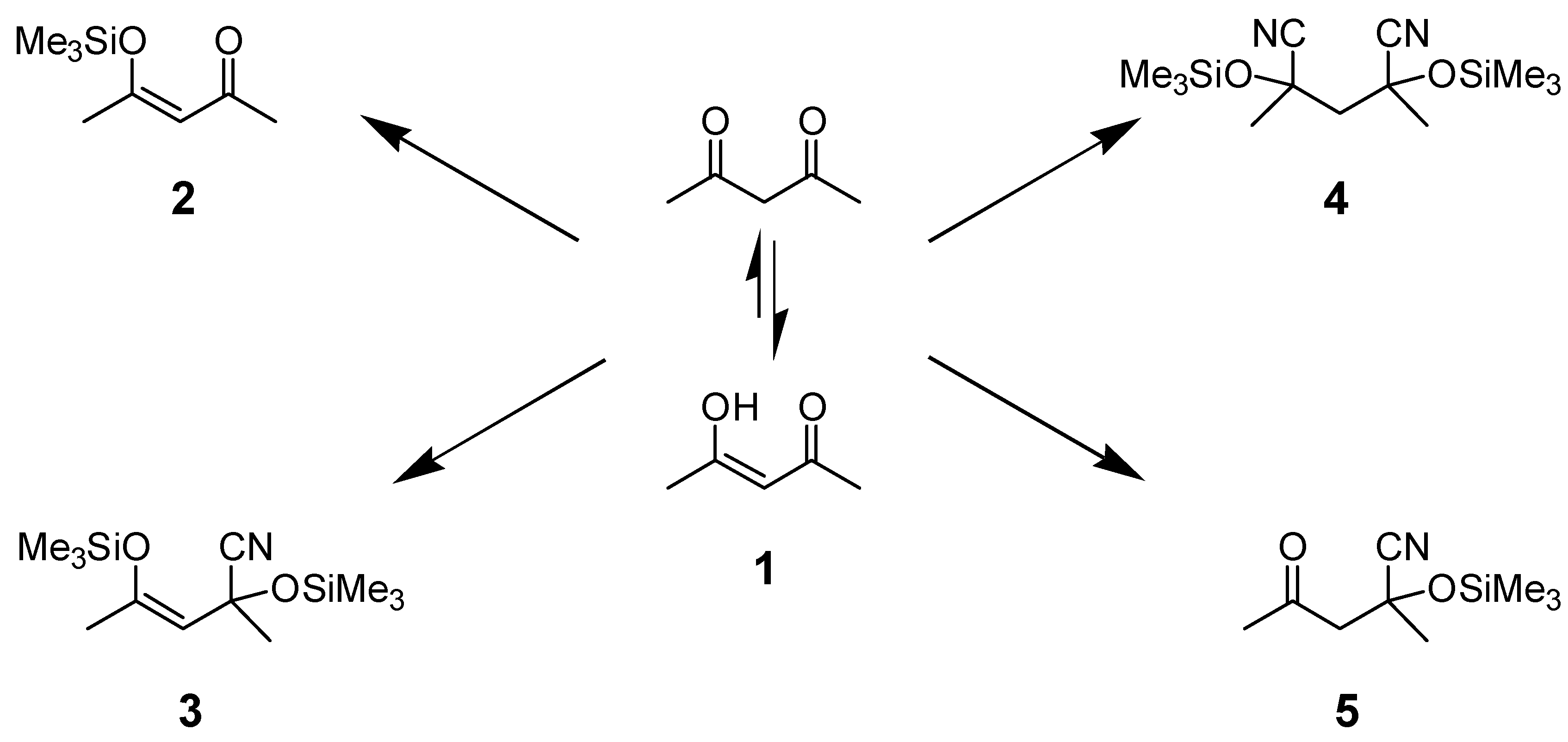
The reaction of Me3SiCN with 1,3-diketones is complicated as shown in Scheme 1. The products formed depend upon the experimental conditions: temperature, time, the stoichiometric amount of Me3SiCN added, and the catalyst used [11]. The reaction of pentan-1,3-dione (1) with excess Me3SiCN formed trimethylsilyl enol ether 2 at room temperature or the adduct 3 at elevated temperatures (90°C) [11,15]. Alternatively, the reaction yielded compounds 4 or/and 5 under different conditions, [15,16,17].
