Hydroxyquinones: Synthesis and Reactivity
Abstract
:Contents
- Introduction
- Synthesis
- Reactivity
- Conclusion
- References
1. Introduction

2. Synthesis
3. Reactivity



4. Conclusions
References
- The Chemistry of the Quinoid Compounds; Patai, S.; Rappoport, Z. (Eds.) Wiley-Interscience: New York, 1988.
- Tisler, M. Heterocyclic Quinones. In Advances in Heterocyclic Chemistry; Katritzky, A. R., Ed.; Academic Press: London, 1989; Volume 45, p. 37. [Google Scholar]
- Mehendale, A.R.; Thomson, R.H. Binaphthoquinones in lomatia ferruginea. Phytochemistry 1975, 14, 801–802. [Google Scholar] [CrossRef]
- Yin, J.; Liebeskind, L. S. A synthesis of trisquinones. J. Org. Chem. 1998, 63, 5726–5727, and references cited therein. [Google Scholar] [CrossRef] [PubMed]
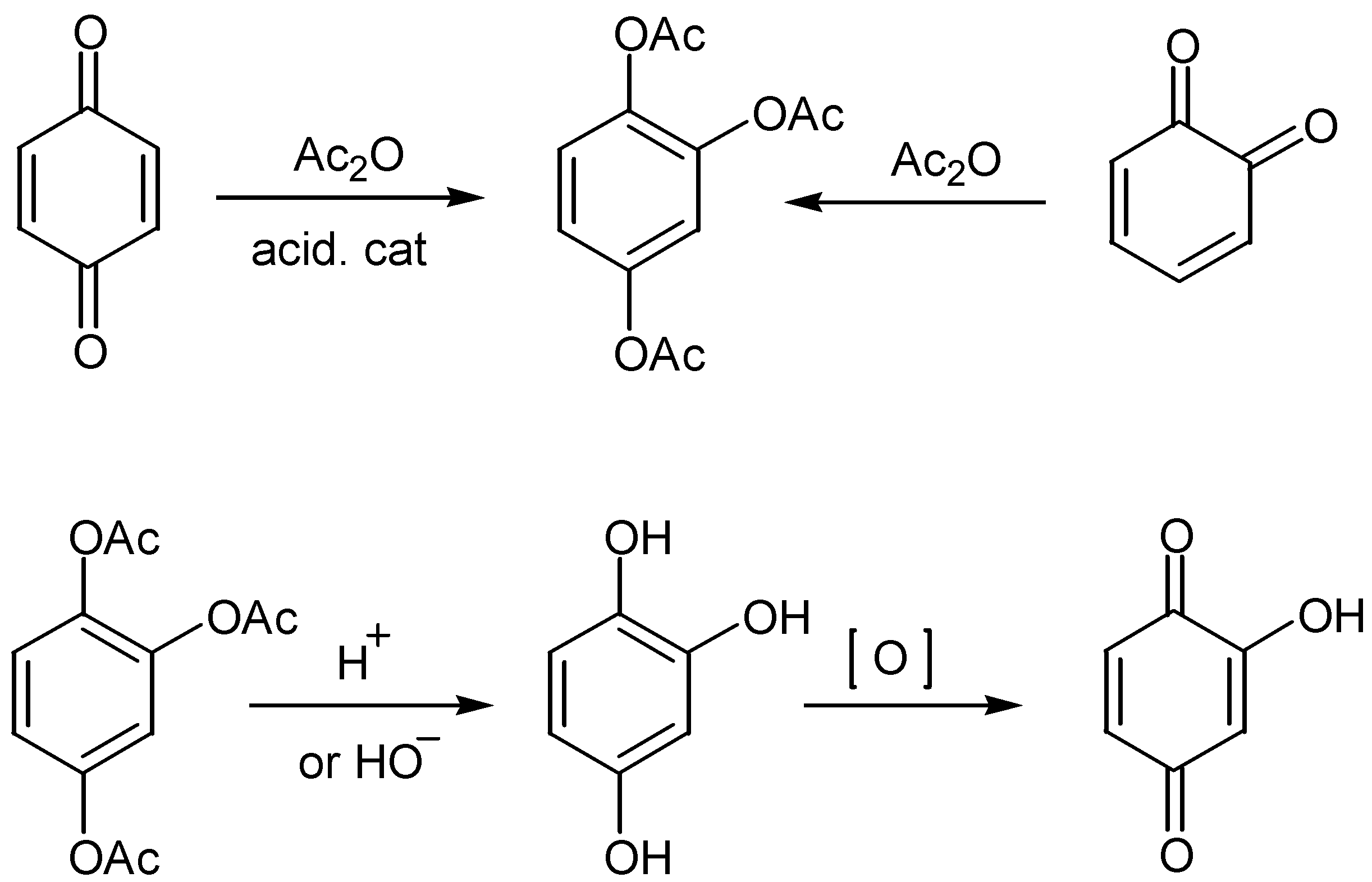
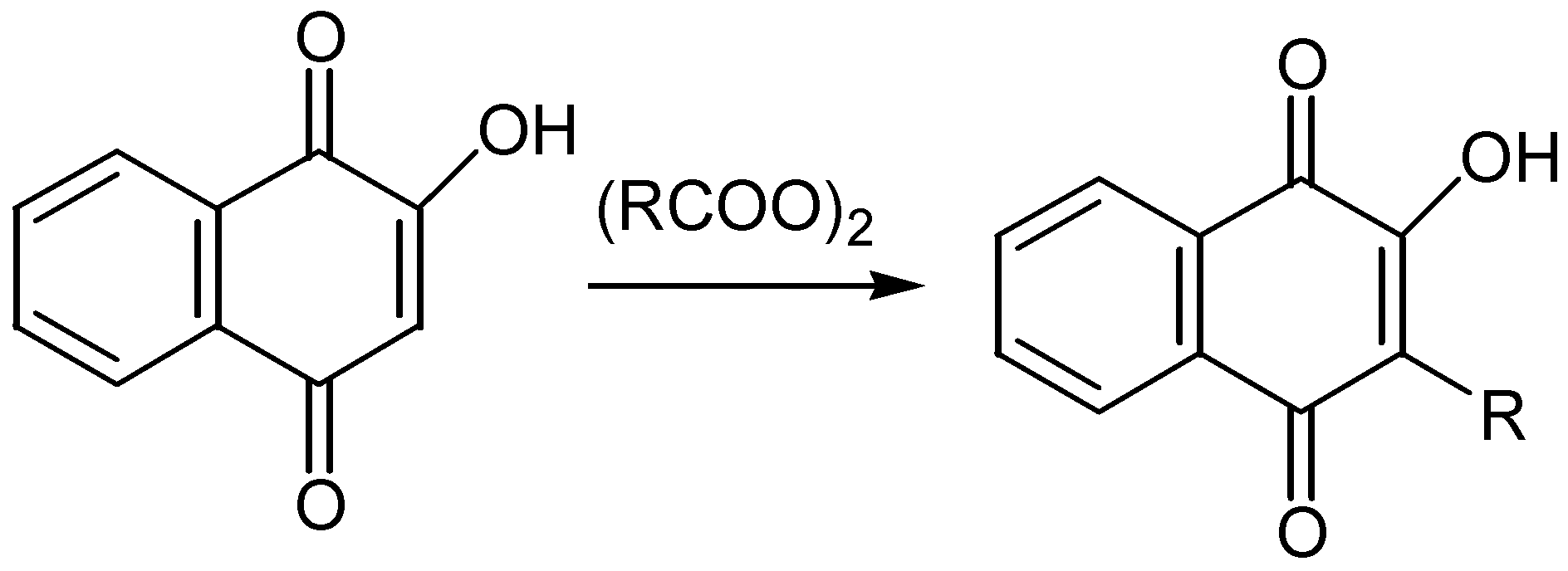
- McOmie, J. F. W.; Blatchly, J.M. The Thiele-Winter acetoxylation of quinones in Organic Reactions. Vol. 19, Wiley: New York, 1972; p. 199. [Google Scholar]
- Finley, K. T. in ref. 1, Vol. 2, p. 537.
- Villemin, D.; Bar, N.; Hammadi, M. Triflic acid an efficient catalyst for the Thiele-Winter reaction. Tetrahedron Lett. 1997, 38, 4777–4778. [Google Scholar] [CrossRef]
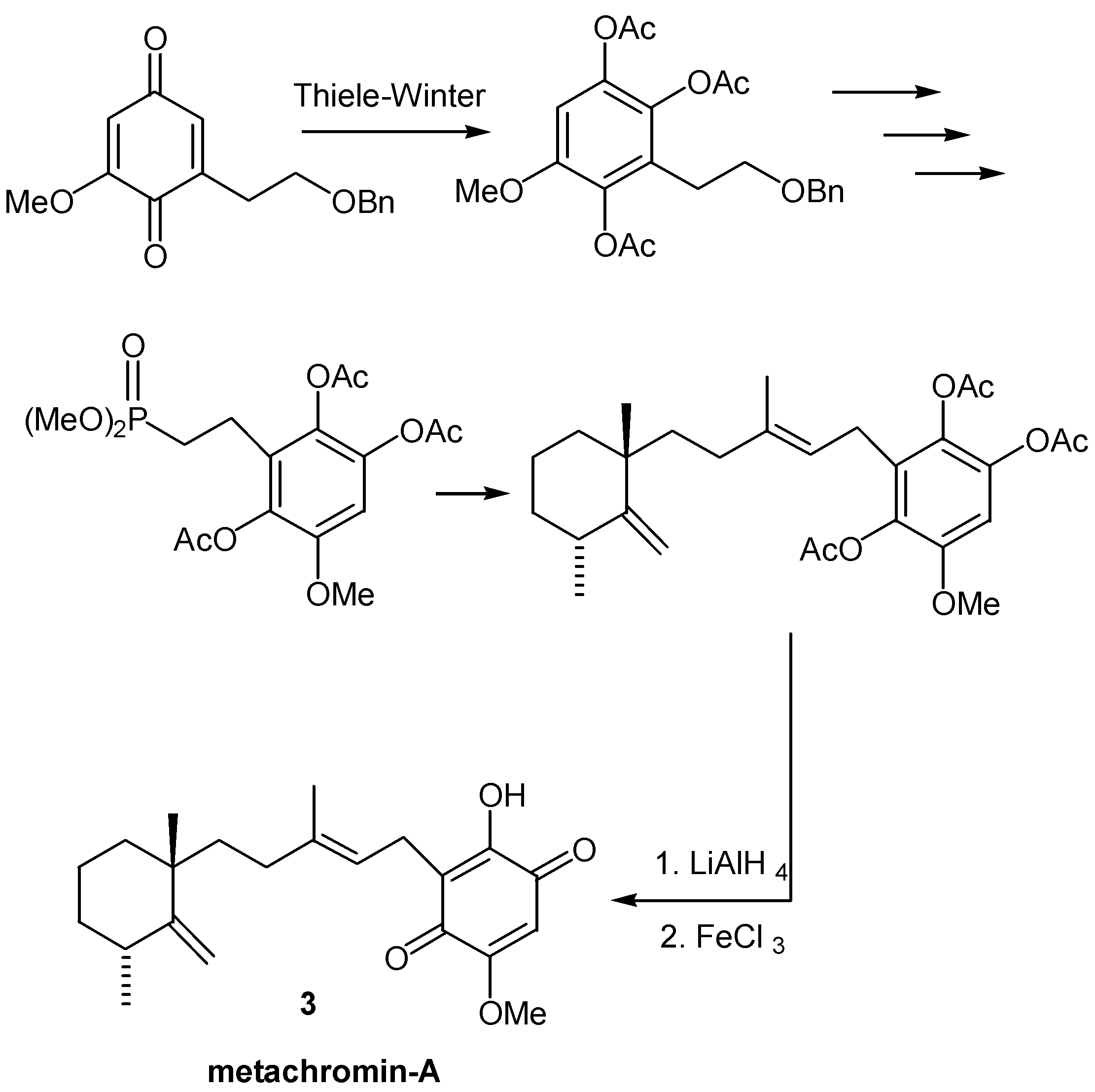
- Almeida, W. P.; Correia, C. R. D. A total synthesis of the sesquiterpene quinone metachromin-A. Tetrahedron Lett. 1994, 35, 1367–1370. [Google Scholar] [CrossRef]
- Sargent, M. V.; Wangchareontrakul, S. The synthesis of the first natural host germination stimulant for Striga asiatica (witchweed). J. Chem. Soc. Perkin Trans. 1 1990, 1429–1434. [Google Scholar] [CrossRef]
- Suginome, H.; Kamekawa, H.; Sakurai, H.; Konishi, A.; Senboku, H.; Kobayashi, K. Photoinduced molecular transformations. Part 145. Regioselective [3+2] photoadditions of 2-hydroxyphenanthrene-1,4-dione with electron rich alkenes and phenylacetylene: New one-step synthesis of 9,10-dihydrophenanthro [2,3-b]furan-7,11-diones and 2-phenylphenanthro[2,3-b] furan-7,11-dione. J. Chem. Soc. Perkin Trans. 1 1994, 471–475. [Google Scholar]
- Huot, R.; Brassard, P. Synthèse de méthyl-3 furoquinones. Can. J. Chem. 1974, 52, 88–94. [Google Scholar] [CrossRef]
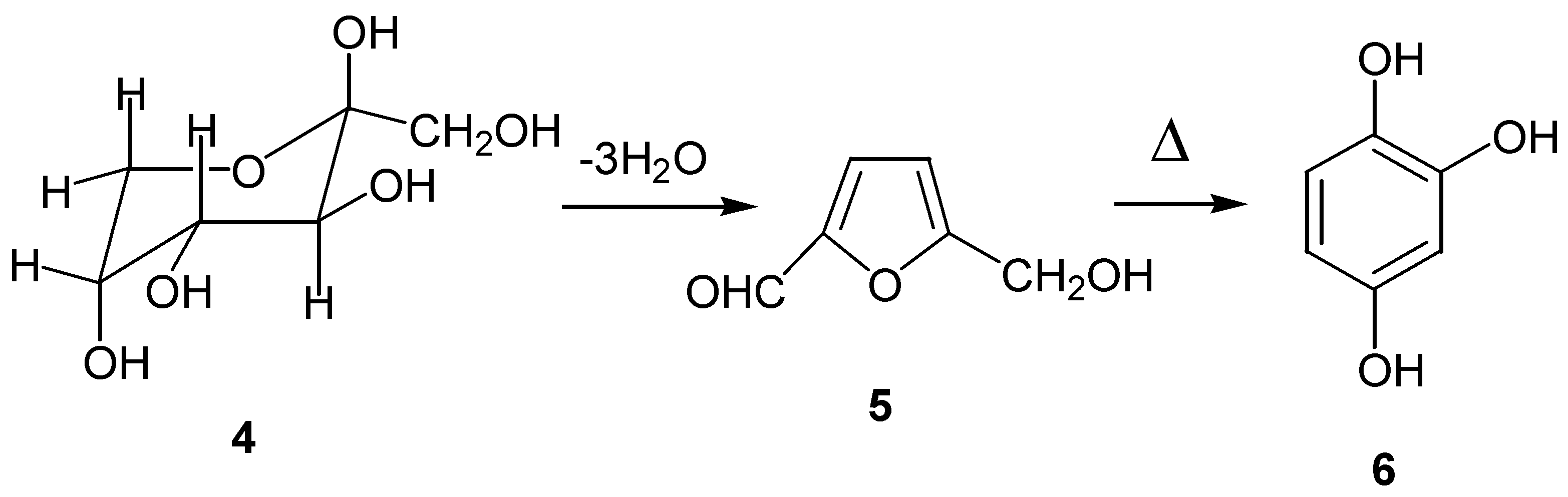
- (a) Waldhör, E.; Schwederski, B.; Kaim, W. Ruthenium(II) coordination to a model for the topasemiquinone cofactor of amine oxidases. Resolution of 1H and 99,101Ru EPR hyperfine structure. J. Chem. Soc. Perkin Trans. 2 1993, 2109–2111. [Google Scholar] ; (b) Luijkx, G. C. A.; Rantwijk, van F.; Bekkum, van H. Hydrothermal formation of 1,2,4-benzenetriol from 5-hydroxymethyl-2-furaldehyde and D-fructose. Carbohydr. Res. 1993, 242, 131–140. [Google Scholar]