Scheme 1.
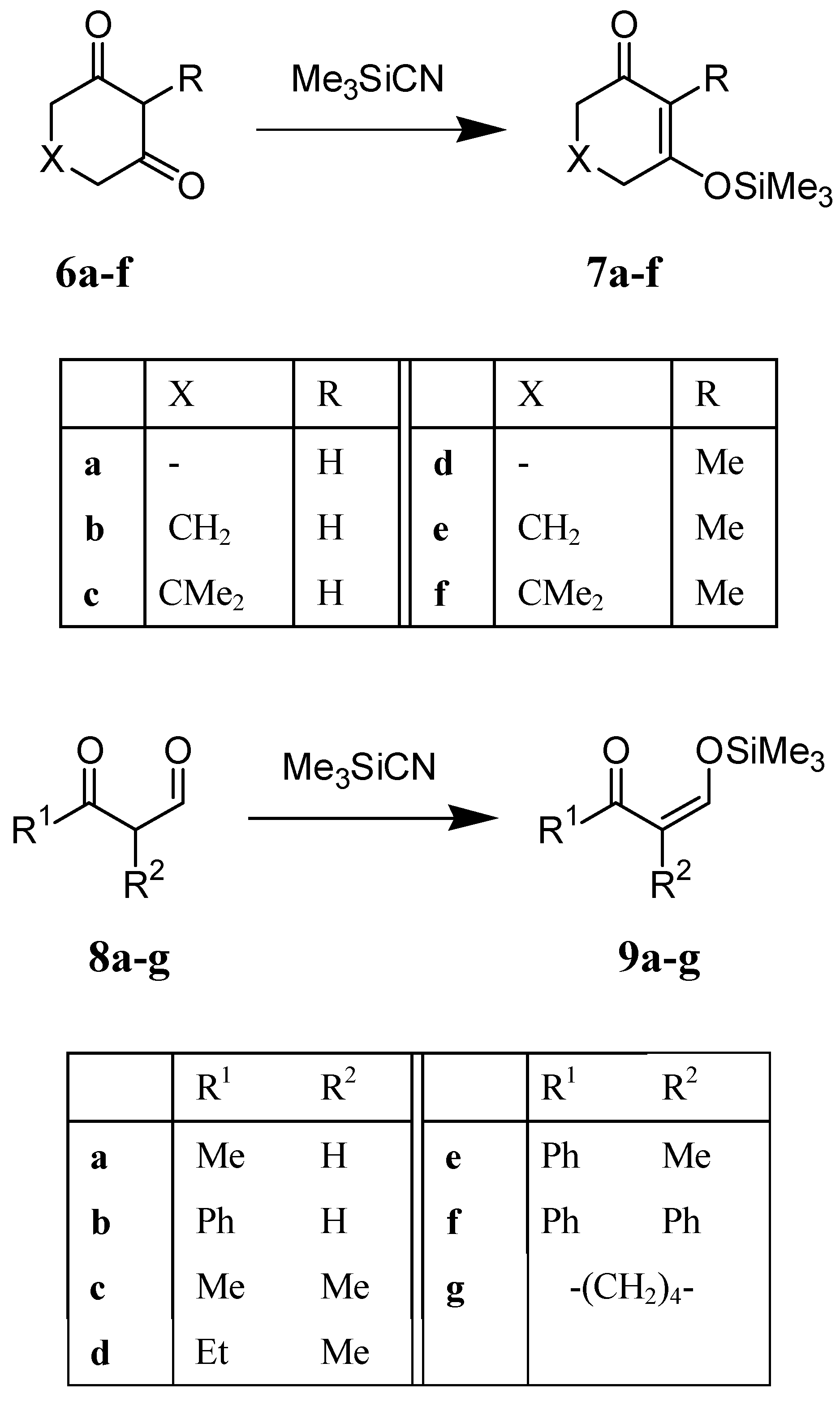
We observed that the silylation proceeded very smoothly at ambient temperature when Me3SiCN was added to the 1,3-diketones 6a-f or 8a-g in CDCl3 solution. The reactions were completed in 10 min (monitored by 1H NMR) and produced the corresponding trimethylsilyl enol ethers 7a-f or 9a-g in quantitative yield (Scheme 2). The reactions of Me3SiCN with 1,3-diketones can be carried out in various solvents. For example, the reaction of Me3SiCN with cyclohexane-1,3-dione 6b proceeded very smoothly in cyclohexane, hexane, benzene, carbon tetrachloride, chloroform, methylene chloride, and acetonitrile, and afforded 7b in quantitative yield.