- Kobayashi, K.; Kanno, Y.; Suginome, H. Photoinduced molecular transformations. Part 141. New one-step general synthesis of benzofuran-4,7-diones by the regioselective (3+2) photo-addition of 2-hydroxy-1,4-benzoquinones with various alkenes. J. Chem. Soc. Perkin Trans. 1 1993, 1449–1452. [Google Scholar] [CrossRef]
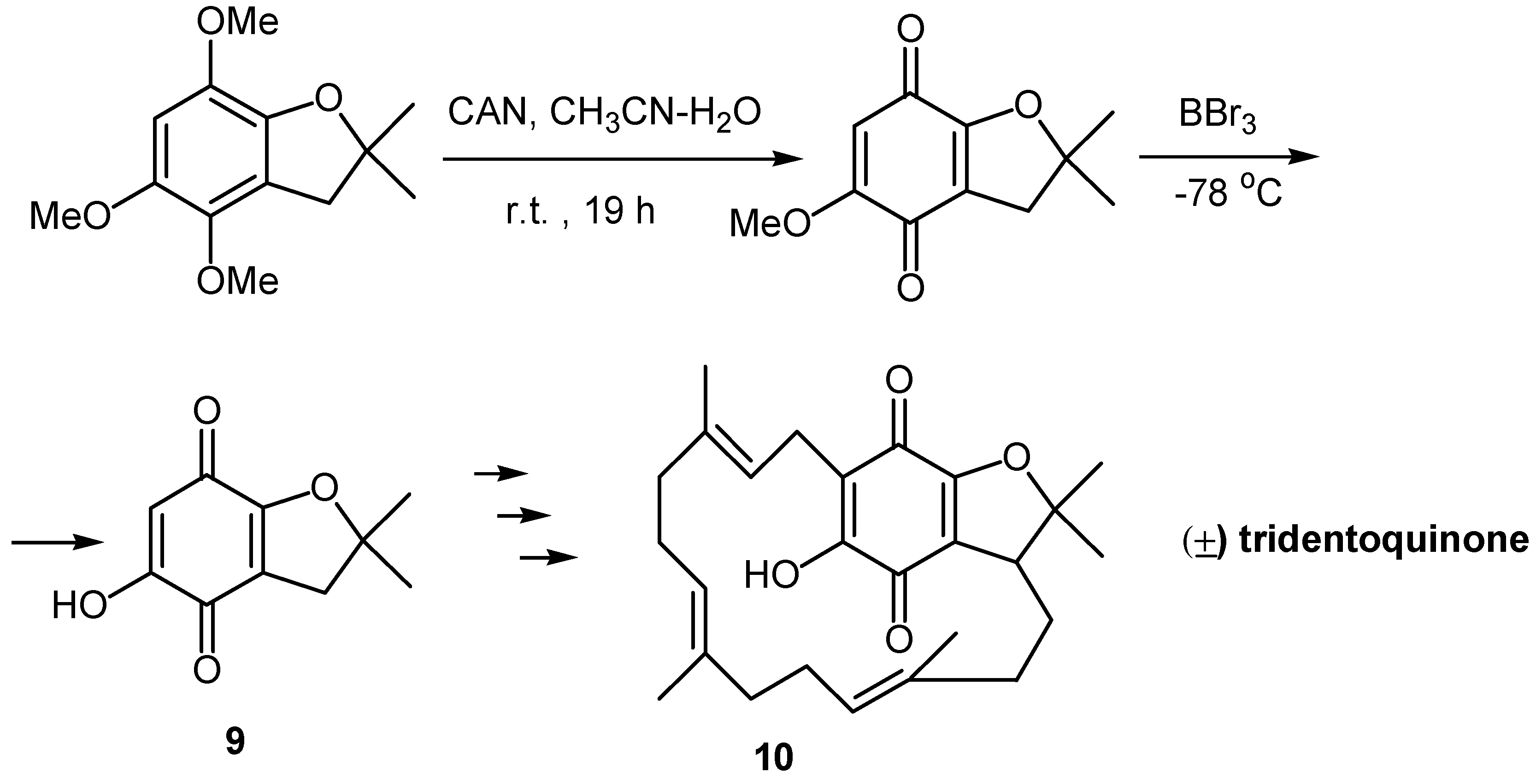
- Brown, R. F. C.; Robinson, A. J. A synthetic approach to (±)-tridentoquinone. Aust. J. Chem. 1995, 48, 515–529. [Google Scholar] [CrossRef]
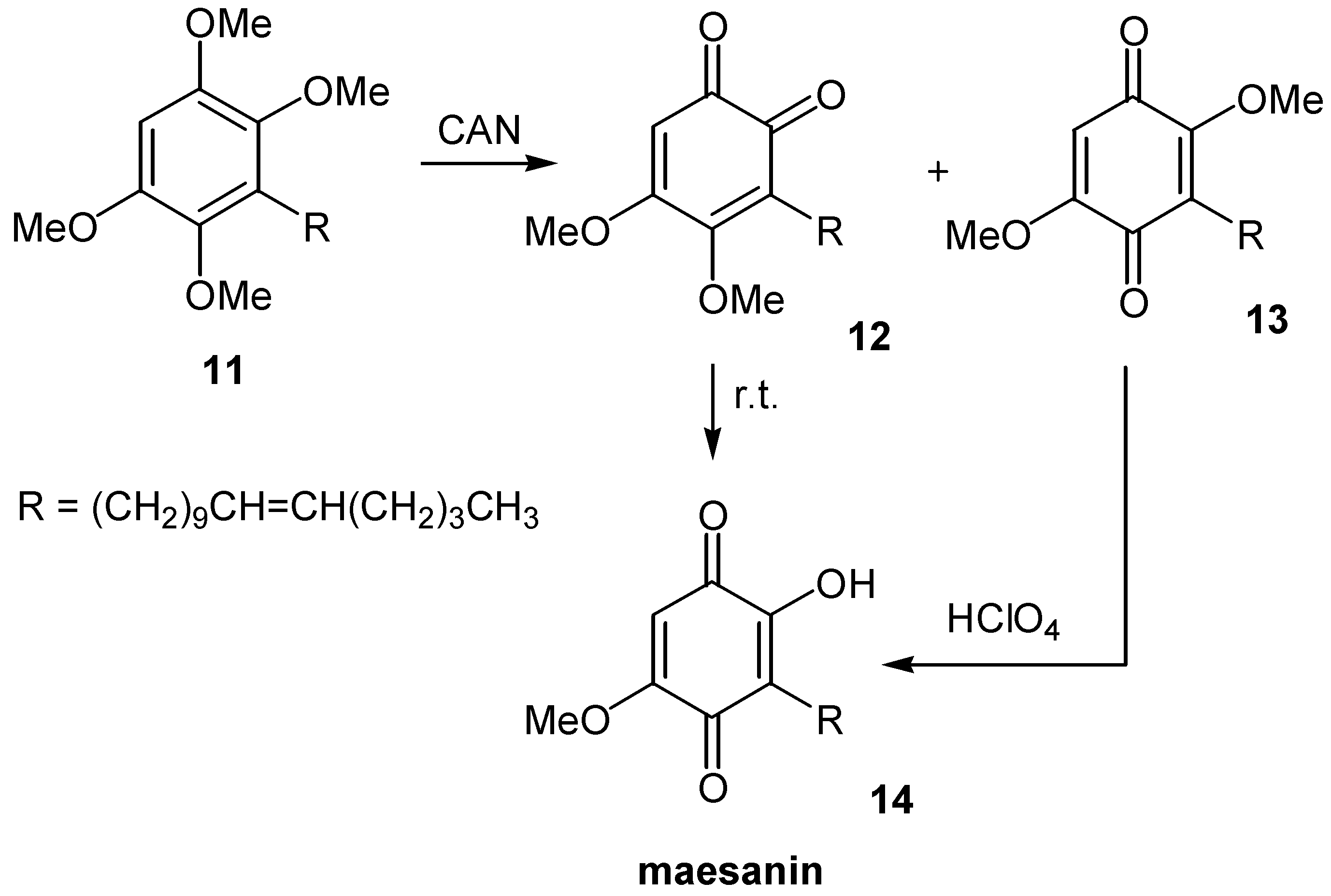
- Poigny, S.; Guyot, M.; Samadi, M. Total synthesis of maesanin and analogues. Tetrahedron 1998, 54, 14791–14802. [Google Scholar] [CrossRef]
- Poigny, S.; Guyot, M.; Samadi, M. Efficient synthesis of (-)-illimaquinone. J. Org. Chem. 1998, 63, 5890–5894. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Yaso, H.; Kiriyama, H.; Takahashi, H.; Minami, H.; Kamikawa, T. Synthesis of cytotoxic maesaquinone bearing a 2,5-dihydroxy-6-methyl-1,4-benzoquinone nucleus. Tetrahedron 1997, 53, 16969–19976. [Google Scholar] [CrossRef]
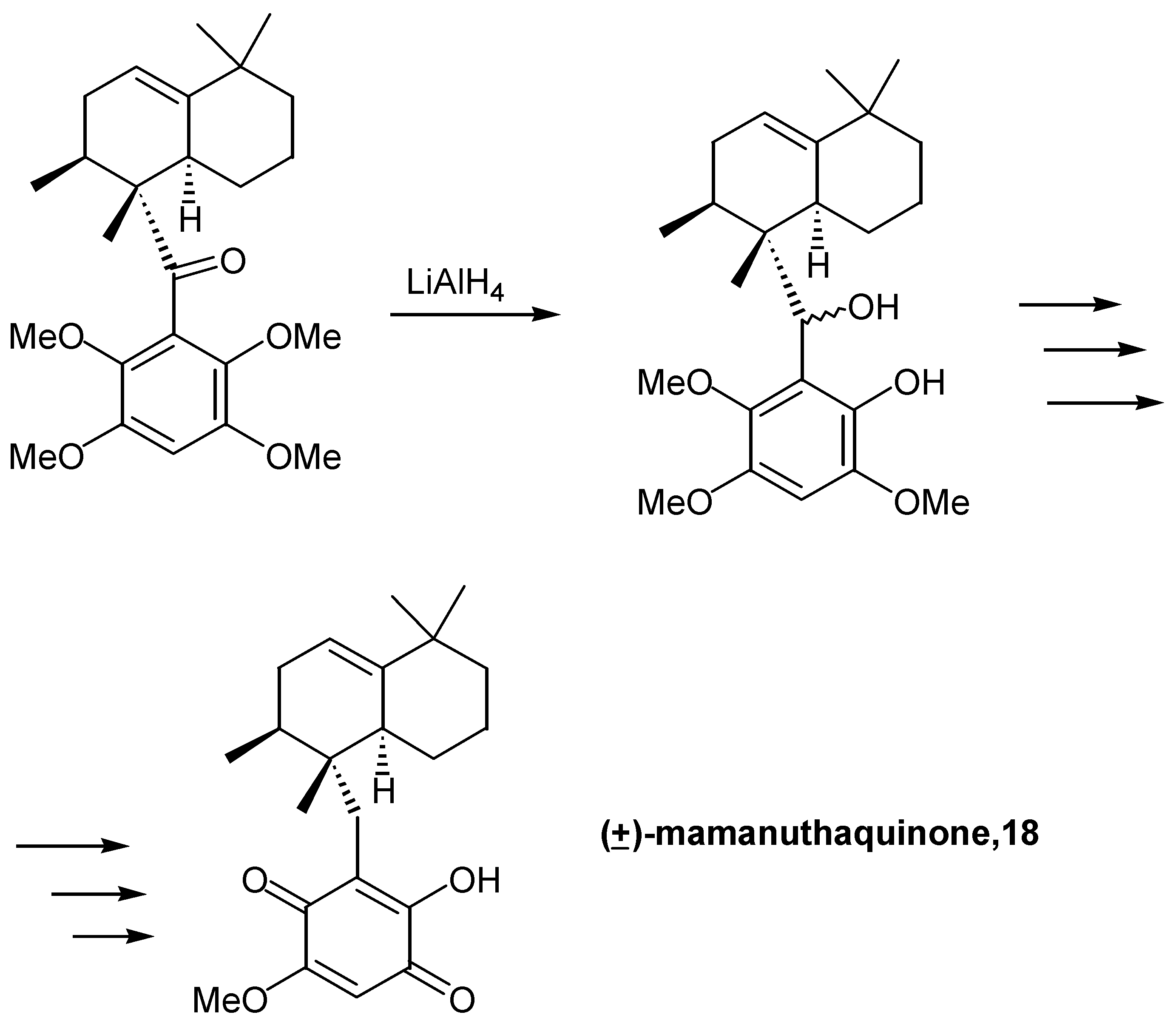
- Yoon, T.; Danishefski, S. J.; Cala de, S. A concise total synthesis of (±)-mamanuthaquinone by using an exo-Diels-Alder reaction. Angew. Chem. Int. Ed. Engl. 1994, 33, 853–855. [Google Scholar] [CrossRef]
- Bruner, S.D.; Radeke, H. S.; Tallarico, J. A.; Snapper, M. L. Total synthesis of (-)-illimaquinone. J. Org. Chem. 1995, 60, 1114–1115. [Google Scholar] [CrossRef]
- Radeke, H. S.; Digits, C.A.; Bruner, S. D.; Snapper, M. L. New tools for studying vesicularmediated protein trafficking: Synthesis and evaluation of illimaquinone analogs in a nonradioisotope-based antisecretory assay. J. Org. Chem. 1997, 62, 2823–2831. [Google Scholar] [CrossRef] [PubMed]
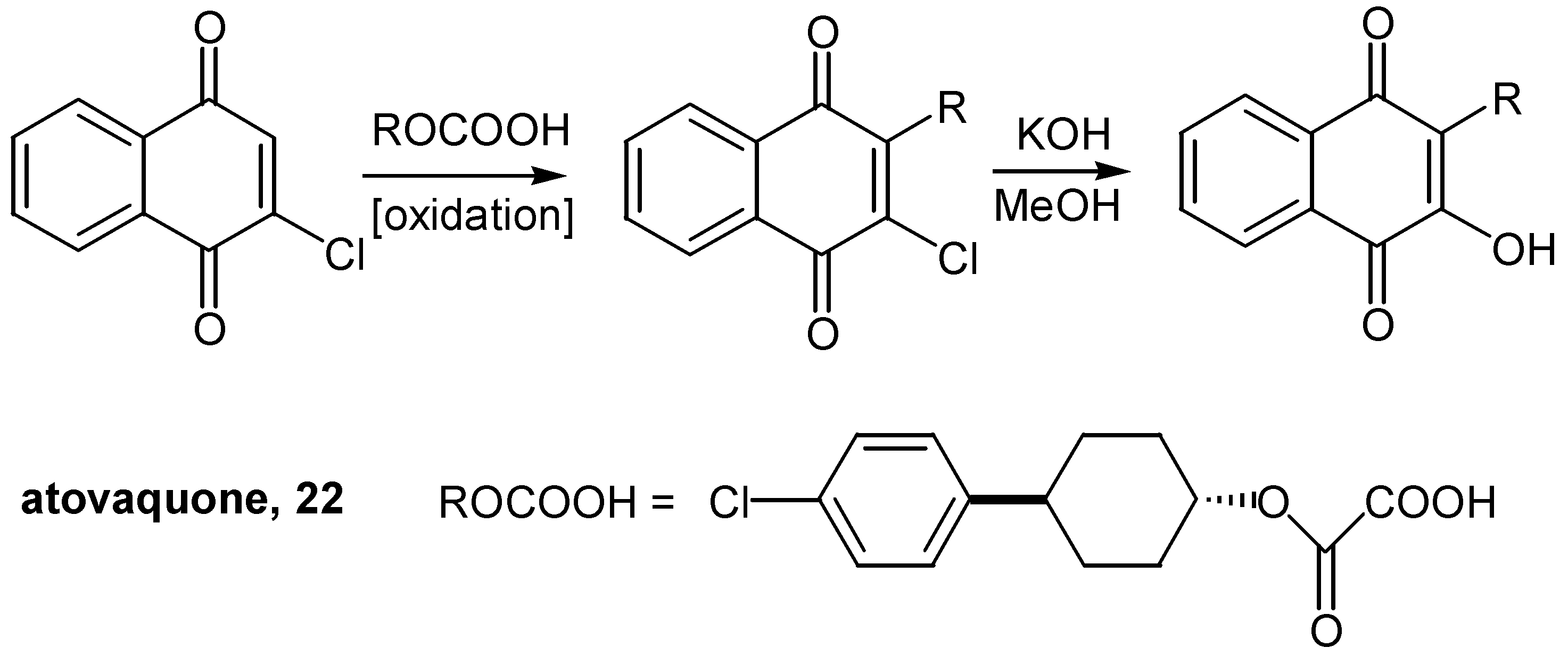
- Williams, D. R.; Clark, M. P. Synthesis of Atovaquone. Tetrahedron Lett. 1998, 39, 7629–7632. [Google Scholar] [CrossRef]
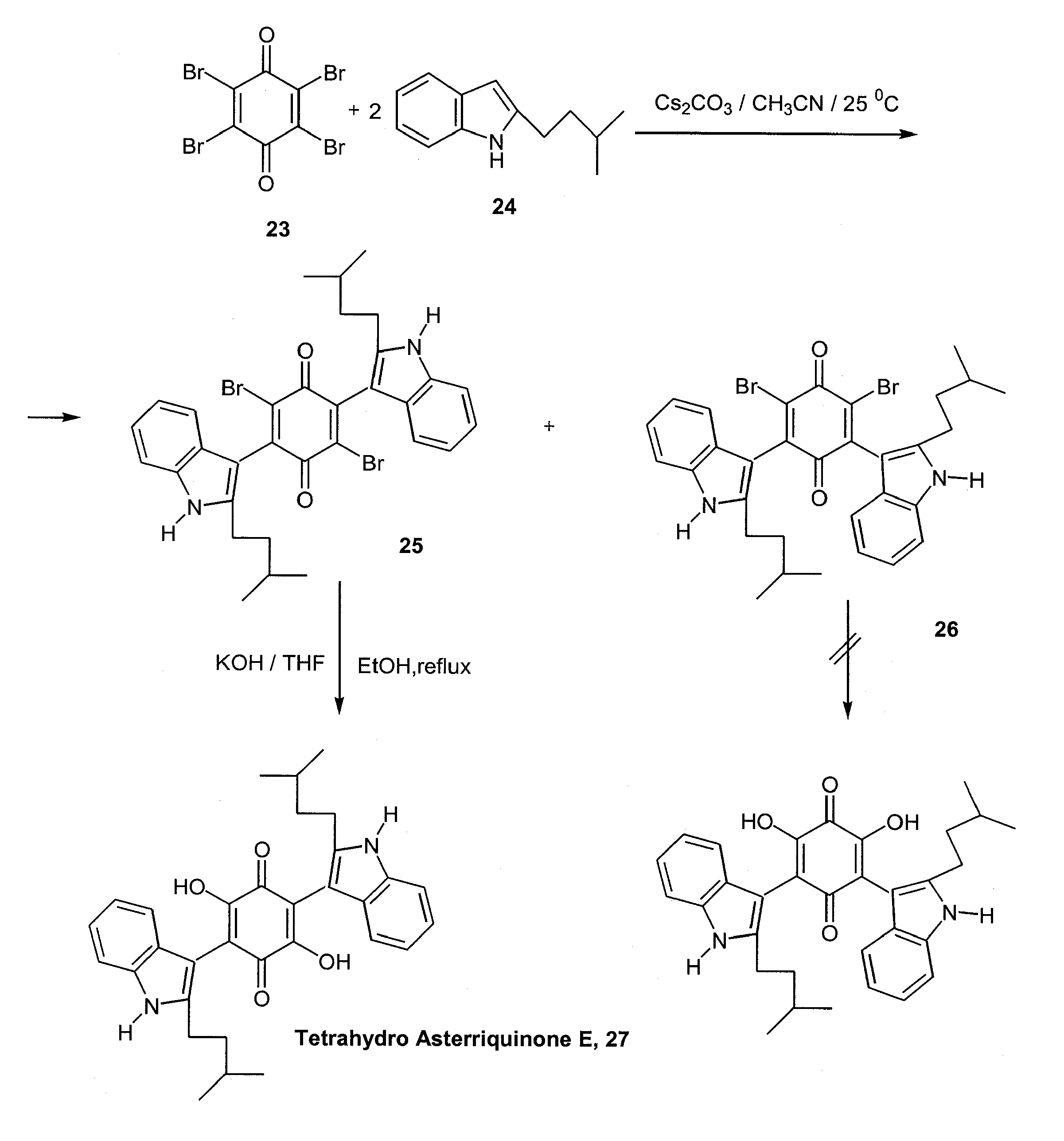
- Harris, G. D., Jr.; Nguen, A.; App, H.; Hirth, P.; McMahon, G.; Tang, C. A one-pot, two-step synthesis of tetrahydro asterriquinone E. Organic Lett. 1999, 1, 434–436. [Google Scholar] [CrossRef]
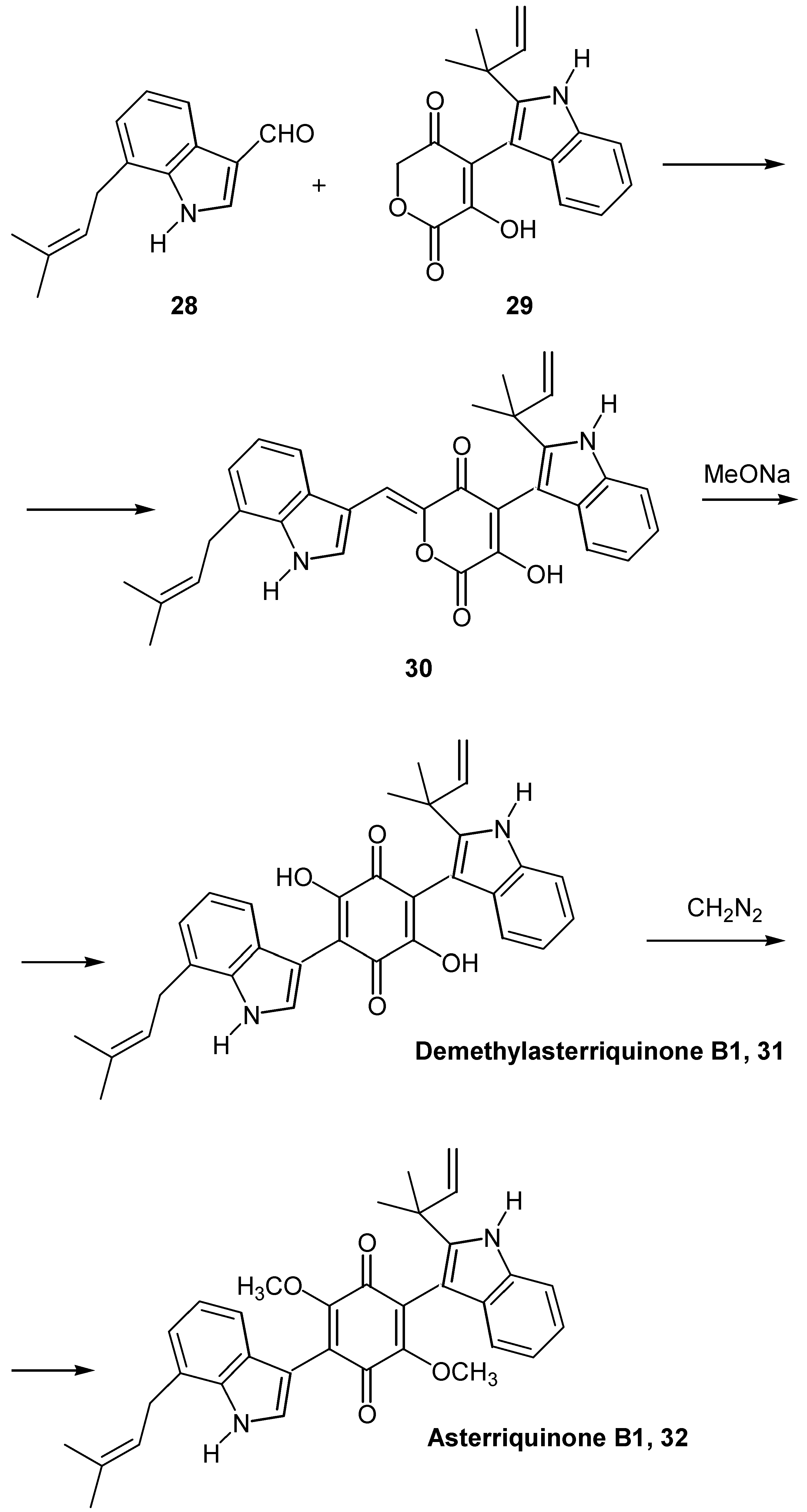
- (a) Liu, K.; Wood, H. B.; Jones, A. B. Total synthesis of asterriquinone B1. Tetrahedron Lett. 1999, 40, 5119–5122. [Google Scholar] ; (b) Zhang, B.; Zhang, G.; Szalkowski, D.; Li, Z.; Zhang, Y.; Royo, I.; Villela, D.; Diez, M.T.; Pelaez, F.; Ruby, C.; Kendall, R. L.; Mao, X.; Griffin, P.; Calaycay, J.; Zierath, J. R.; Heck, J. V.; Smith, R. G.; Moller, D. E. Discovery of a small molecule insulin mimetic with antidiabetic activity in mice. Science 1999, 284, 284–977. [Google Scholar]
- Ref. 6, p. 552.