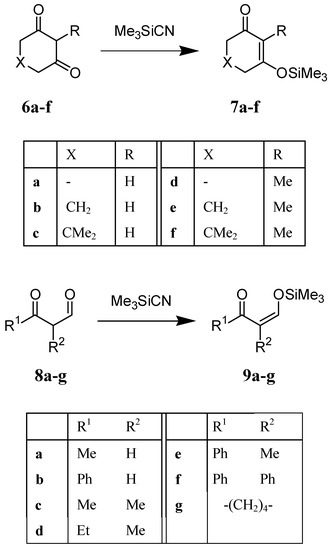
Scheme 2.
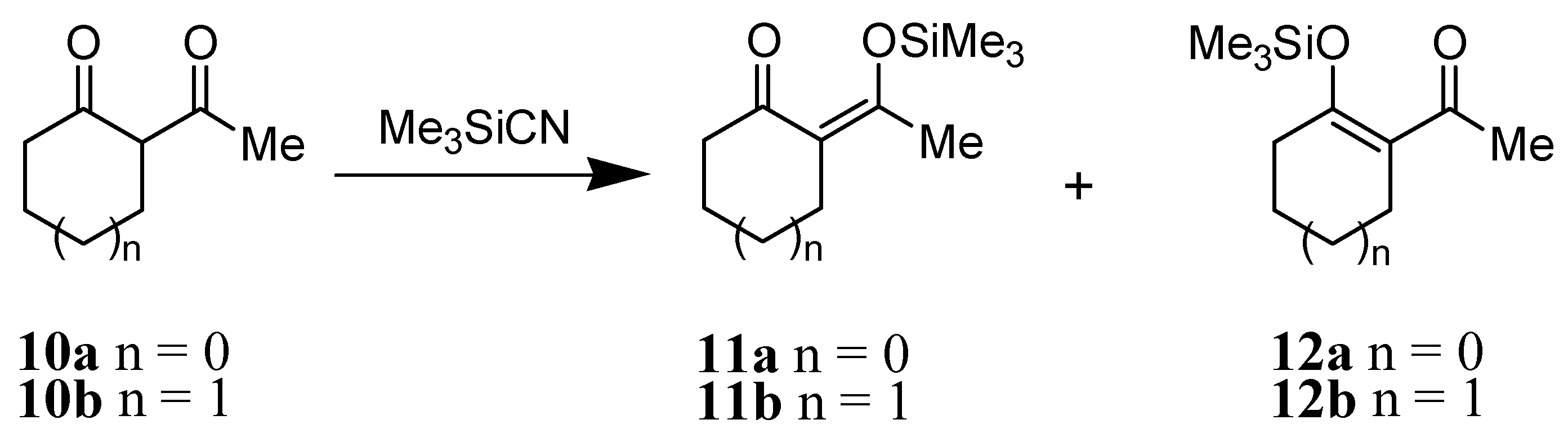
The reaction of Me3SiCN with 2-acetylcyclopentanone 10a gave a ca. 1:1 ratio mixture of the two trimethylsilyl enol ethers 11a and 12a in quantitative yield (Scheme 3). Interestingly, the reaction of Me3SiCN toward 2-acetylcyclohexanone 10b was slow and produced only trimethylsilyl enol ether 12b in 50% yield after 24 h.