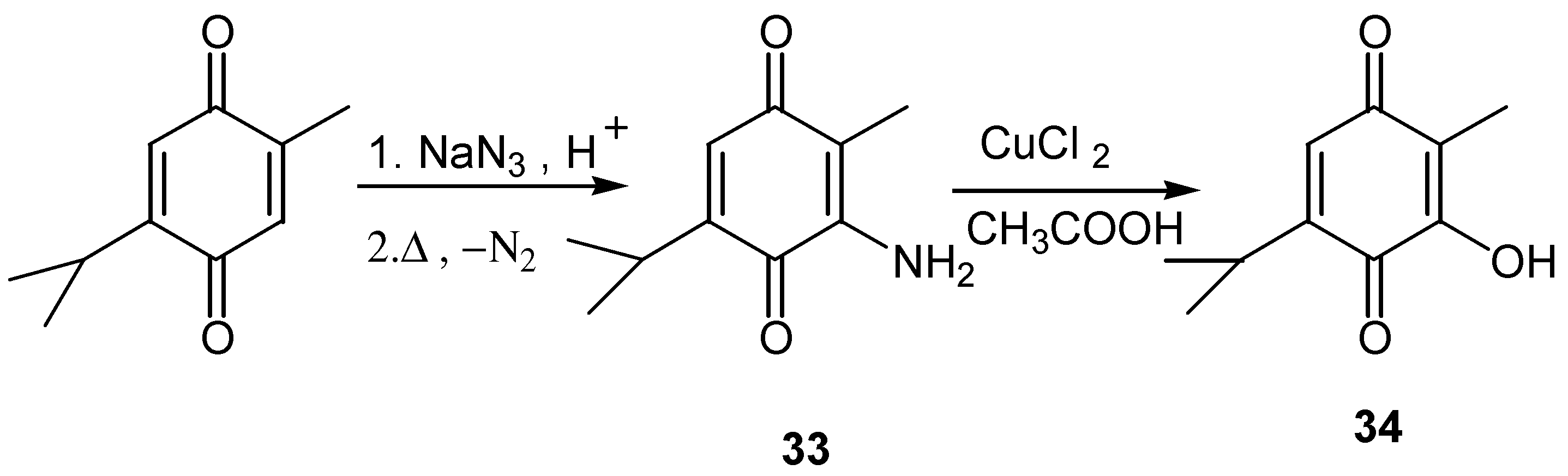
- Moore, H. W.; Shelden, H. R. Rearrangement of azidoquinones. Reaction of thymoquinone and 2-5-dimethyl-1,4-benzoquinone with sodium azide in trichloroacetic acid. J. Org. Chem. 1968, 33, 4019–4024. [Google Scholar] [CrossRef]
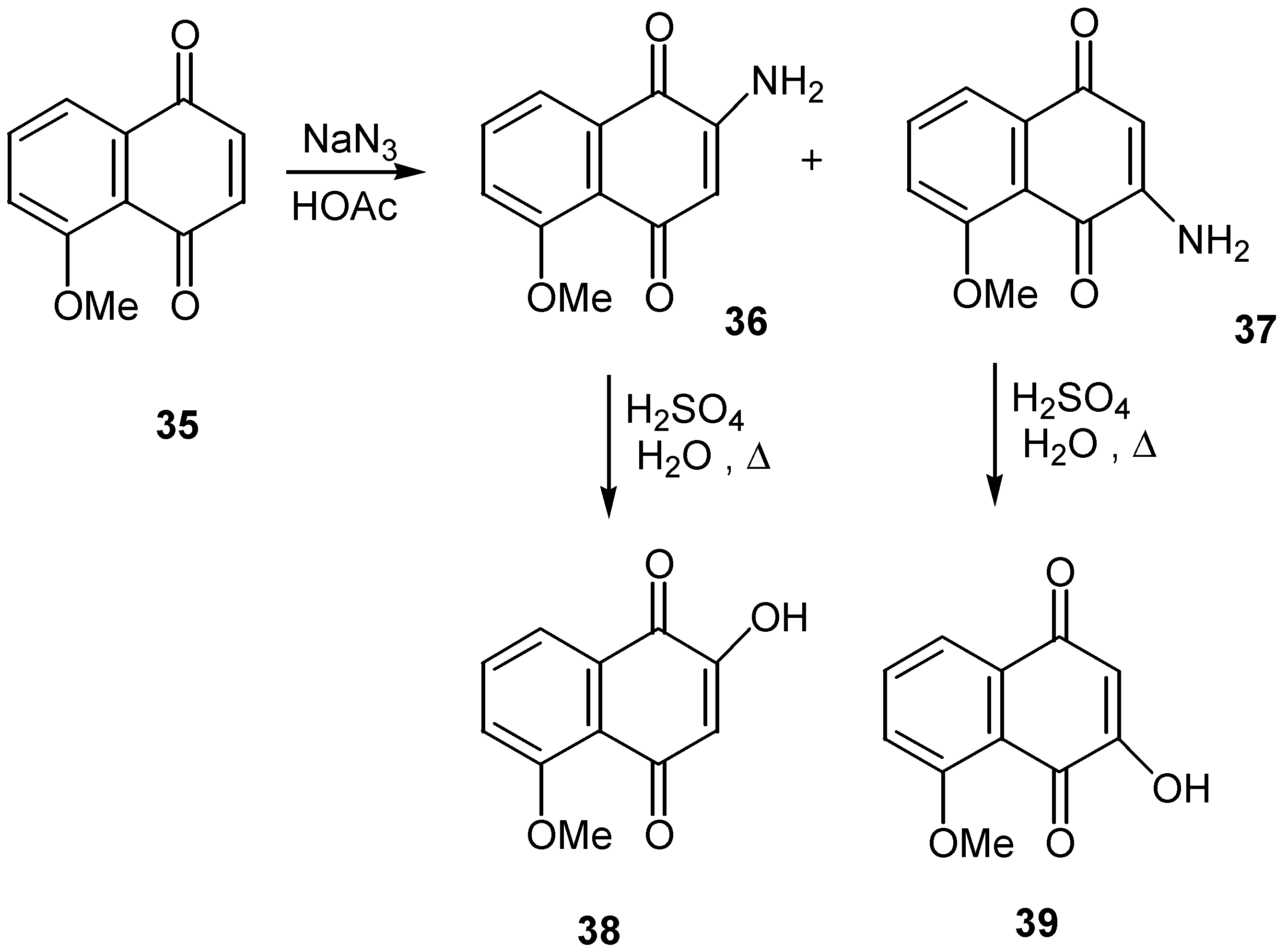
- Parker, K. A.; Sworin, M. E. Assignment of regiochemistry to substituted naphthoquinones by chemical and spectroscopic methods. Amino-, hydroxy-, and bromojuglone derivatives. J. Org. Chem. 1981, 46, 3218–3223. [Google Scholar]
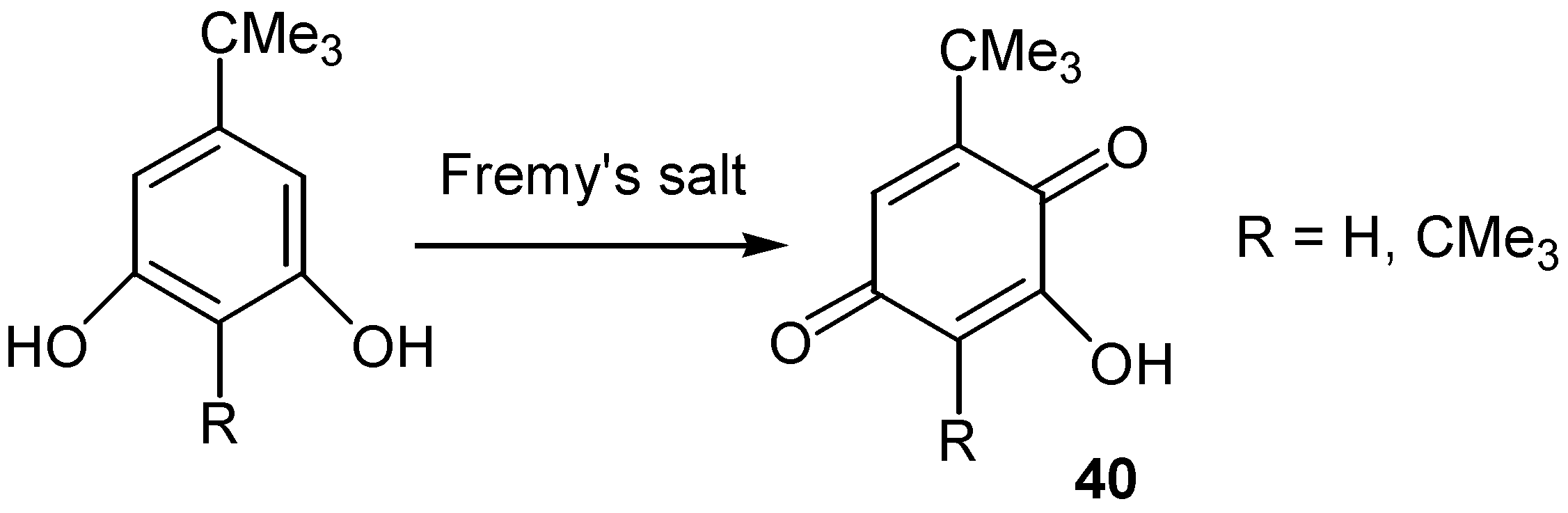
- Cuntze, U.; Maasen, D.; Musso, H. Uber die bildung von hydroxy-tert.-butyl-chinonen und deren abbau zu cyclopentenon-derivaten durch alkali. Chem. Ber. 1969, 102, 2851–2861. [Google Scholar] [CrossRef]
- Croux, S.; Maurette, M.-T.; Hocquaux, M.; Ananides, A.; Braun, A. M.; Oliveros, E. Kinetic parameters of the reactivity of dihydroxynaphthalenes with singlet oxygen. New J. Chem. 1990, 14, 161–167. [Google Scholar]
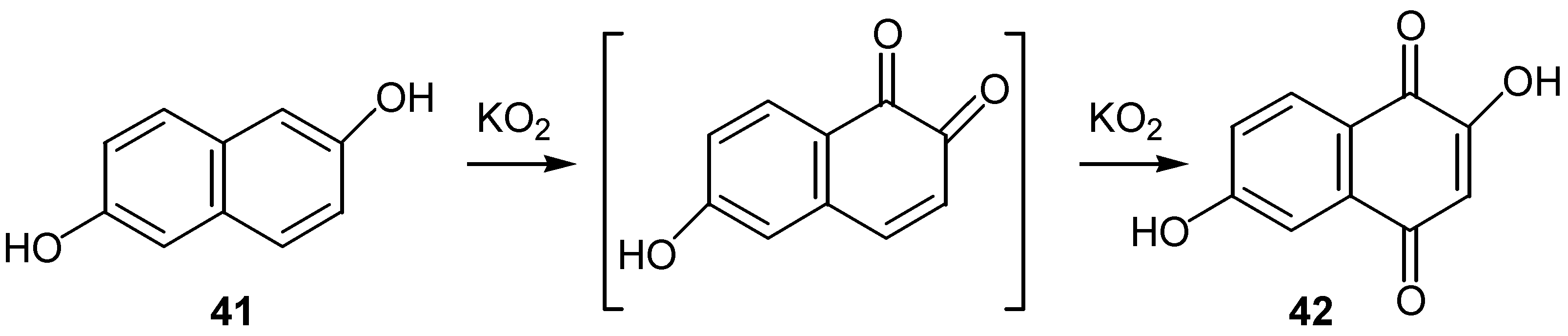
- Min, D. M.; Croux, S.; Tournair, C.; Hocquaux, M.; Jacquet, B.; Oliveros, E.; Maurette, M.-T. Réactivité du superoxyde de potassium en phase hétérogène: Oxydation de naphtalénediols en Naphtoquinones hydroxylées. Tetrahedron 1990, 48, 1869–1882. [Google Scholar] [CrossRef]
- Spyroudis, S.; Xanthopoulou, N. unpublished results.
- (a) Kasturi, T. R.; Arunachalam, T. General method of preparation of substituted 2-hydroxy-1,4-quinones. Can. J. Chem 1966, 44, 1086. [Google Scholar] ; (b) Baillie, A. C.; Thomson, R. H. Quinones. Part VII. New routes to 2-hydroxy-1,4-naphthaquinones. J. Chem. Soc. Sec. C 1966, 2184–2186. [Google Scholar]
- Pettit, G. R.; Fleming, W. C.; Paull, K. D. Synthesis of the 6- and 7-hydroxy-5,8-dioxocarbostyrils. J. Org. Chem. 1968, 33, 1089–1092. [Google Scholar] [CrossRef]
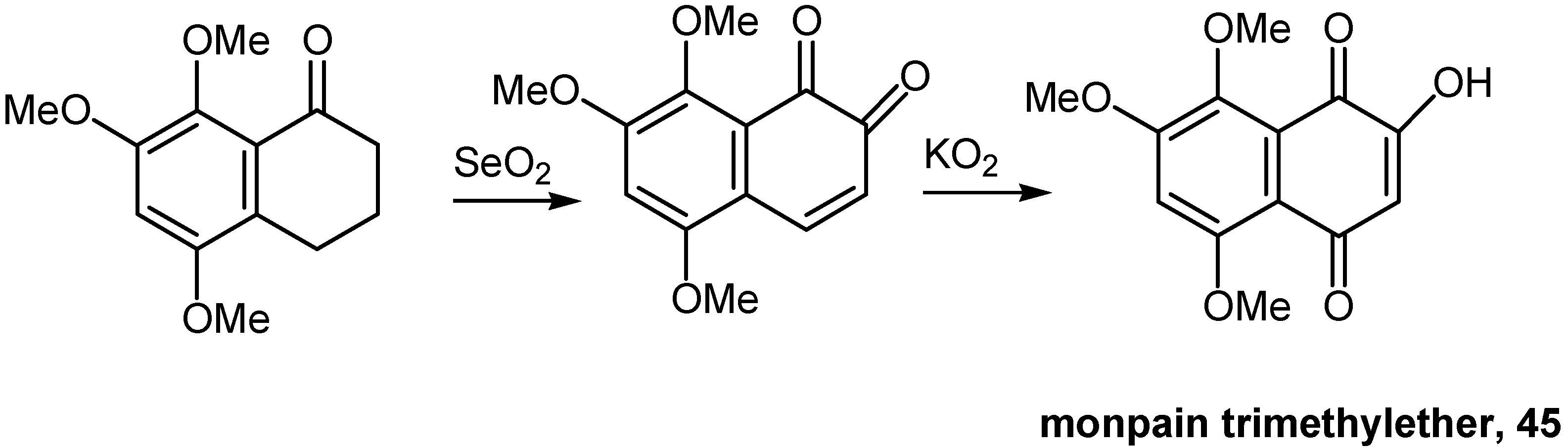
- Bekaert, A.; Andrieux, J.; Plat, M.; Brion, J-D. A convenient synthesis of monpain trimethylether. Tetrahedron Lett. 1997, 38, 4219–4220. [Google Scholar] [CrossRef]
- Takada, T.; Akiba, M. Synthesis of 1H-pyrrolo[1,2-a]indole derivatives. III. Synthesis of 2,3-dihydro-7-hydroxy-6,9-dimethyl-5,8-dioxo-1H-pyrrolo[1,2-a]indole. Chem. Pharm. Bull. 1972, 20, 1785–1792. [Google Scholar] [CrossRef]
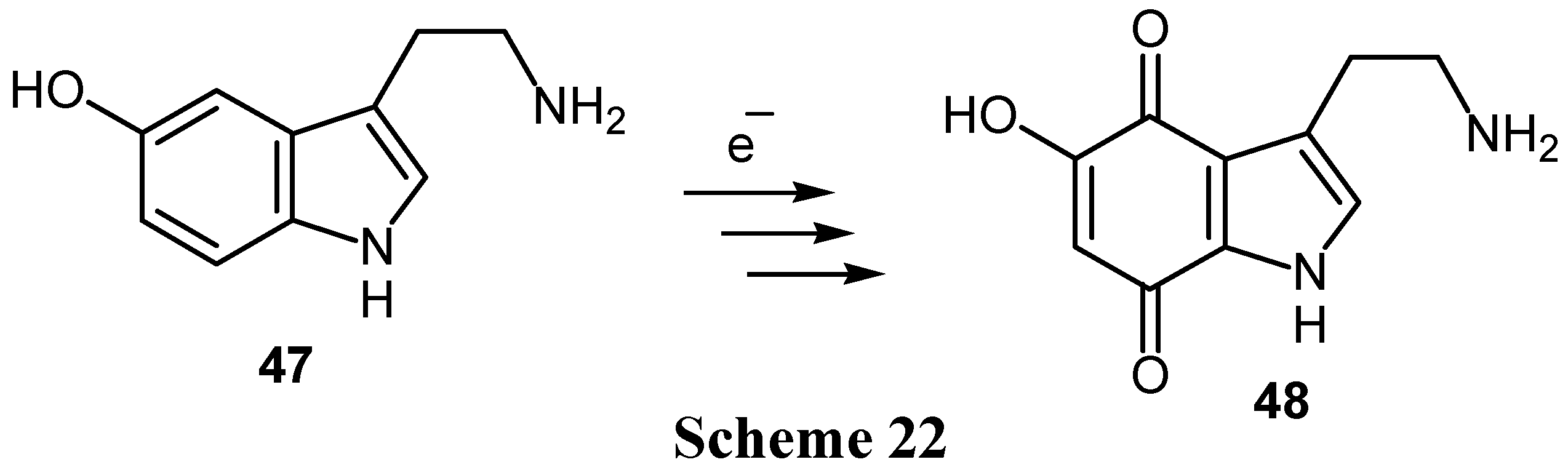
- Senoh, S.; Witkop, B. Formation and rearrangements of aminochromes from a new metabolite of dopamine and some of its derivatives. J. Am. Chem. Soc. 1959, 81, 6231–6235. [Google Scholar] [CrossRef]
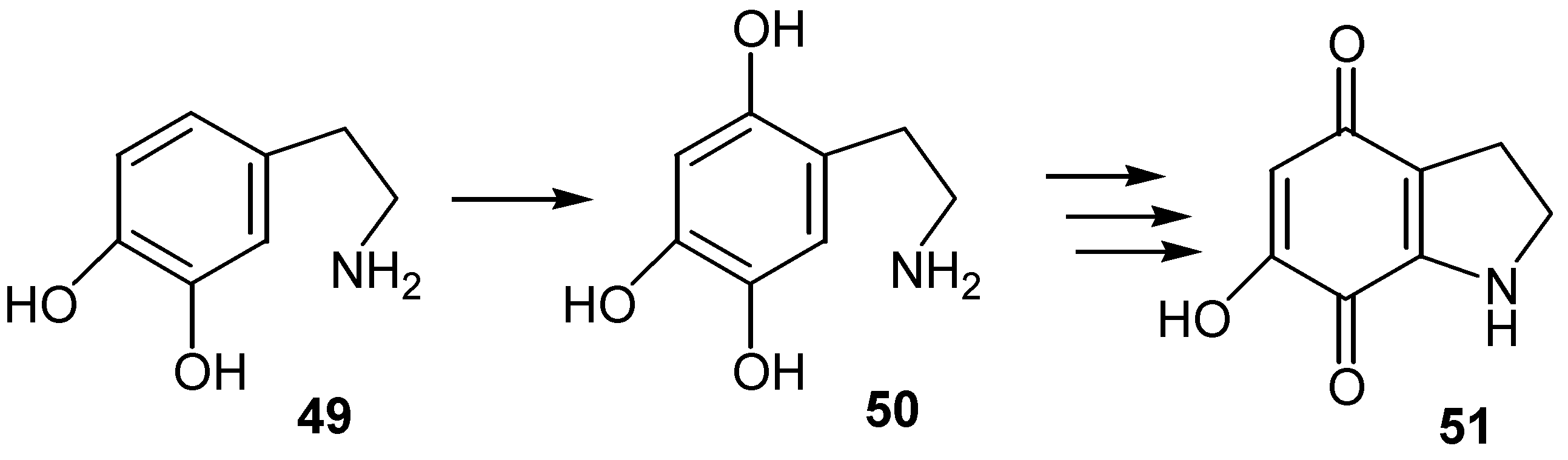
- Pezzella, A.; Ischia, d' M.; Napolitano, A.; Misuraca, G.; Prota, G. Iron-Mediated generation of the neurotoxin 6-hydroxydopamine quinone in the reaction of fatty acid hydroperoxides with dopamine: A possible contributory mechanism for neuronal degeneration in Parkinson’s disease. J. Med. Chem. 1997, 40, 2211–2216. [Google Scholar] [PubMed]
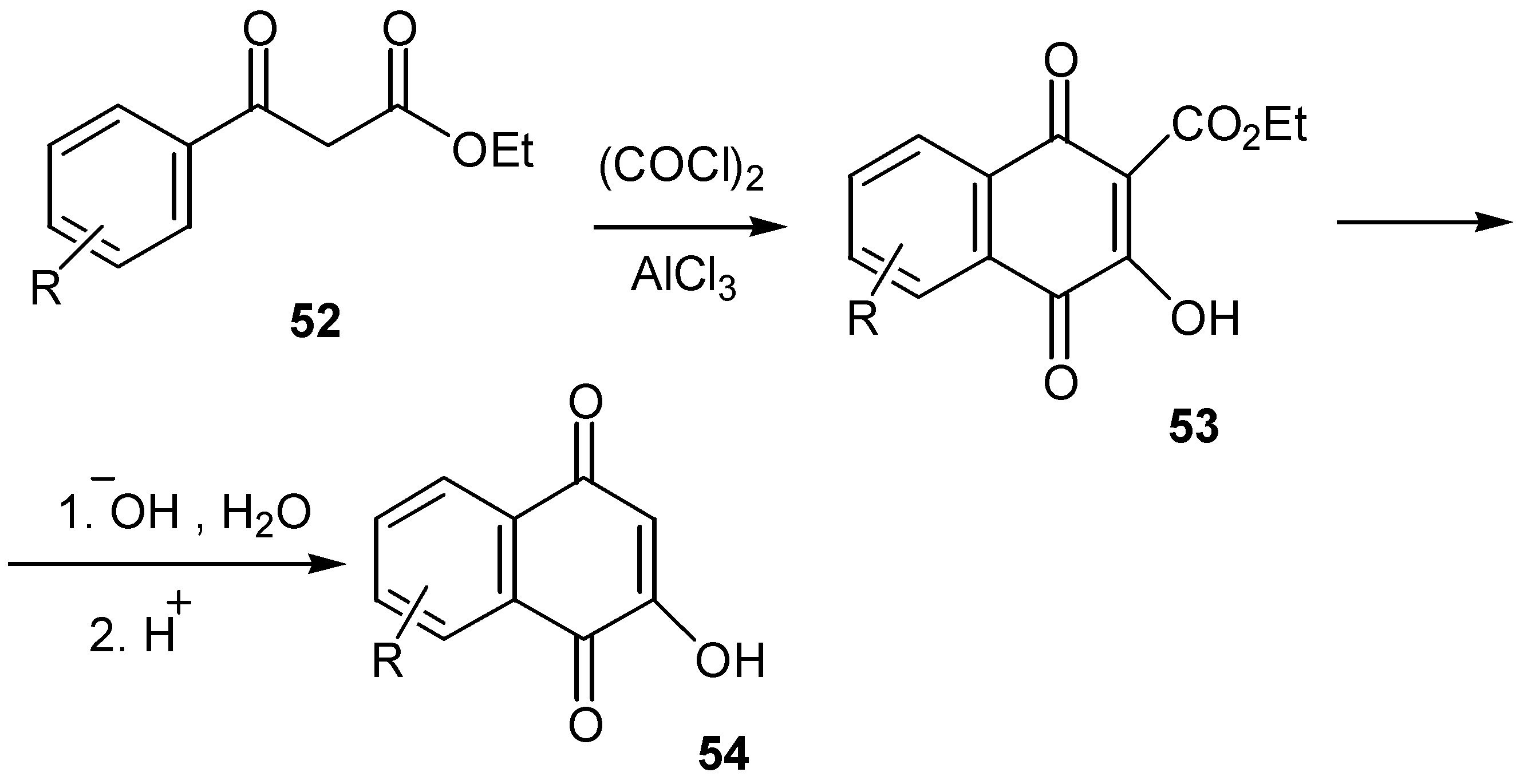
- Sartori, G.; Bigi, F.; Canali, G.; Maggi, R.; Casnati, G.; Tao, X. Friedel-Crafts coordinated processes: Highly selective synthesis of hydroxynaphthoquinones. J. Org. Chem. 1993, 58, 840–843. [Google Scholar] [CrossRef]
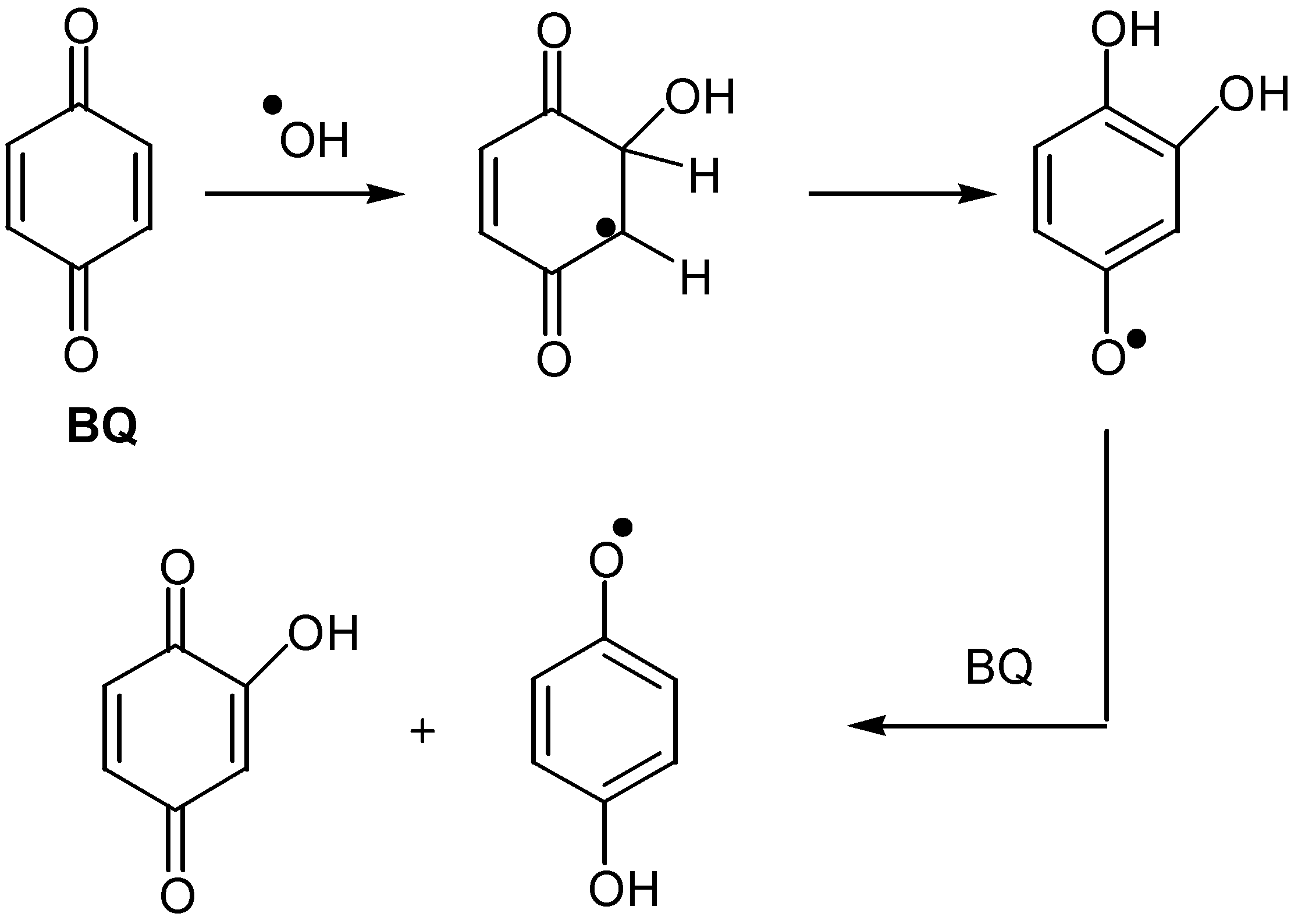
- Schuchman, M. N.; Bothe, E.; Sonntag, v. J.; Sonntag, v. C. Reaction of OH radicals withbenzoquinone in aqueous solutions. A pulse radiolysis study. J. Chem. Soc. Perkin II 1998, 791–796. [Google Scholar] [CrossRef]
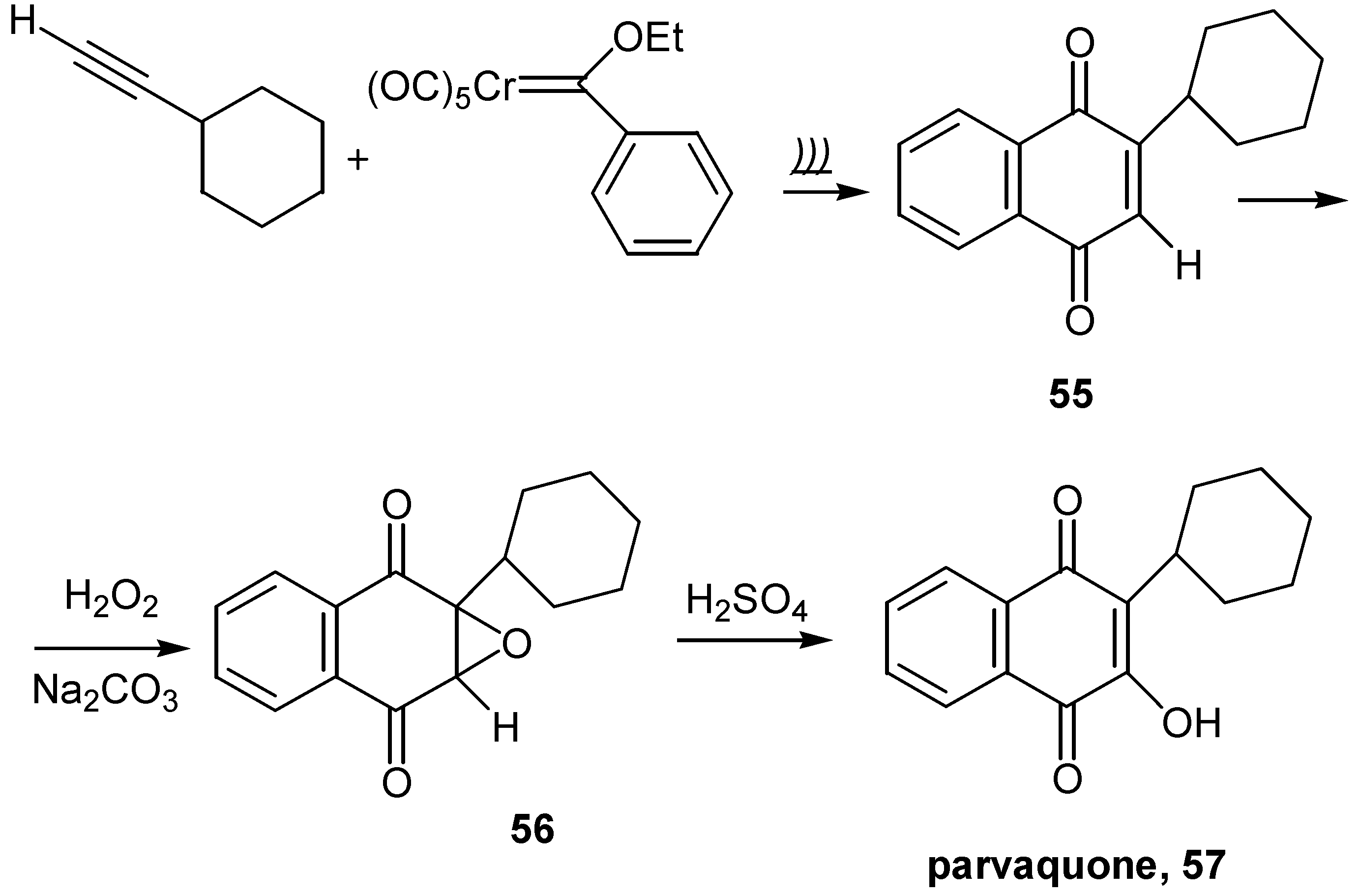
- Harrity, J. P. A.; Kerr, W. J.; Middlemiss, D.; Scott, J. S. Total synthesis of parvaquone and the serendipitous discovery of a novel chromium-mediated method for a-lactone formation. J. Organomet. Chem. 1997, 532, 219–227. [Google Scholar] [CrossRef]
- Singh, I.; Moore, R. E.; Chang, C. W. J.; Ogata, R. T.; Scheuer, P. J. Spinochrome synthesis. Tetrahedron 1968, 24, 2969–2978. [Google Scholar] [CrossRef]
- Jones, R. G.; Shonle, H. A. The preparation of 2,5-dihydroxyquinone. J. Am. Chem. Soc. 1945, 67, 1034–1035. [Google Scholar] [CrossRef]
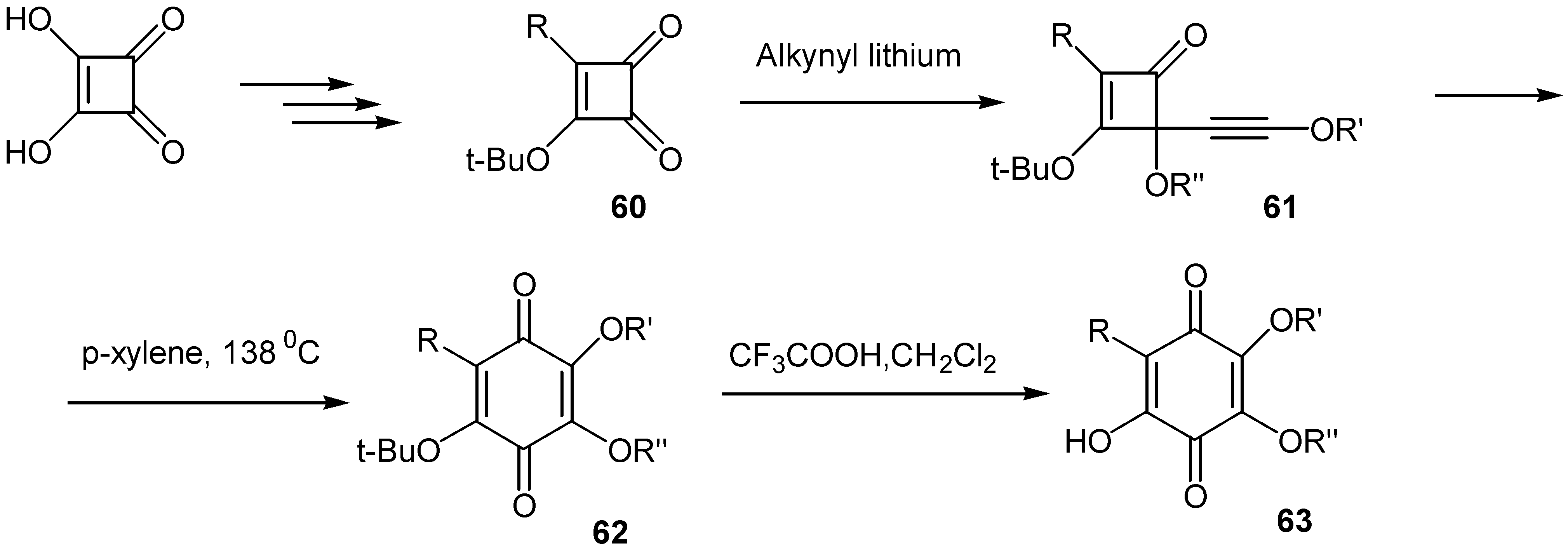
- (a) Enhsen, A.; Karabelas, K.; Heerding, J. M.; Moore, H. W. Synthesis of hydroxyquinones and related compounds: Semisquaric acids, (±)-terreic acid, (±)-perezone, and (±)-isoperezone. J. Org. Chem. 1990, 55, 1177–1185. [Google Scholar] ; (b) Heerding, J. M.; Moore, H. W. Regiospecific synthesis of hydroxyquinones and related compounds from 3-tert-butoxycyclobutene-1,2-dione. J. Org. Chem. 1991, 56, 4048–4050. [Google Scholar]
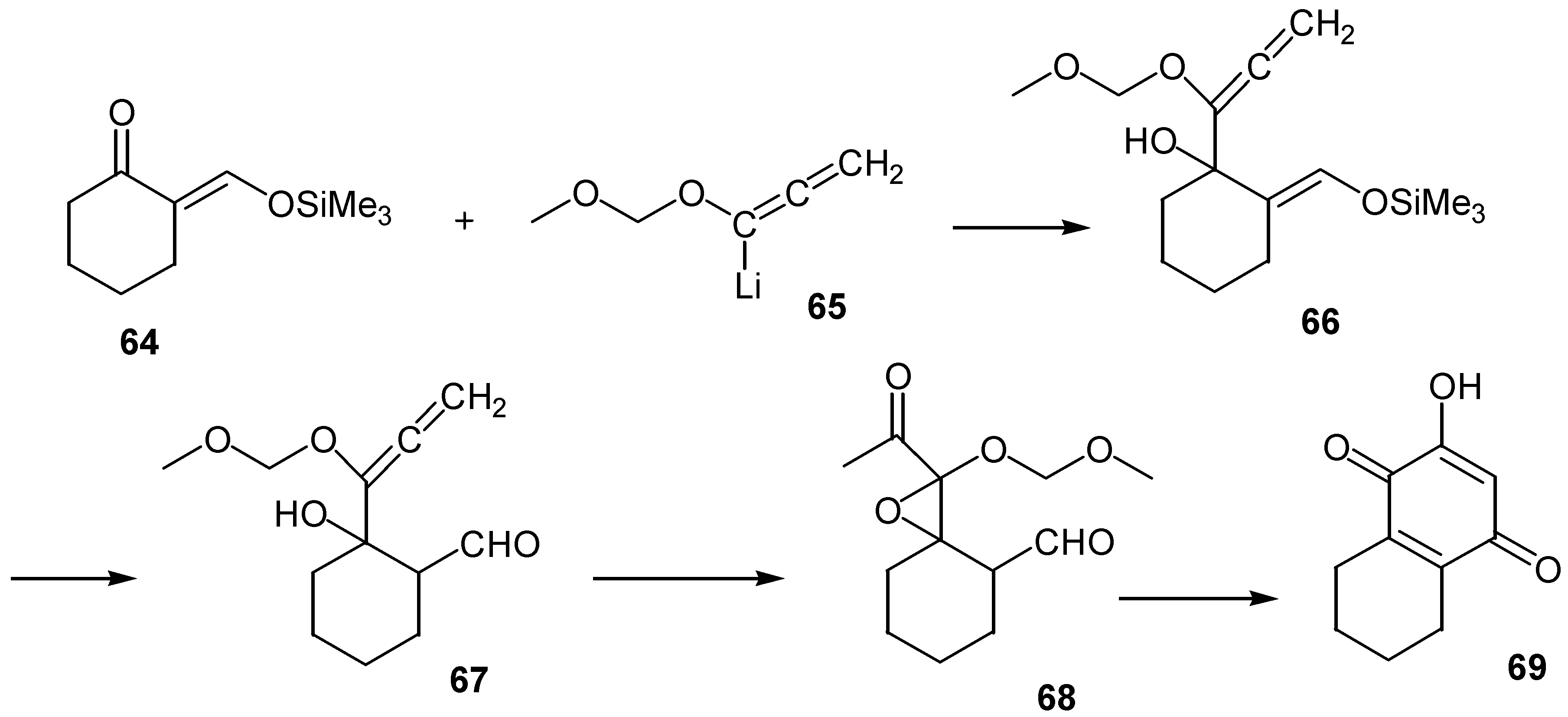
- Tius, M. A.; Cullingham, J. M.; Ali, S. Hydroxyquinone annelation. J. Chem. Soc. Chem. Commun. 1989, 867–869. [Google Scholar] [CrossRef]
- Horiuchi, C. A.; Suzuki, Y. A new synthesis of 3-hydroxy-2,5-dialkyl-1,4-benzoquinone from 3-halo-3,6-dialkyl-1,2-cyclohexanedione using iodine-copper(II) acetate. Bull. Chem. Soc. Jpn. 1989, 62, 2919–2922. [Google Scholar] [CrossRef]
- Reinaud, O.; Capdevielle, P.; Maumy, M. Synthesis of new 3-(-2-alkenyl)-2-hydroxy-5-methoxy-p-benzoquinones via Claisen rearrangement of original 5-methoxy-4-(2-propenyloxy)-o-benzoquinones. Synthesis 1988, 293–300. [Google Scholar] [CrossRef]
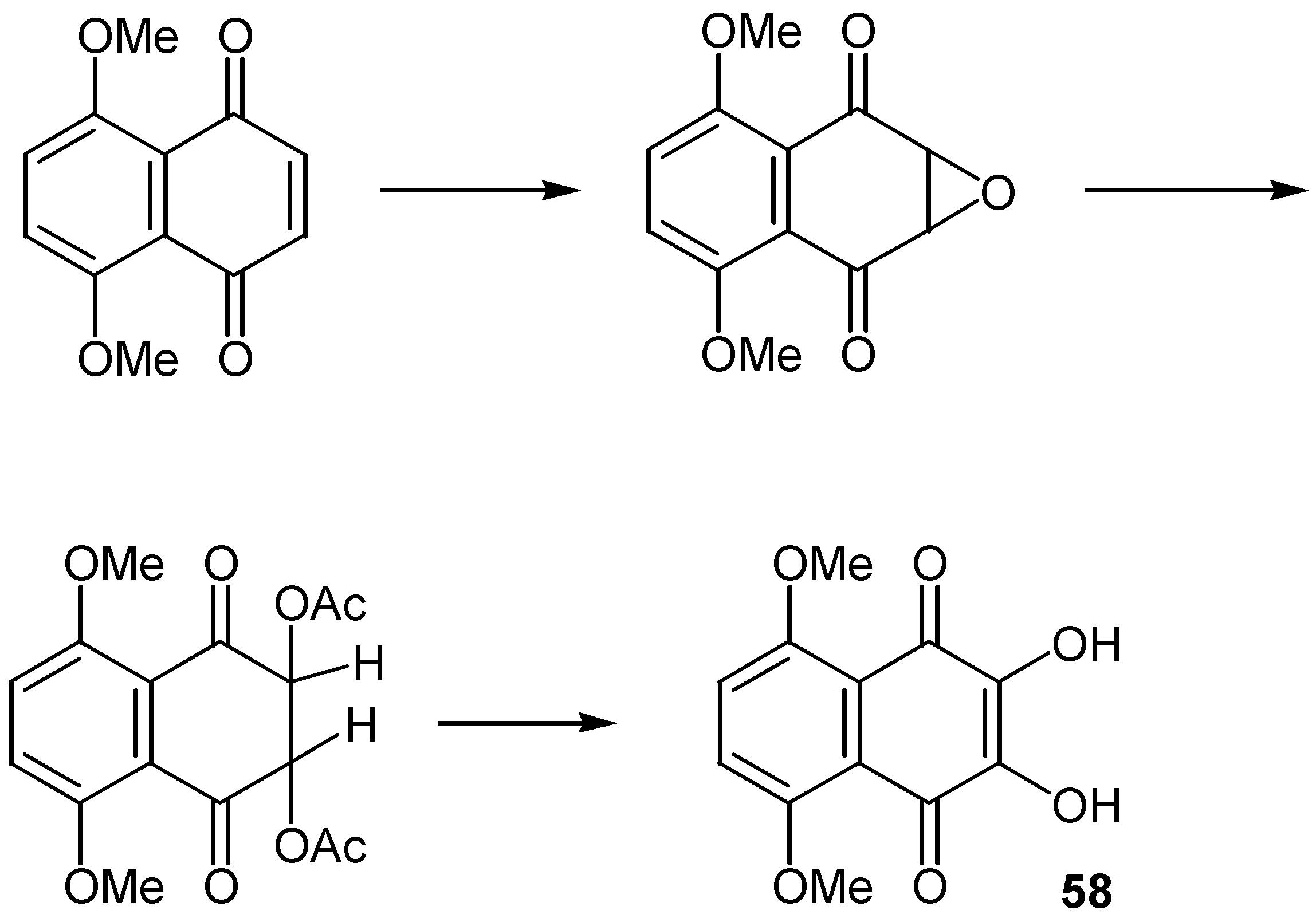
- Finley, K. T. in ref. 1, Vol 2, p 603.