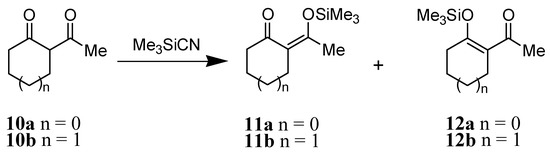
Scheme 3.
Conclusion
In summary, we have shown that Me3SiCN is an efficient silylation reagent for silylating 1,3-diketones, especially for the cyclic 1,3-diketones and 2-(hydroxymethylene)ketones. The method provides a convenient way to trimethylsilyl enol ethers of 1,3-diketones under neutral conditions.
Experimental
The general procedure for the preparation of trimethylsilyl enol ethers of 1,3-diketones is as follows: Me3SiCN (0.205 g, 2.05 mmol) was added to 1,3-diketone (2 mmol) in dry CDCl3 (3 ml) or other suitable solvent under a nitrogen atmosphere. The progress of the reaction was directly monitored by 1H-NMR. The reaction is generally completed at room temperature in 5-10 min. After the completion of the reaction, a stream of nitrogen was allowed to pass through the mixture for 5 min. The resulting solution contains the trimethylsilyl enol ether. The solvent was evaporated under reduced pressure. The residue is the desired compound.
3-(Trimehylsilyloxy)cyclopent-2-en-1-one (7a): 1H NMR(CDCl3): 5.30 (t, J = 1.0, H-2), 2.57 (m, 2H, H-4), 2.42 (m, 2H, H-5), 0.33 (s, 9H, OSiMe3).
3-(Trimehylsilyloxy)cyclohex-2-en-1-one (7b): 1H NMR(CDCl3): 5.37 (t, J = 0.7, H-2), 2.36 (td, J = 6.2, 0.7, 2H, H-4), 2.32 (t, J = 6.7, 2H, H-6), 1.97 (m, 2H, H-5), 0.30 (s, 9H, OSiMe3).
5,5-Dimethyl-3-(trimehylsilyloxy)cyclohex-2-en-1-one (7c): 1H NMR(CDCl3): 5.35 (t, J = 0.6, H-2), 2.24 (d, J = 0.6, 2H, H-4), 2.17 (s, 2H, H-6), 1.07 (s, 6H, 2 Me), 0.30 (s, 9H, OSiMe3).
2-Methyl-3-(trimehylsilyloxy)cyclopent-2-en-1-one (7d): 1H NMR(CDCl3): 2.51 (m, 2H, H-4), 2.43 (m, 2H, H-5), 1.58 (t, J = 1.8, Me), 0.33 (s, 9H, OSiMe3).
2-Methyl-3-(trimehylsilyloxy)cyclohex-2-en-1-one (7e): 1H NMR(CDCl3): 2.40 (tq, J = 6.4, 1.6, 2H, H-4), 2.35 (t, J = 6.7, 2H, H-6), 1.94 (m, 2H, H-5), 1.66 (t, J = 1.6, Me), 0.28 (s, 9H, OSiMe3).
5,5-Dimethyl-2-methyl-3-(trimehylsilyloxy)cyclohex-2-en-1-one (7f): 1H NMR(CDCl3): 2.26 (q, J = 1.5, 2H, H-4), 2.23 (s, 2H, H-6), 1.67 (t, J = 1.5, 3H, Me), 1.07 (s, 6H, 2 Me), 0.28 (s, 9H, OSiMe3).