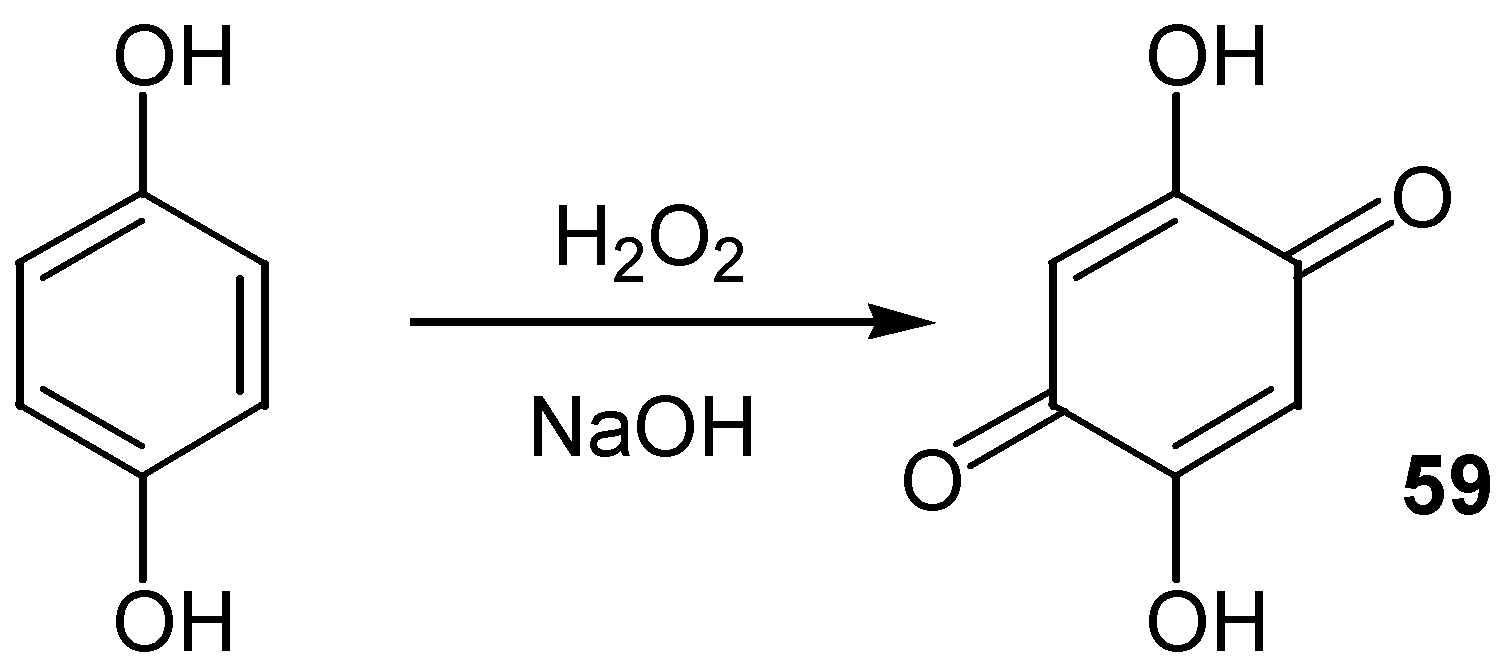
- Fieser, L.F.; Berliner, E.; Bondhus, F.J.; Chang, F.C.; Dauben, W.G.; Ettlinger, M.G.; Fawaz, G.; Fields, M.; Heidelberger, C.; Heymann, H.; Vaughan, W.R.; Wilson, E.; Moore, E.E.; Moore, M.B.; Zaugg, H.E. Naphthoquinone antimalarials. IV-XI. Synthesis. J. Am. Chem. Soc. 1948, 70, 3174. [Google Scholar] [CrossRef] [PubMed]
- Khambay, B.P.S.; Batty, D.; Beddie, D.G.; Denholm, I.; Cahill, M.R. A new group of plant-derived naphthoquinone pesticides. Pestic. Sci. 1997, 50, 291–296. [Google Scholar] [CrossRef]
- Sun, J.S.; Geiser, A.H.; Frydman, B. A preparative synthesis of lapachol and related naphthoquinones. Tetrahedron Lett. 1998, 39, 8221–8224. [Google Scholar] [CrossRef]
- Bieber, L.W.; Neto, P.J.R.; Generino, R.M. Regioselective alkylation of substituted quinones by trialkylboranes. Tetrahedron Lett. 1999, 40, 4473–4476. [Google Scholar] [CrossRef]
- Brassard, P.; L’ Ecuyer, P. Arylation of quinones by diazonium salts. IV. Reaction of these salts with 2,5-dihydroxy-p-benzoquinone and the synthesis of 3-hydroxy-2,5-diphenyl-p-benzoquinone. Can. J. Chem. 1958, 36, 1346. [Google Scholar] [CrossRef]
- Kobayashi, K.; Taki, T.; Kawakita, M.; Uchida, M.; Morikawa, O.; Konishi, H. A simple synthesis of benzocarbazolequinones via o-nitroarylation of 2-hydroxy-1,4-naphthoquinones. Heterocycles 1999, 51, 349–354. [Google Scholar] [CrossRef]
- Hagiwara, H.; Sato, K.; Suzuki, T.; Ando, M. Tandem nucleophilic reaction leading to hydrofurans. Application to one-pot synthesis of antitumor naphthofuran natural product. Heterocycles 1999, 51, 497–500. [Google Scholar] [CrossRef]
- Kobayashi, K.; Shimizu, H.; Sasaki, A.; Suginome, H. New one-step synthesis of 2,3-dihydronaphtho[2,3-b]furan-4,9-diones by regioselective [3+2] photoaddition of 2-hydroxy-1,4-naphthoquinones with various alkenes and its application to a two-step synthesis of maturinone. J. Org. Chem. 1991, 56, 3204–3206. [Google Scholar] [CrossRef]
- Kobayashi, K.; Shimizu, H.; Sasaki, A.; Suginome, H. Photoinduced molecular transformations. 140. New one-step general synthesis of naphtho[2,3-b]furan-4,9-diones and their 2,3-dihydro derivatives by the regioselective [3+2] photoaddition of 2-hydroxy-1,4-naphthoquinones with various alkynes and alkenes: Application of the photoaddition to a two-step synthesis of maturinone. J. Org. Chem. 1993, 58, 4614–4618. [Google Scholar]
- Suginome, H.; Konishi, A.; Sakurai, H.; Minakawa, H.; Takeda, T.; Senboku, H.; Tokuda, M.; Kobayashi, K. Photoinduced molecular transformations. Part 156. New photoadditions of 2-hydroxy-1,4-naphthoquinones with naphthols and their derivatives. Tetrahedron 1995, 51, 1377–1386. [Google Scholar] [CrossRef]
- Chuang, C.-P.; Wang, S.-F. Manganese(III) acetate initiated free radical reaction between 1,4-naphthoquinones and a-alkylmalonates. Tetrahedron 1998, 54, 10043–10052. [Google Scholar] [CrossRef]
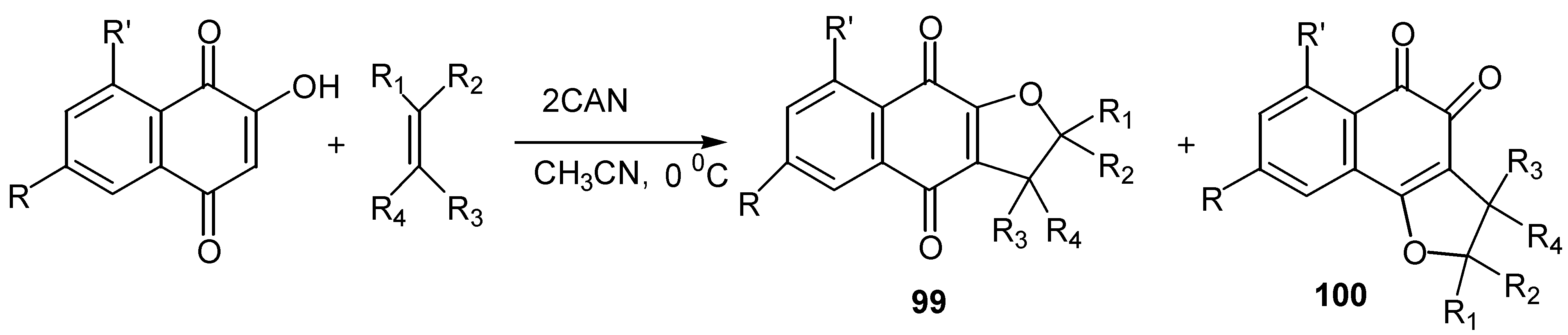
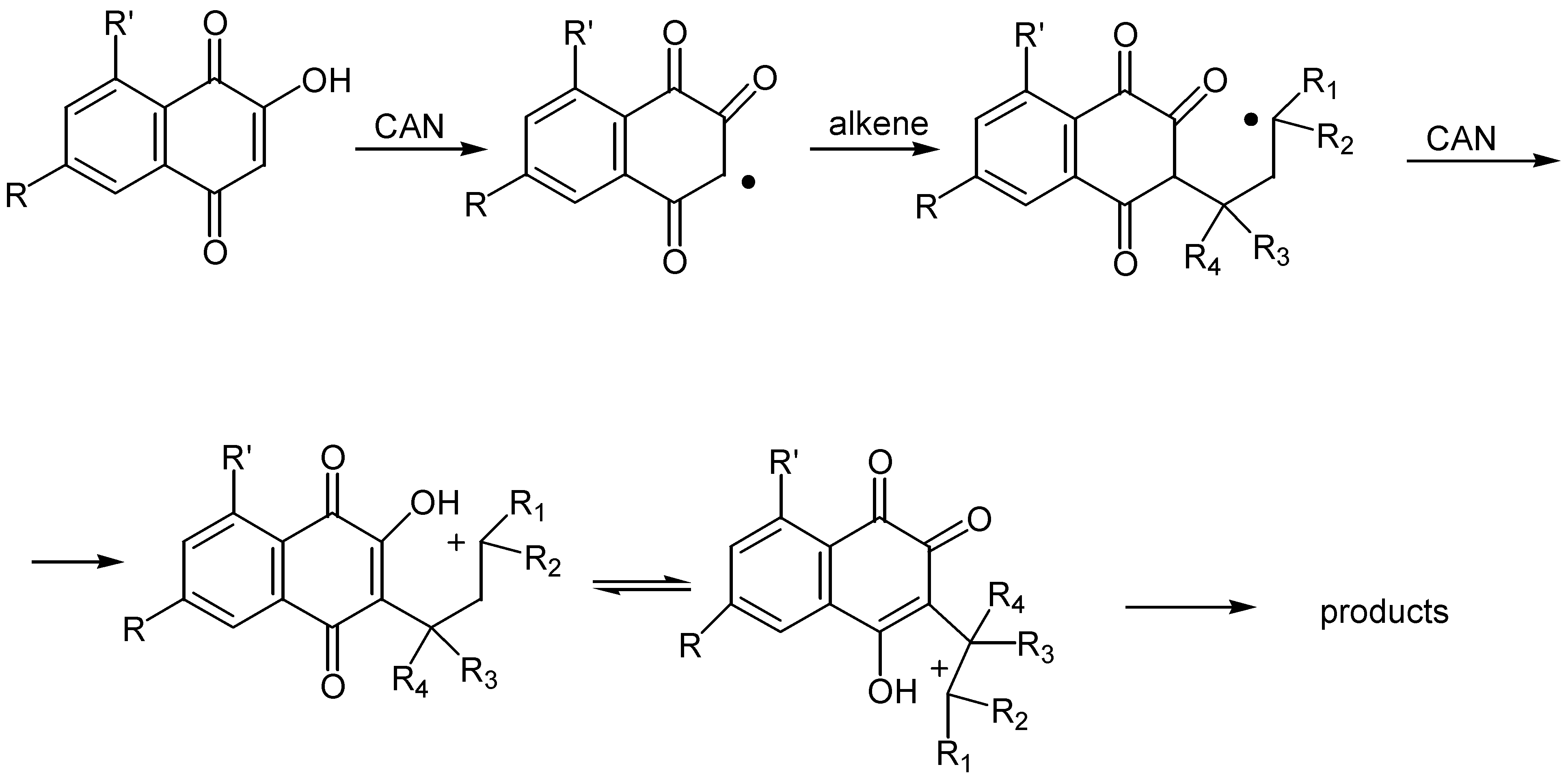
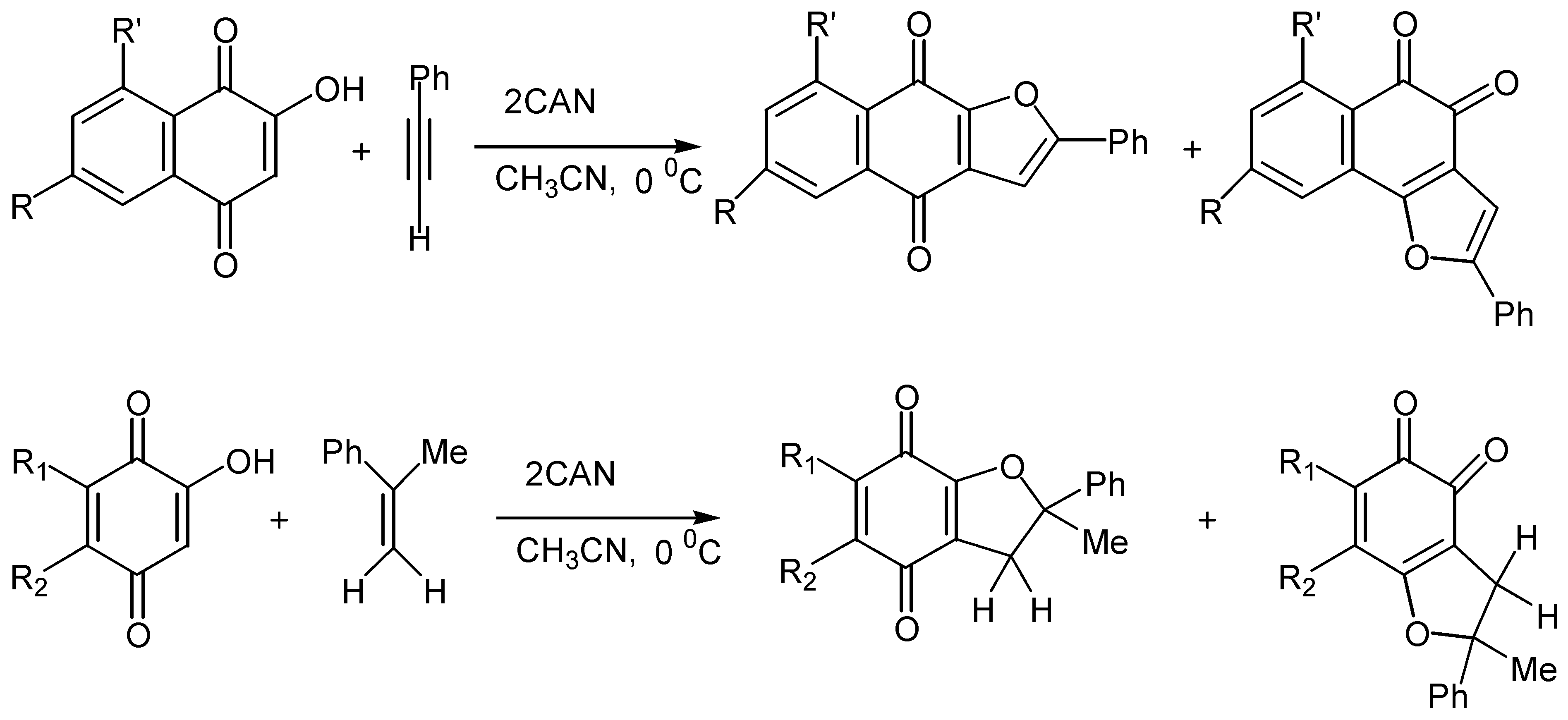
- (a) Kobayashi, K.; Mori, M.; Uneda, T.; Morikawa, O.; Konishi, H. Ceric ammonium nitrate mediated cycloaddition of hydroxyquinones with alkenes for the one-step construction of furoquinone derivatives. Chem. Lett. 1996, 451–452. [Google Scholar] ; (b) Kobayashi, K.; Uneda, T.; Uneda, K.; Mori, M.; Tanaka, H.; Morikawa,, O.; Konishi, H. One-step synthesis of naphthofurandione, benzofurandione, and phenalenofuranone derivatives by the CAN-mediated cycloaddition. Bull. Chem. Soc. Jpn. 1998, 71, 1691–1697. [Google Scholar]
- Kobayashi, K.; Tanaka, K.; Uneda, T.; Maeda, K.; Morikawa, O.; Konishi, H. A direct one-pot preparation of naphtho[2,3-b]furan-4,9-diones from 2-hydroxy-1,4-naphthoquinones and enamines. Synthesis 1998, 1243–1245. [Google Scholar] [CrossRef]
- Kobayashi, K.; Uneda, T.; Kawakita, M.; Morikawa, O.; Konishi, H. One-pot synthesis of naphtho[2,3-b]furan-4,9-diones by sequential coupling/ring-closing reactions. Tetrahedron Lett. 1997, 38, 837–840. [Google Scholar] [CrossRef]
- Shu, T.; Chen, D.-W.; Ochiai, M. Direct synthesis of 2-substituted furotropones fromtropolones utilizing alkynyl(phenyl)iodonium salts. Tetrahedron Lett. 1996, 37, 5539–5542. [Google Scholar] [CrossRef]
- (a) Martínez, E.; Martínez, L.; Estévez, J.C.; Estévez, R.J.; Castedo, L. New, simple total syntheses of benzo[b]naphtho[2,3-d]furan-6,11-diones and benzo[b]naphtho[2,1-d]furans. Tetrahedron Lett. 1998, 39, 2175–2176. [Google Scholar] ; (b) Martínez, A.; Estévez, J.C.; Estévez, R.J.; Castedo, L. 1,2- and 1,4-naphthoquinones: general synthesis of benzo[b]naphtho[2,3-d]furan-6,11-diones. Tetrahedron Lett. 2000, 41, 2365–2367. [Google Scholar]
- Thomson, R.H. Naturally occurring quinones; Blackie Academic and Professional: London, 1997. [Google Scholar]
- Kopanski, L.; Karbach, D.; Selbitschka, G.; Steglich, W. Vesparion, ein naphtho[2,3-b] pyrandion-derivat aus dem schleimpilz metrtichia vesparium (myxomycetes). Liebigs Ann. Chem. 1987, 793–796. [Google Scholar] [CrossRef]
- Gabaja, C.; Perchelet, E.M.; Perchellet, J.-P.; Jones, G.B. Regioselective lactonization of naphtho-quinones: synthesis and antitumorial activity of the WS-5995 antibiotics. Tetrahedron Lett. 2000, 41, 3007–3010. [Google Scholar]
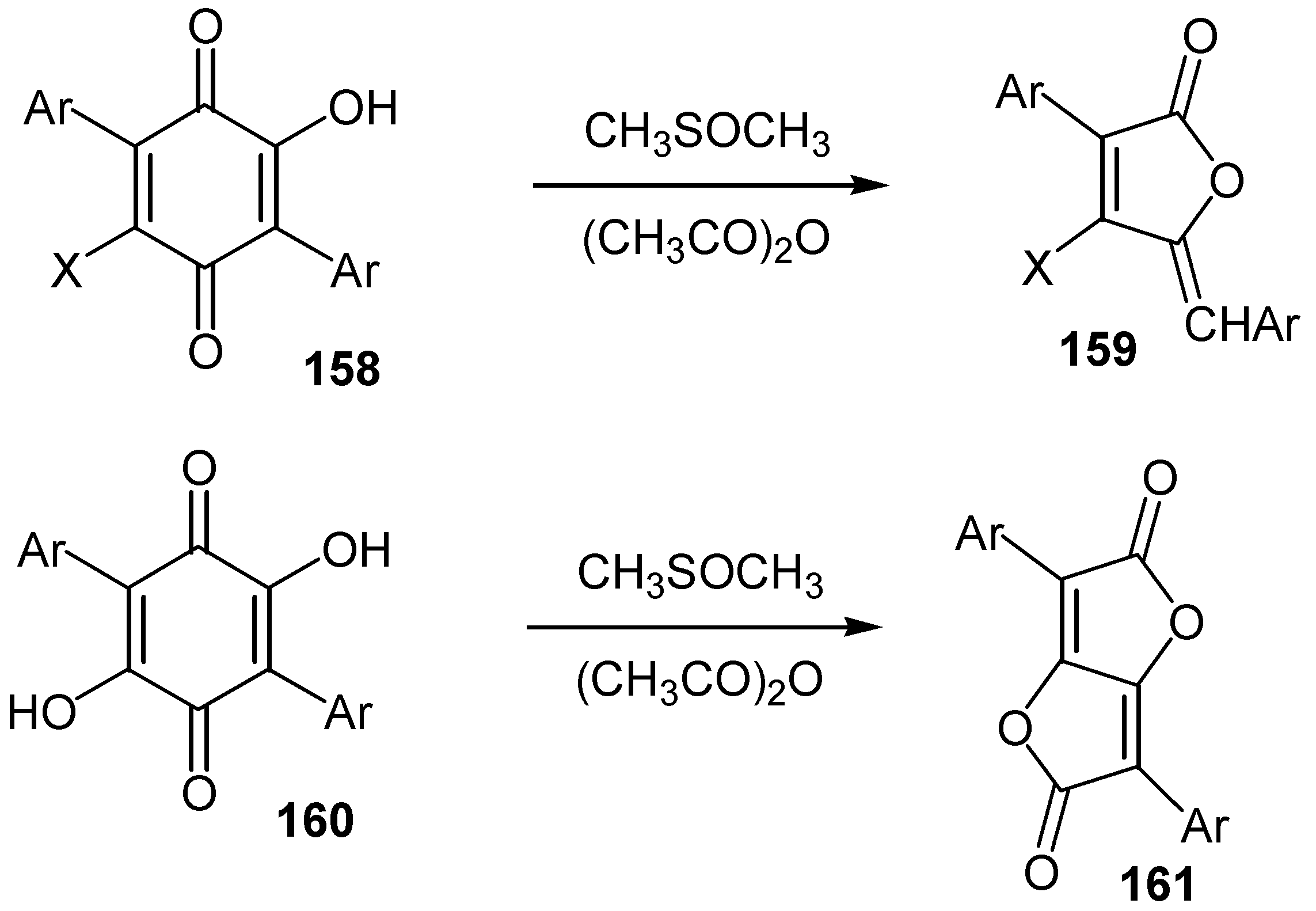