4-(Trimehylsilyloxy)but-3-en-2-one (9a). 1H NMR(CDCl3): 7.51 (d, J = 12.2, 1H, H-4), 5.73 (d, J = 12.2, 1H, H-3), 2.17 (s, 3H, Me), 0.29 (s, 9H, OSiMe3).
1-Phenyl-3-(trimehylsilyloxy)prop-2-en-1-one (9b). 1H NMR(CDCl3): 7.87-7.91 (m, 2H, Ph), 7.75 (d, J = 11.6, 1H, H-3), 7.50-7.55 (m, 1H, Ph), 7.42-7.47 (m, 2H, Ph), 6.53 (d, J = 11.6, 1H, H-2), 0.32 (s, 9H, OSiMe3).
3-Methyl-4-(trimehylsilyloxy)but-3-en-2-one (9c). 1H NMR(CDCl3): 7.46 (q, J = 1.2, 1H, H-4), 2.17 (s, 3H, MeCO), 1.70 (d, J = 1.2, Me-3), 0.30 (s, 9H, OSiMe).
2-Methyl-1-(trimehylsilyloxy)pent-1-en-3-one (9d). 1H NMR(CDCl3): 7.48 (q, J = 1.3, 1H, H-4), 2.55 (q, J = 7.4, 2H, H-4), 1.72 (d, J = 1.3, Me-2), 1.10 (t, J = 7.4, 3H, H-5), 0.29 (s, 9H, OSiMe3).
2-Methyl-1-phenyl-3-(trimehylsilyloxy)prop-2-en-1-one (9e). 1H NMR(CDCl3): 7.53 (m, 2H, Ph), 7.48 (m, 1H, Ph), 7.41 (m, 2H, Ph), 7.15 (q, J = 1.3, 1H, H-3), 1.88 (d, J = 1.3, 3H, Me-2), 0.22 (s, 9H, OSiMe3).
1,2-Diphenyl-3-(trimehylsilyloxy)prop-2-en-1-one (9f). 1H NMR(CDCl3): 7.63-7.67 (m, 2H), 7.21-7.49 (m, 9H), 0.24 (s, 9H, OSiMe3).
2-(Trimehylsilyloxymethylene)cyclohexan-1-one (9g). 1H NMR(CDCl3): 7.46 (t, J = 2.0, 1H, =CH), 2.44 (td, J = 6.7, 2.0, 2H, H-3), 2.34 (t, J = 6.7, 2H, H-6), 1.77-1.90 (m, 2H, H-5), 1.64-1.76 (m, 2H, H-4), 0.26 (s, 9H, OSiMe3).
2-(1-Trimehylsilyloxyethylene)cyclopentan-1-one (11a). 1H NMR(CDCl3): 2.59 (tq, J = 7.3, 1.8, 2H, H-3), 2.48-2.52 (m, 2H, H-5), 2.32 (t, J = 1.8, 3H, Me), 1.76-1.87 (m, 2H, H-4), 0.26 (s, 9H, OSiMe3).
2-(1-Oxoethyl)-1-(Trimehylsilyloxy)cyclopent-1-ene (12a). 1H NMR(CDCl3): 2.47-2.52 (m, 4H, H-3 and H-5), 2.34 (s, 3H, MeCO), 1.76-1.87 (m, 2H, H-4), 0.32 (s, 9H, OSiMe3).
2-(1-Oxoethyl)-1-(Trimehylsilyloxy)cyclohex-1-ene (12b). 1H NMR(CDCl3): 2.37 (s, 3H, MeCO), 2.30-2.36 (m, 2H), 2.20-2.28 (m, 2H), 1.64-1.71 (m, 2H), 1.51-1.58 (m, 2H), 0.29 (s, 9H, OSiMe3).
Acknoledgement
The author is indebted to Professor Hugo Wyler for valuable comments.