- Wilkholm, R.J.; Moore, H.W. Dimethyl sulfoxide-acetic anhydride oxidative rearrangements of hydroxyterphenylquinones. A possible biosynthetic model. J. Am. Chem. Soc. 1972, 94, 6152–6158. [Google Scholar] [CrossRef]
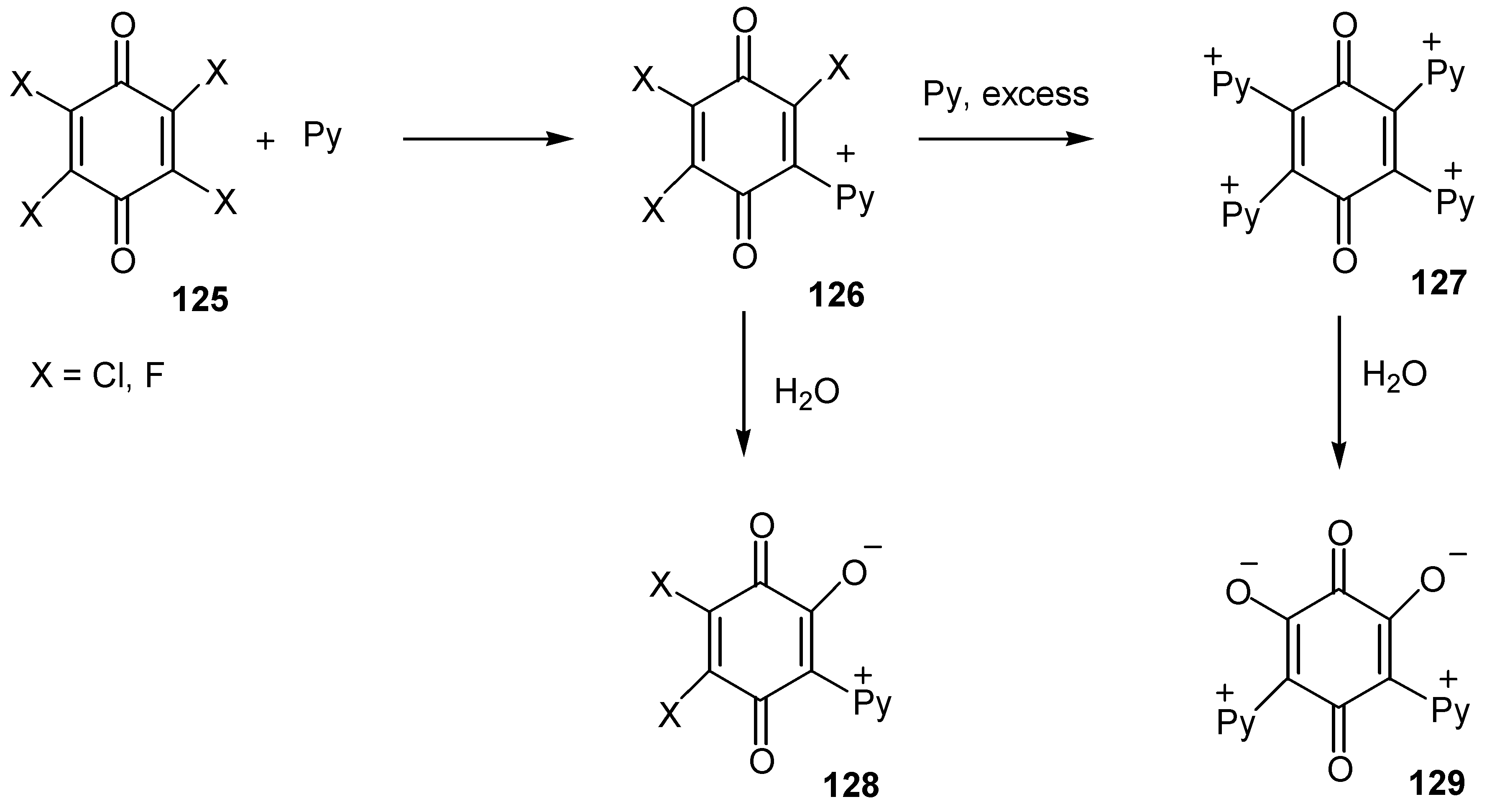
- Koch, A.S.; Harbison, W.G.; Hubbard, J. M.; de Kort, M.; Roe, B. A. Stability of pyridiniumylquinones to aqueous media: The formation of pyridinium-oxy zwitterionic quinones. J. Org. Chem. 1996, 61, 5959–5963. [Google Scholar] [CrossRef]
- Citterio, A.; Fichi, M.; Maronati, A.; Sebastiano, R.; Mele, A. Synthesis of 2-oxy-3-(pyridinium1’-yl)-1,4-naphthoquinone derivatives by iodine or hydrogen peroxide oxidation of 1,4-naphthoquinones in the presence of substituted pyridines. Synthesis 1997, 614–616. [Google Scholar] [CrossRef]
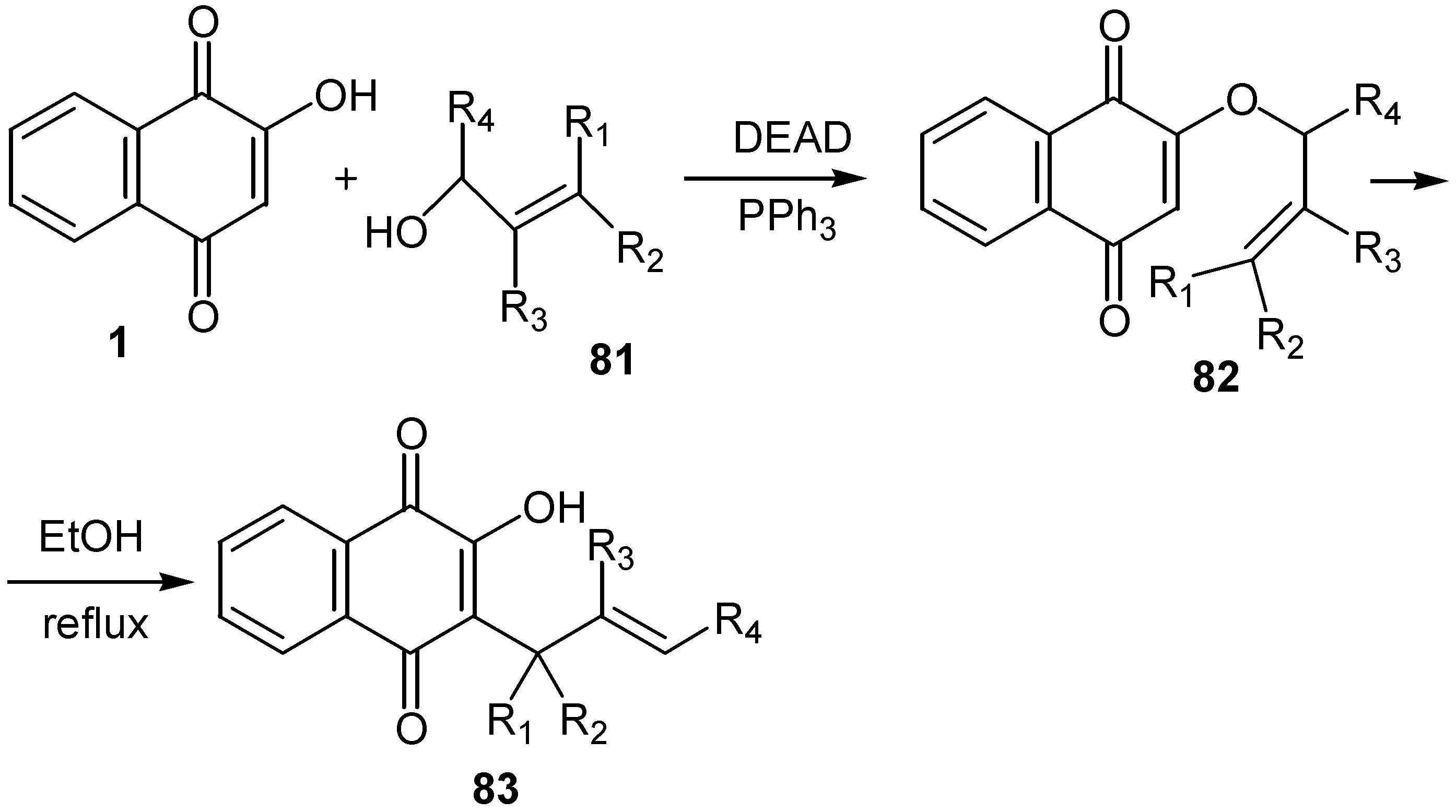
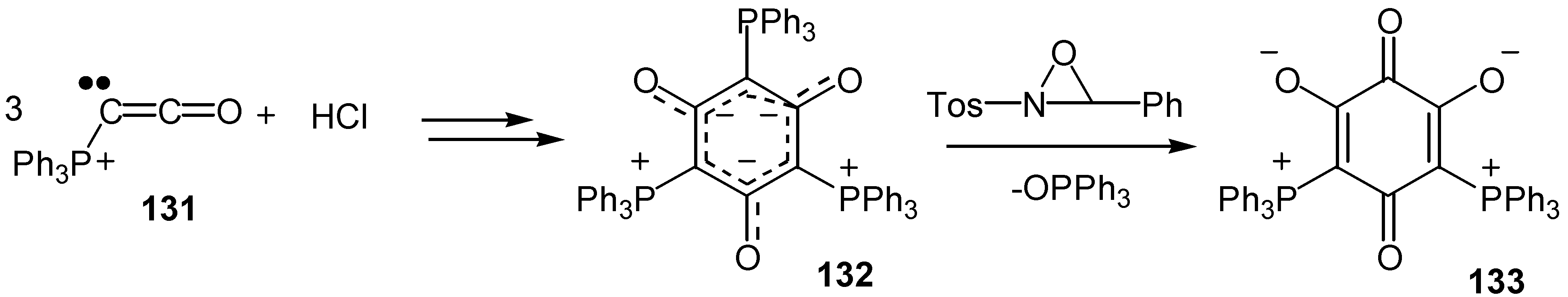
- Bestmann, H. J.; Furst, T. G.; Schier, A. Trimeric ketenylidene(triphenyl)phosphorane: A hybrid between an arene and an ylide. Angew. Chem. Int. Ed. Engl. 1993, 32, 1747–1750. [Google Scholar] [CrossRef]
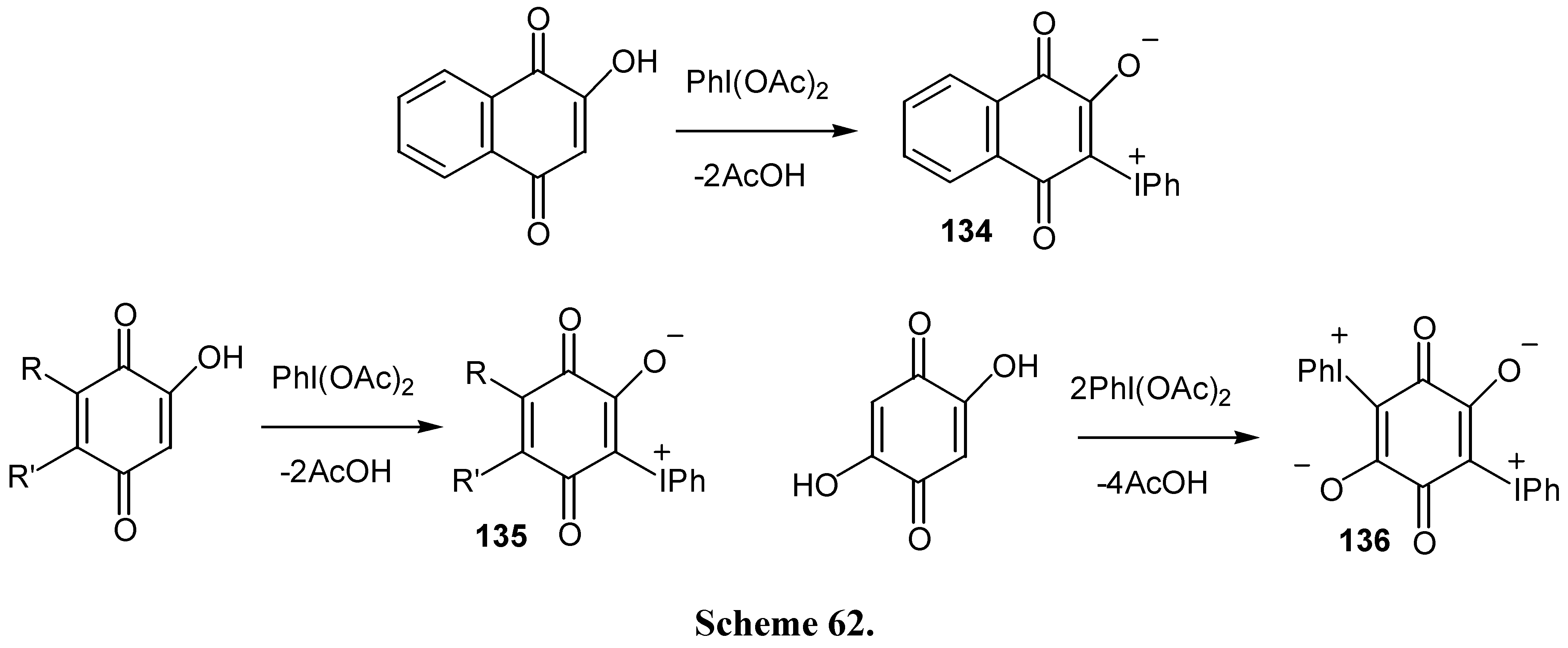
- Hatzigrigoriou, E.; Spyroudis, S.; Varvoglis, A. Derivatives of 1,4-naphthoquinone via 3-(phenyliodonio)-1,2,4-trioxo-1,2,3,4-tetrahydronaphthalenide. Liebigs. Ann. Chem. 1988, 167–170. [Google Scholar] [CrossRef]
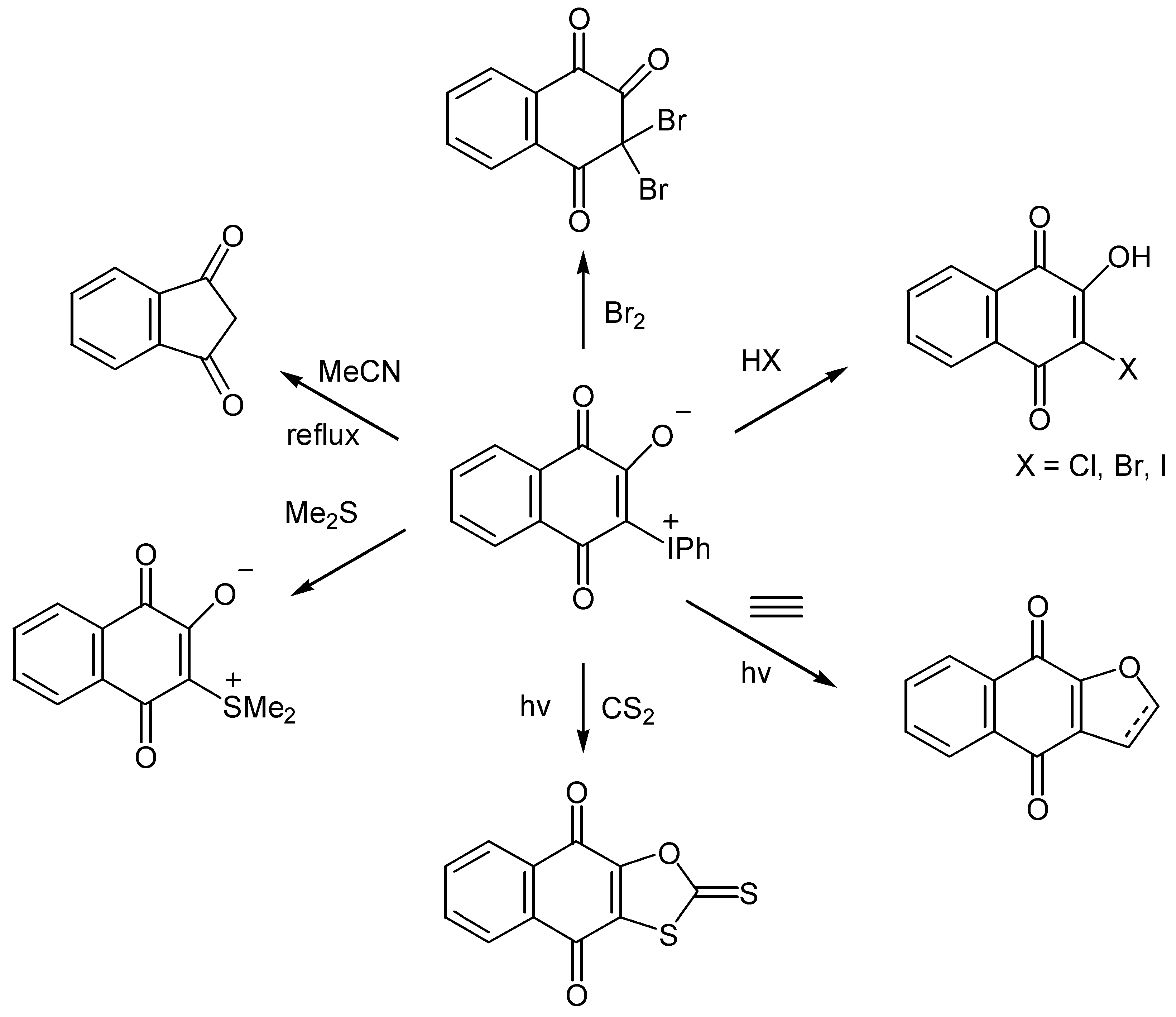
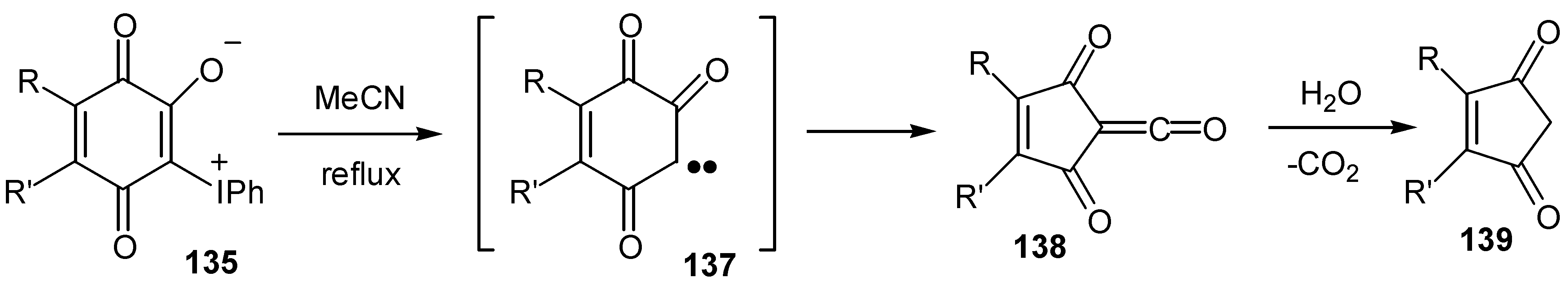
- Papoutsis, I.; Spyroudis, S.; Varvoglis, A. The chemistry of 2-oxido-3-phenyliodonio-1,4-benzoquinones: Transformation to 2-cyclopentene-1,4-diones and cycloadditions. Tetrahedron Lett. 1994, 35, 8449–8452. [Google Scholar] [CrossRef]
- Spyroudis, S.; Xanthopoulou, N. To be published.
- Stagliano, K.W.; Malinakova, H. C. Regiospecific synthesis of unsymmetrical 2,3-diaryl-quinones via stepwise Pd(0)-catalyzed couplings of arylstannanes to doubly activated quinone equivalents. Tetrahedron Lett. 1997, 38, 6617–6620. [Google Scholar] [CrossRef]
- Stagliano, K.W.; Malinakova, H. C. Regiospecific synthesis of 2,3-bisnaphthopyranyl quinones related to conocurvone. Effect of substituents on palladium-catalyzed cross coupling of organostannanes to naphthopyranyl hydroxyquinone triflates. J. Org. Chem. 1999, 64, 8034–8040. [Google Scholar] [CrossRef]
- Farfán, N.; Ortega, E.; Contreras, R. New heterocycles derived from a simultaneous substitution and reductive acetylation of 2,5-dihydroxy-1,4-benzoquinone by N-containing heterocycles. J. Heterocycl. Chem. 1985, 23, 1609–1612. [Google Scholar] [CrossRef]
- Kaji, A.; Kimura, K.; Teranishi, M.; Kiriyama, N.; Nomura, M.; Miyamoto, K. Preparation and structure-activity relationships of novel asterriquinone derivatives. Chem. Pharm. Bull. 1998, 46, 1325–1329. [Google Scholar] [CrossRef]