References and Notes
- West, R. J. Am. Chem. Soc. 1958, 80, 3246–3249. House, H. O.; Czuba, L. J.; Gall, M.; Olmstead, H. D. J. Org. Chem. 1969, 34, 2324–2336. Pawlenko, S. Houben-Weyl, Methoden der Organischen Chemie, 4th Edn; Muller, E., Bayer, O., Eds.; Vol. XIII/5, Georg Thieme Verlag: Stuttgart, 1980; pp. 193–201. [Google Scholar]
- Kantlehner, W.; Kugel, W.; Bredereck, H. Chem. Ber. 1972, 105, 2264–2270.
- Dedier, J.; Gerval, P.; Frainnet, E. J. J. Organomet. Chem. 1980, 185, 183–197. Klebe, J. F.; Finkbeiner, H.; White, D. M. J. Am. Chem. Soc. 1966, 88, 3390–3395.
- Ojima, I.; Nagai, Y. J. Organomet. Chem. 1973, 57, C42–C44.
- Emde, H.; Domsch, D.; Feger, H.; Frick, U.; Goetz, A.; Hergott, H. H.; Hofmann, K.; Kober, W.; Kraegeloh, K.; Oesterle, T.; Steppan, W.; West, W.; Schimchen, G. Synthesis 1982, 1–26. Emde, H.; Goetz, A.; Hofmann, K.; Simchen, G. Justus Liebigs Ann. Chem. 1981, 1643–1657; Simchen, G.; Kober, W. Synthesis 1976, 259–261.
- Nakamura, E.; Murotushi, T.; Shimizu, M.; Kuwajima, I. J. Am. Chem. Soc. 1976, 98, 2346–2348.
- Chu, D. T.; W. Huckin, S. N. Can. J. Chem. 1980, 58, 138–142.
- Torkelson, S.; Ainsworth, C. Synthesis 1976, 722–724.
- Aizpurua, J. M.; Palomo, C. Synthesis 1982, 280–281.
- Yamamoto, Y.; Matui, C. Organometallics 1997, 16, 2204–2206.
- Rasmussen, J. K.; Heilmann, S. M.; Krepski, L. R. Advances in Silicon Chemistry; Larson, G.L., Ed.; JAI Press INC.: London, England, 1991; Volume 1, pp. 64–187. [Google Scholar]
- Furin, G. G.; Vyazankina, O. A.; Gostevsky, B. A.; Vyazankin, N. S. Tetrahedron 1988, 44, 2675–2749. Colvin, E. W. Chem. Soc. Rev. 1978, 7, 15. Groutas, W. C.; Felker, D. Synthesis 1980, 861–868.
- Mai, K.; Paril, G. J. Org. Chem. 1986, 51, 3545–3548. Corey, E. J.; Wu, Y.-J. J. Am. Chem. Soc. 1993, 115, 8871–8872. Becu, C.; Reyniers, M.-F.; Anteuuuunis, M. J. O. Bull. Soc. Chim. Belg. 1990, 99, 779–782. Antenuis, M. J. O.; Becu, C.; Becu, F. Bull. Soc. Chim. Belg. 1990, 99, 361–377.
- Zhuo, J.-C.; Wyler, H. Helv. Chim. Acta 1993, 76, 1916–1927.
- Gostevskii, B. A.; Kruglaya, O. A.; Albanov, A. I.; Vyazankin, N. S. Zh. Obshch. Khim. 1981, 51, 817–820. Gostevskii, B. A.; Vyazankina, O. A.; Vyazankin, N. S. Zh. Obshch. Khim. 1983, 53, 1843–1846.
- Foley, L. H. J. Org. Chem. 1985, 50, 5204–5209.
- Neef, H.; Müller, R. J. Prakt. Chem. 1973, 315, 367–374.
- Samples Availability: Not available.
© 1999 by the authors. Reproduction of this article, by any means, is permitted for noncommercial purposes.