- Frontana, B.; Cárdenas, J.; Rodríguez-Hahn, L.; Baeza, A. Preparative electrochemicalreductive methylation of ortho-hydroxy-para-benzoquinones. Tetrahedron 1997, 53, 469–478. [Google Scholar] [CrossRef]
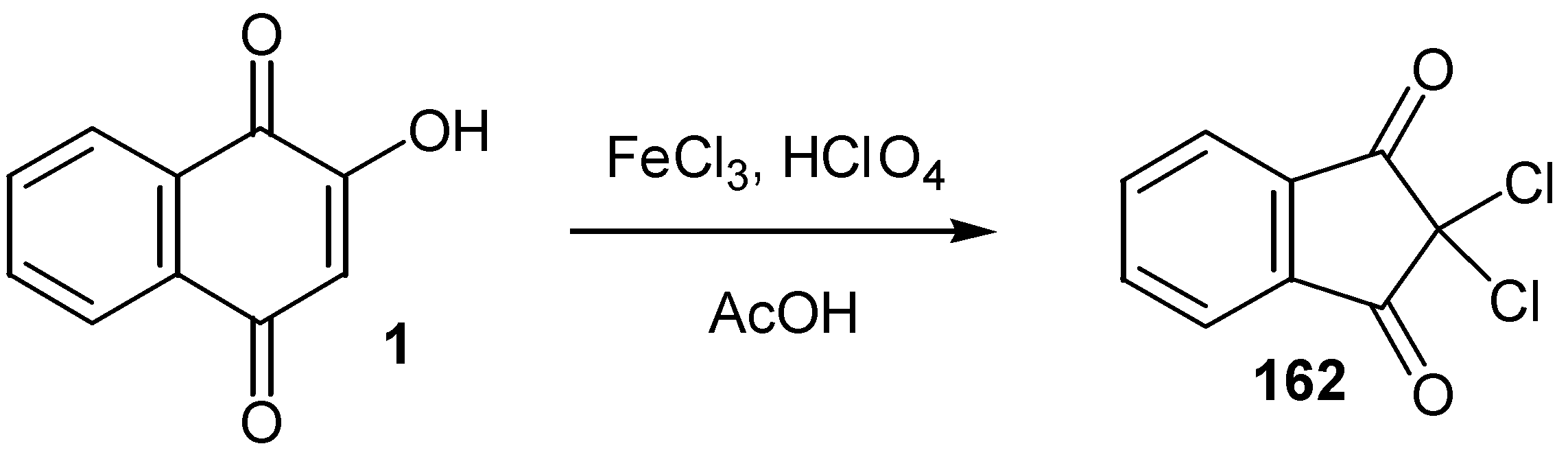
- Singh, P.; Khanna, R. Synthesis of 2,2-dichloroindane-1,3-diones from 1,4-naphthoquinones. Tetrahedron Lett. 1994, 35, 3753–3754. [Google Scholar] [CrossRef]
- Khajuria, R.; Jain, S.; Phar, K. Studies on cycloaddition reactions of 2,4-dihydroxy-3-undecyl-1,4-benzoquinone (embelin) and 2,3-dimethylbuta-1,3-diene. Indian J. Chem., Section B. 1996, 35B(8), 860–861. [Google Scholar]
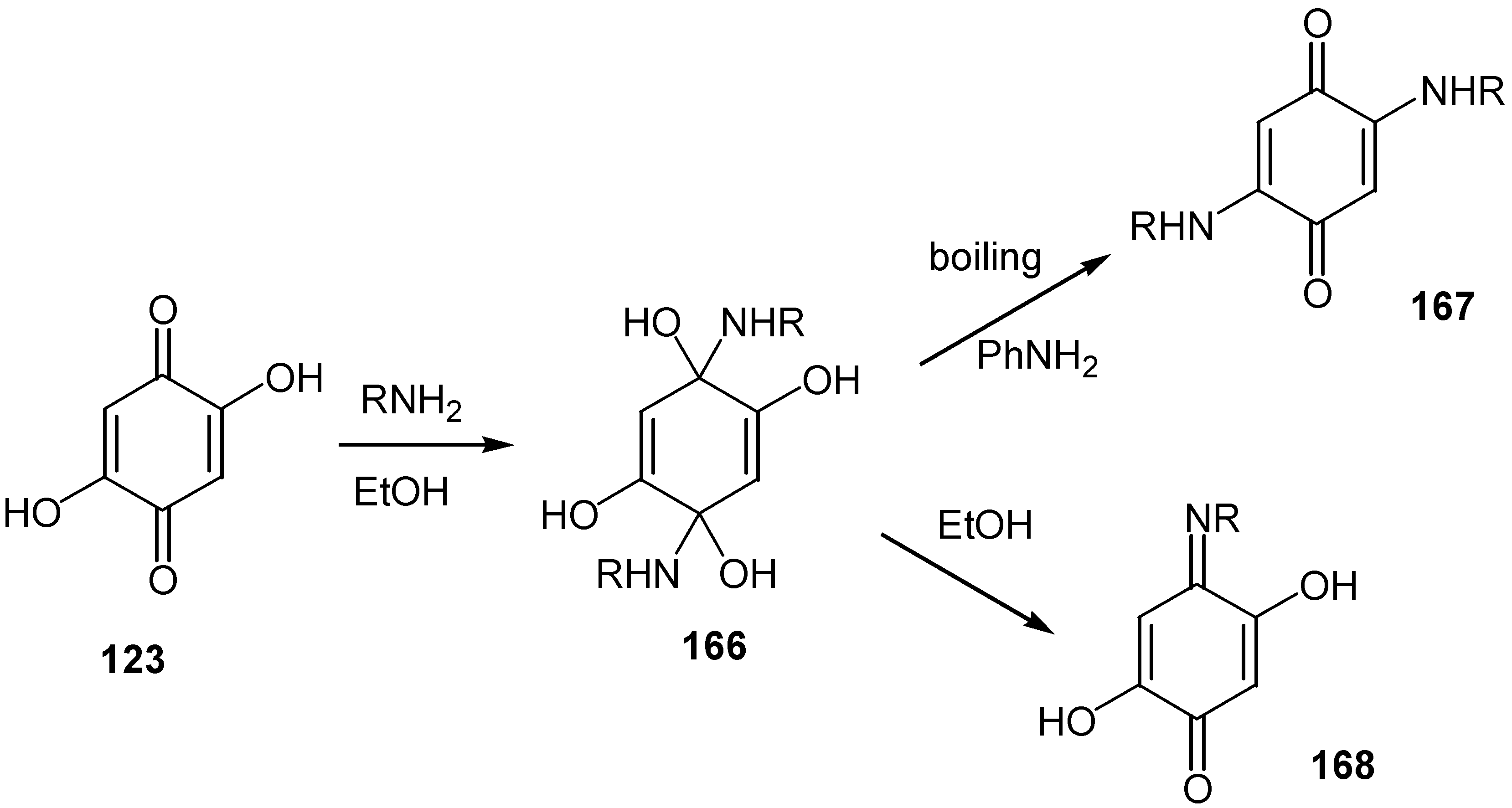
- Tindale, C.R. Reactions of biogenic amines with quinones. Aust. J. Chem. 1984, 37, 611–617. [Google Scholar] [CrossRef]
- (a) Mure, M.; Klinman, J.P. Model studies of topaquinone-dependent amine oxidases. 1. Oxidation of benzylamine by topaquinone analogs. J. Am. Chem. Soc. 1995, 117, 8698–8706. [Google Scholar] ; (b) Mure, M.; Klinman, J.P. Model studies of topaquinone-dependent amine oxidases. 2. Characterization of reaction intermediates and mechanism. J. Am. Chem. Soc. 1995, 117, 8707–8718. [Google Scholar]
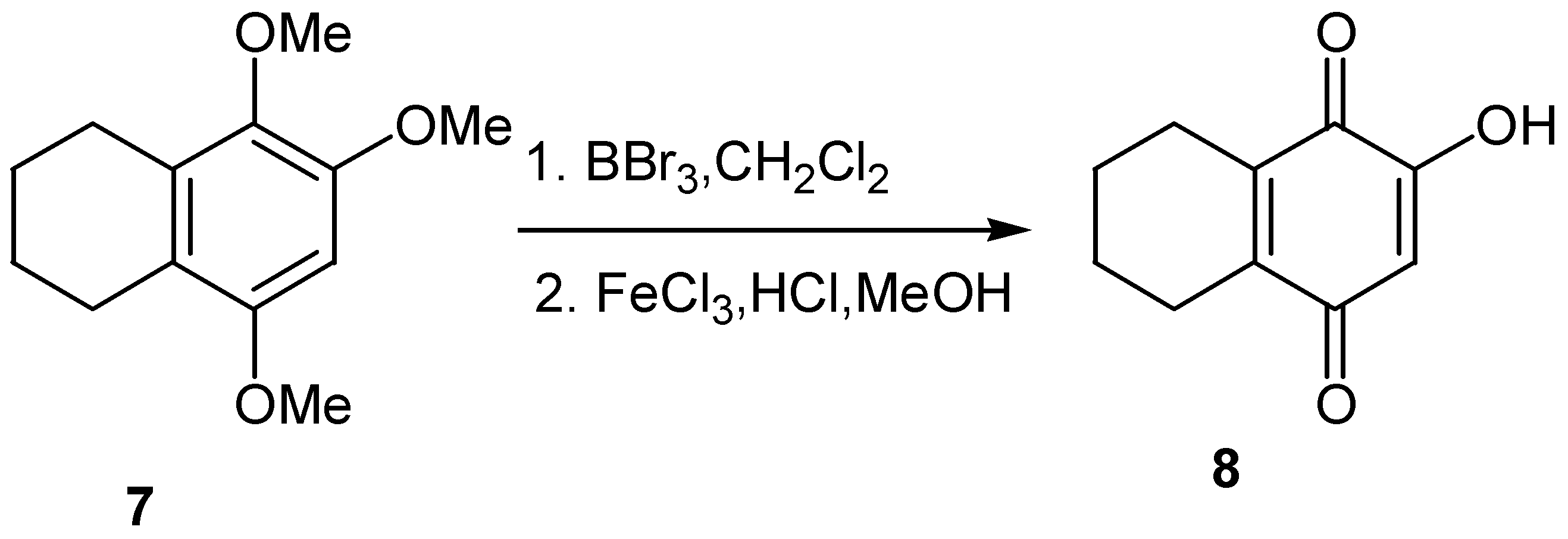



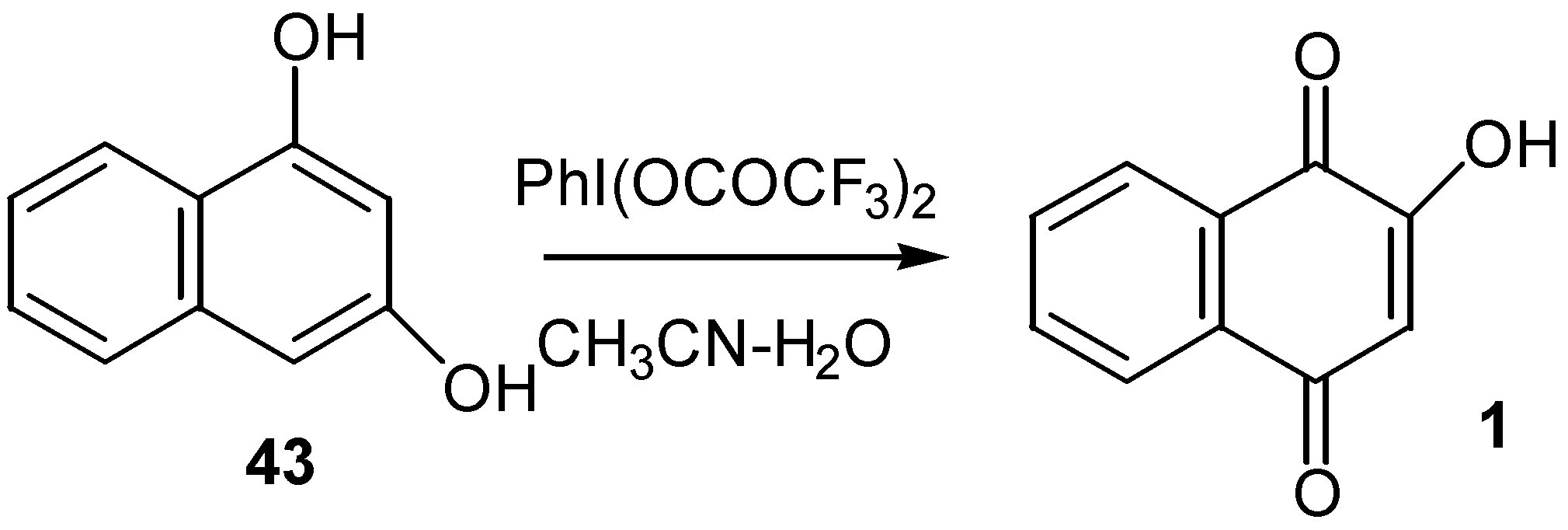

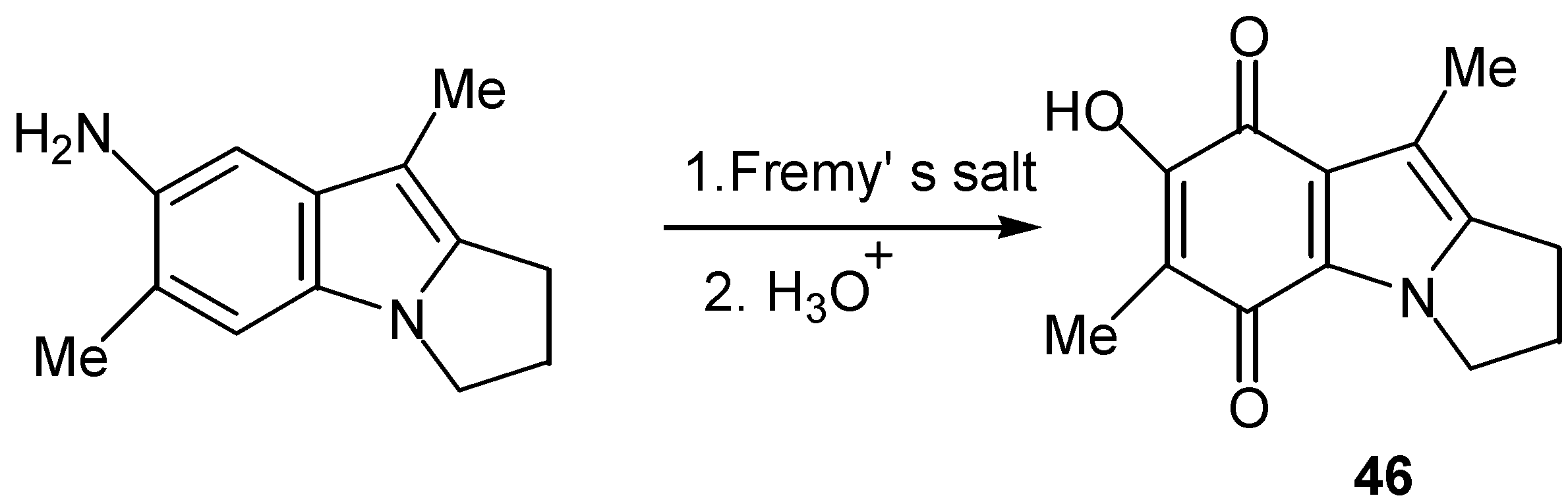
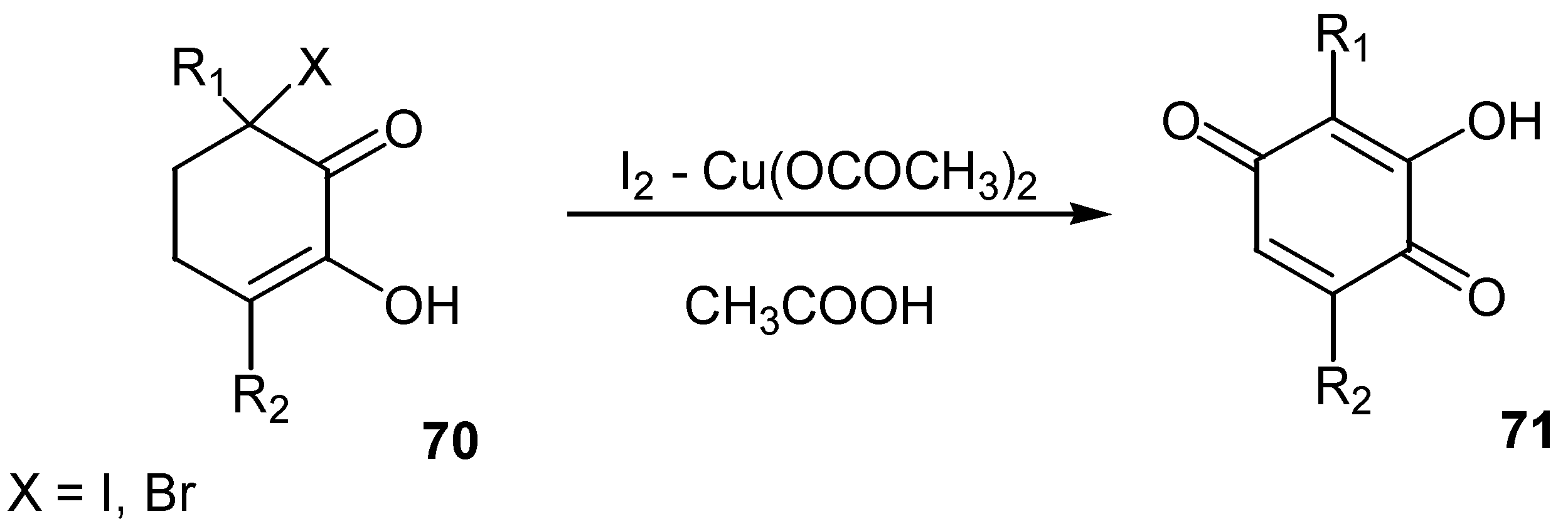



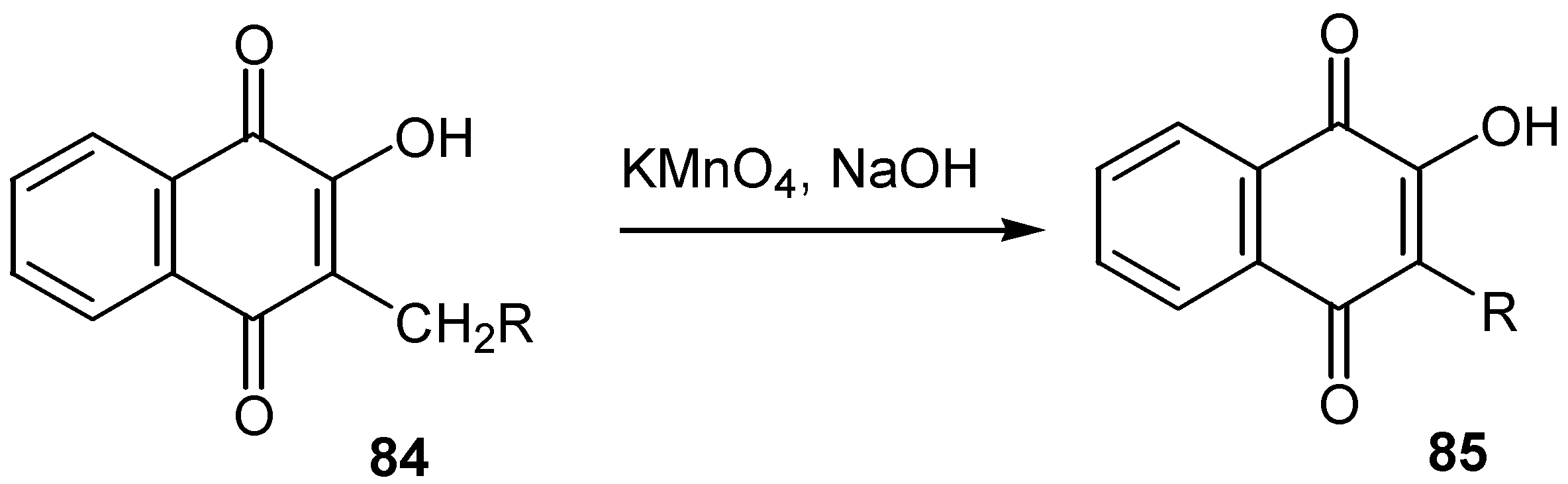

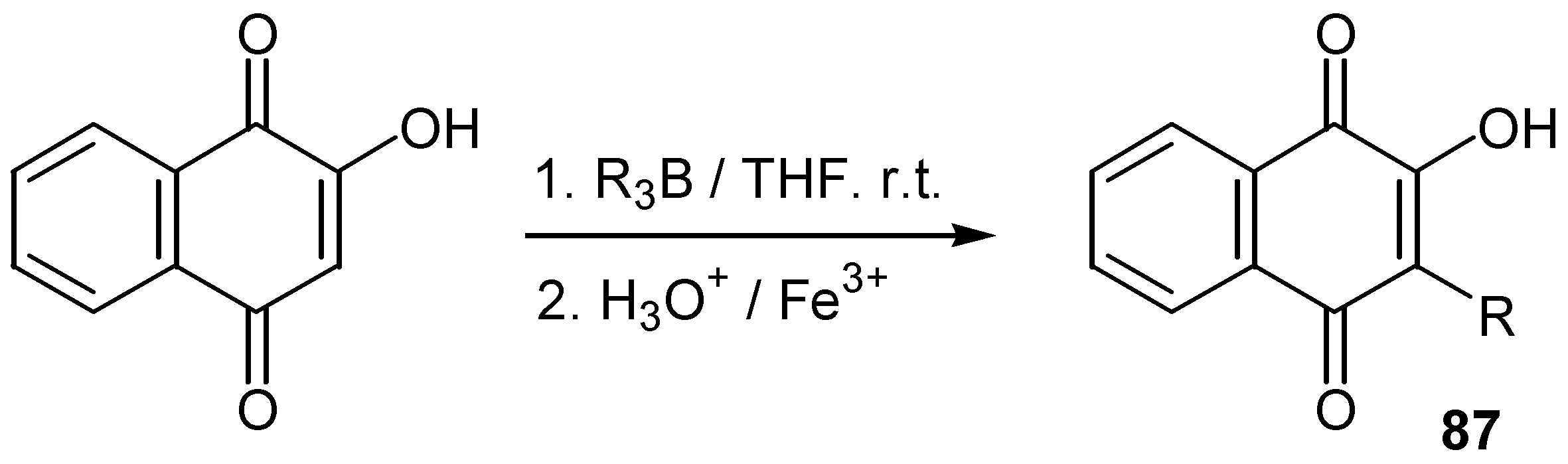
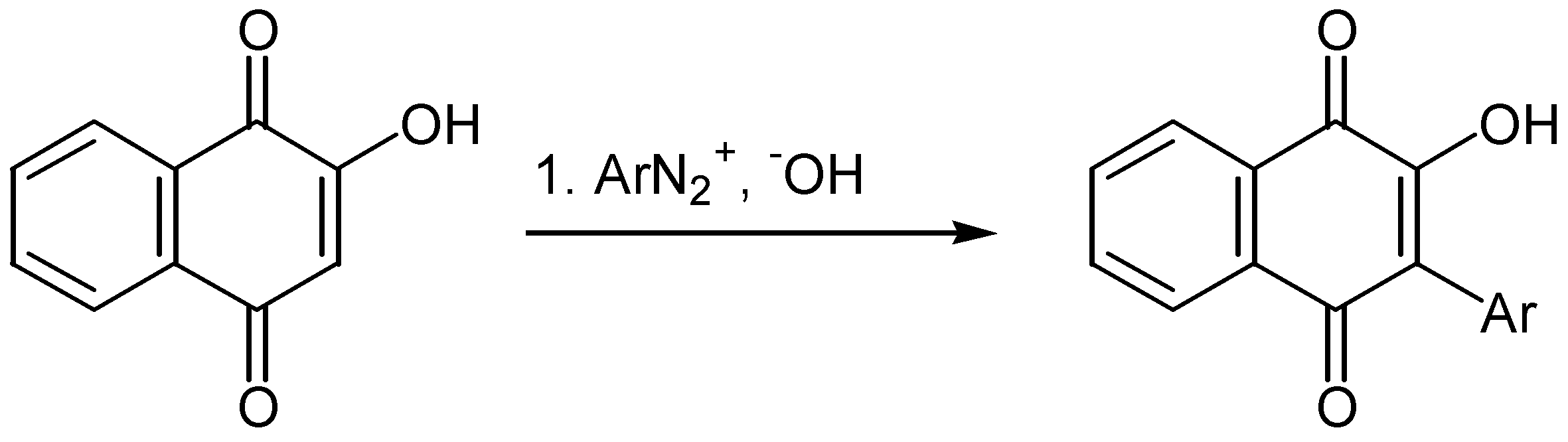


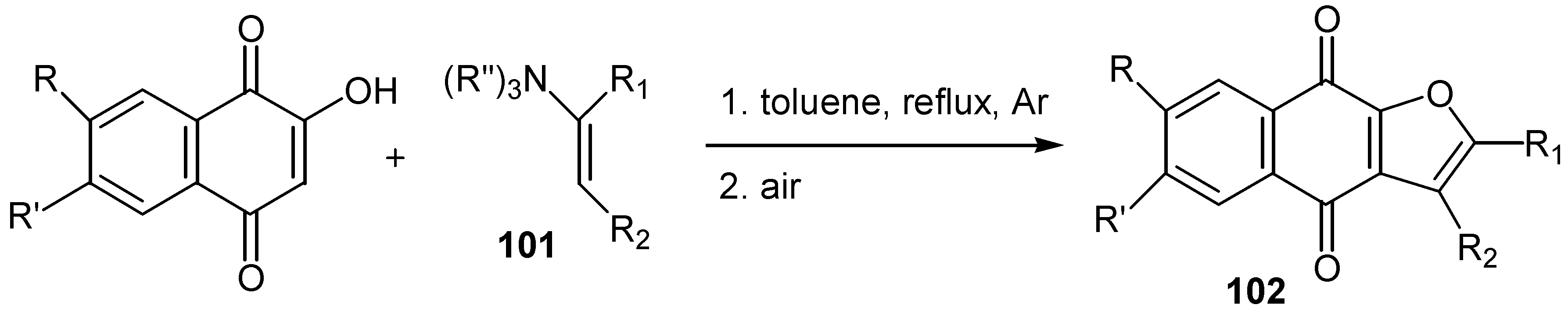
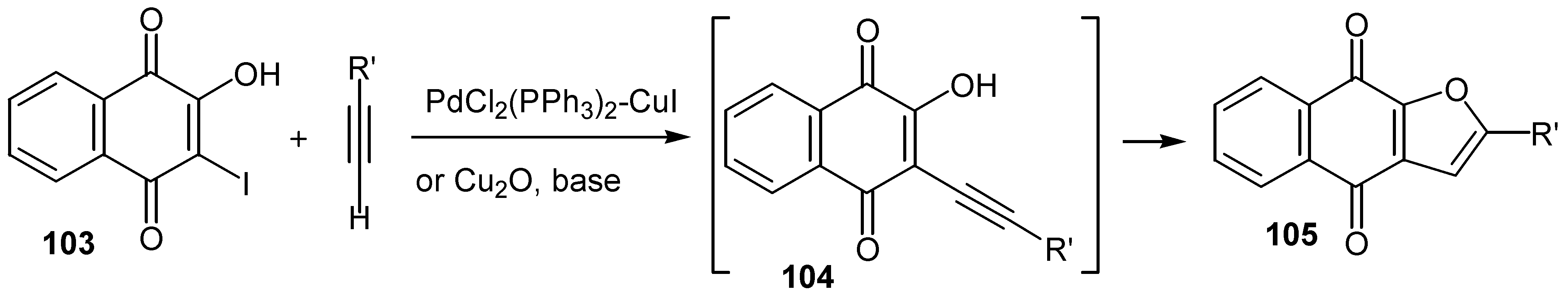
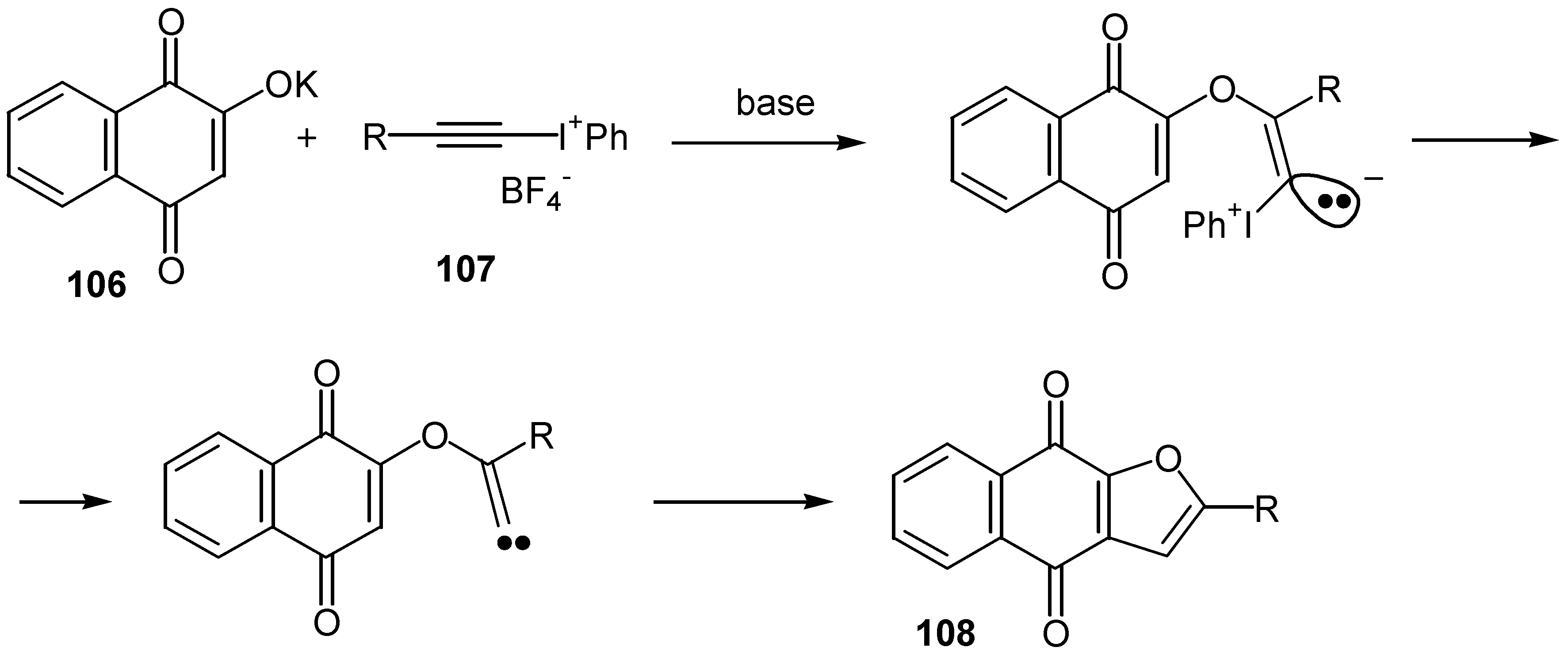
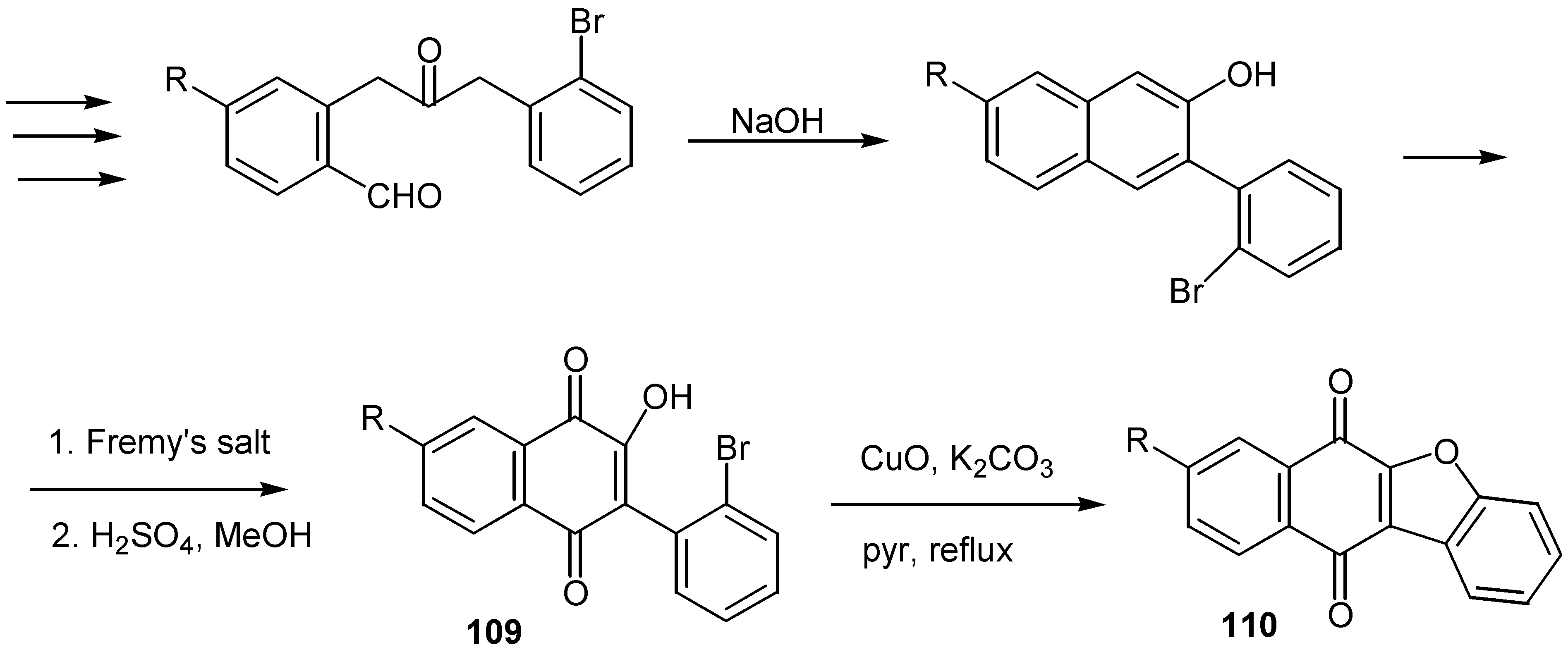
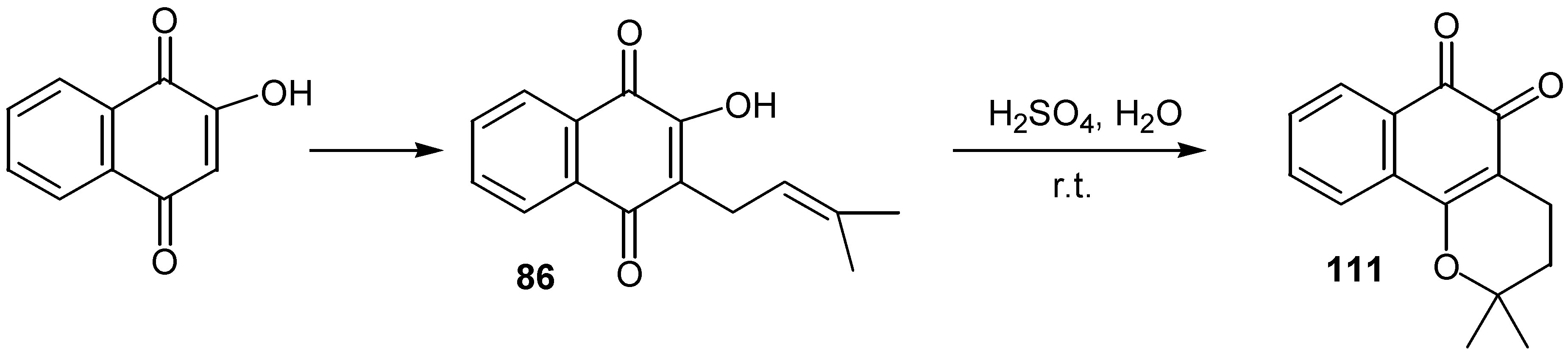


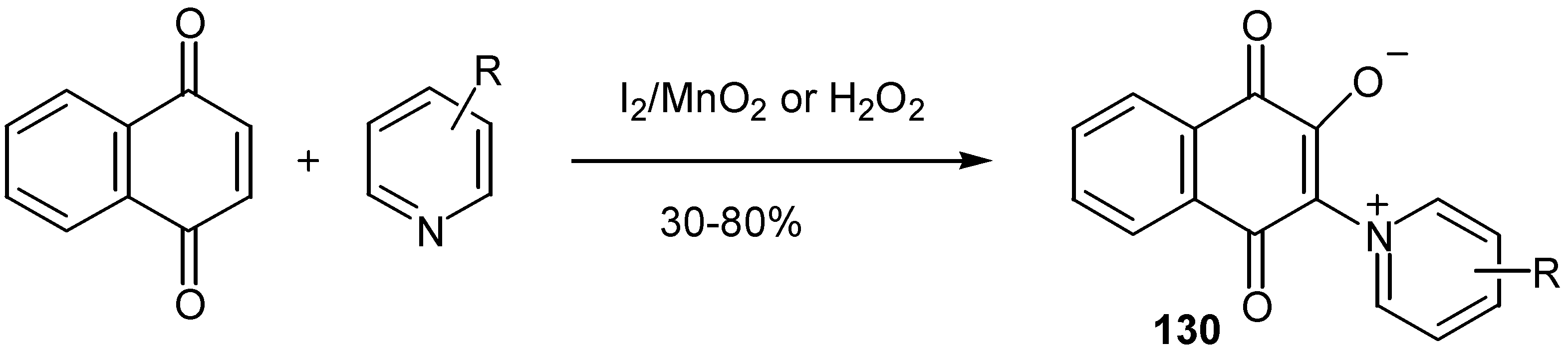
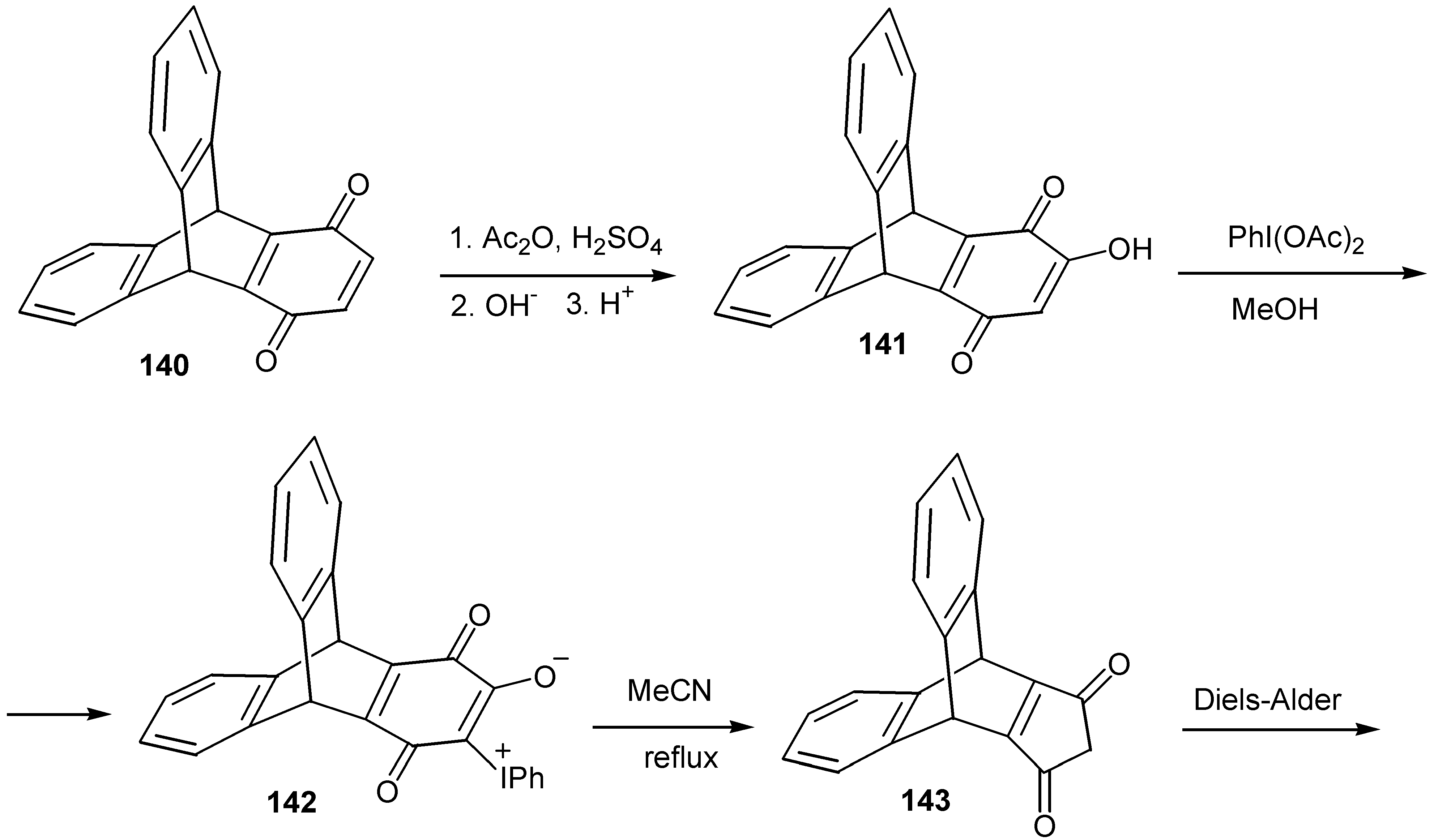
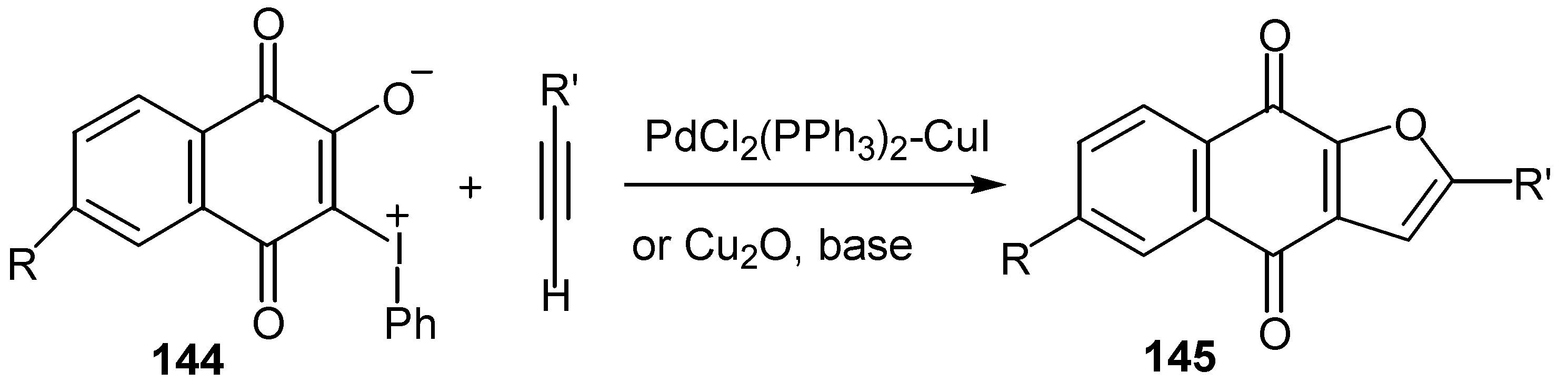

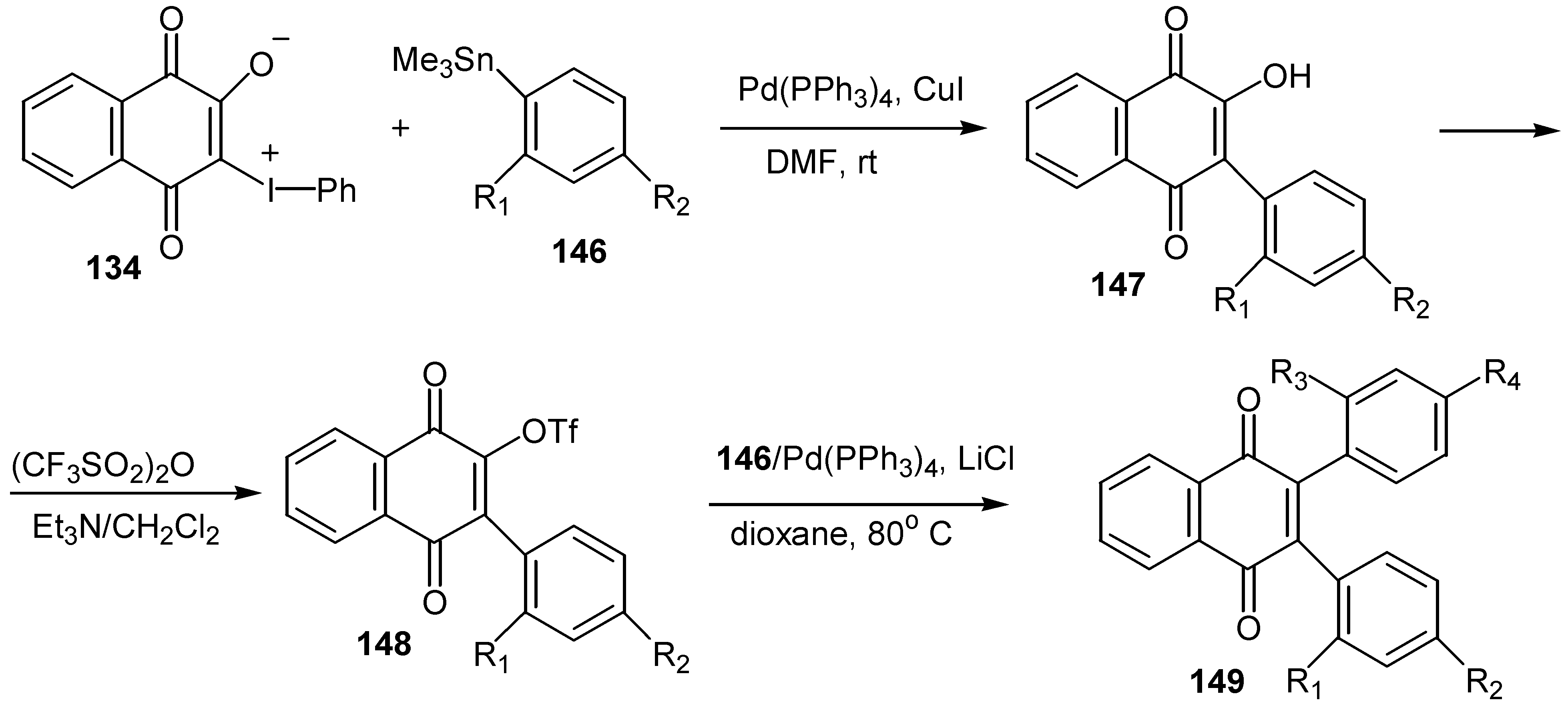

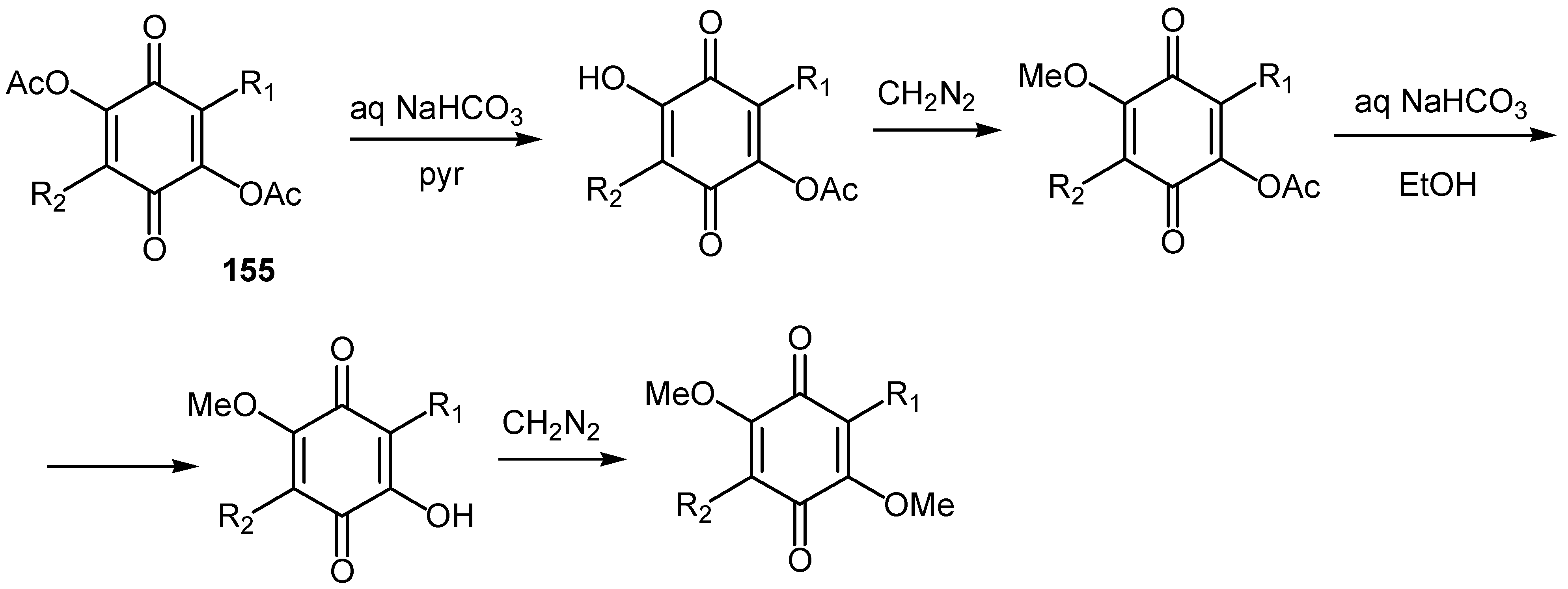
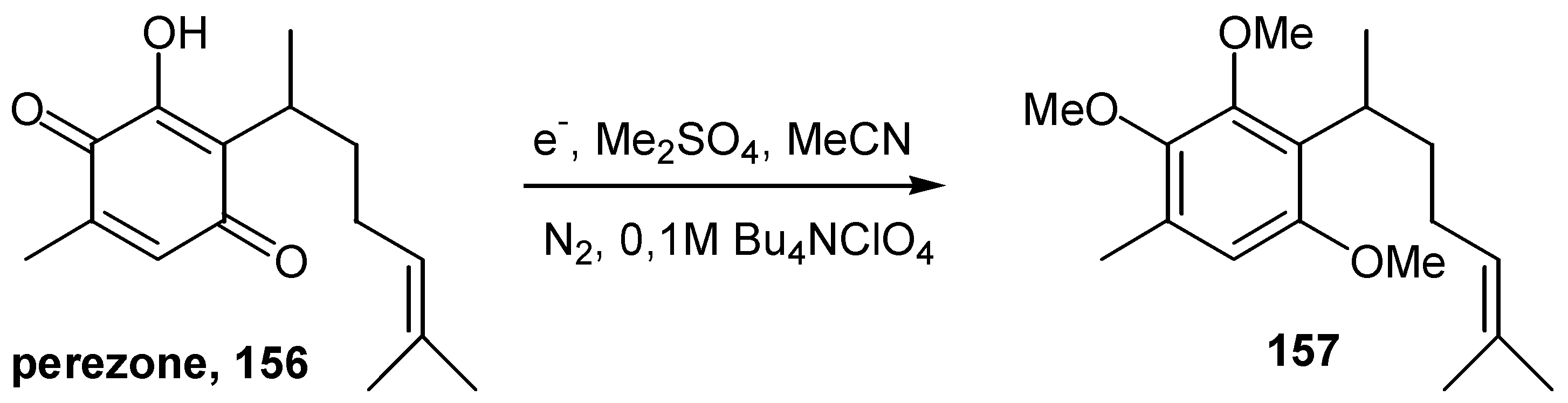


© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Spyroudis, S. Hydroxyquinones: Synthesis and Reactivity. Molecules 2000, 5, 1291-1330. https://doi.org/10.3390/51201291
Spyroudis S. Hydroxyquinones: Synthesis and Reactivity. Molecules. 2000; 5(12):1291-1330. https://doi.org/10.3390/51201291
Chicago/Turabian StyleSpyroudis, Spyros. 2000. "Hydroxyquinones: Synthesis and Reactivity" Molecules 5, no. 12: 1291-1330. https://doi.org/10.3390/51201291
APA StyleSpyroudis, S. (2000). Hydroxyquinones: Synthesis and Reactivity. Molecules, 5(12), 1291-1330. https://doi.org/10.3390/51201291



