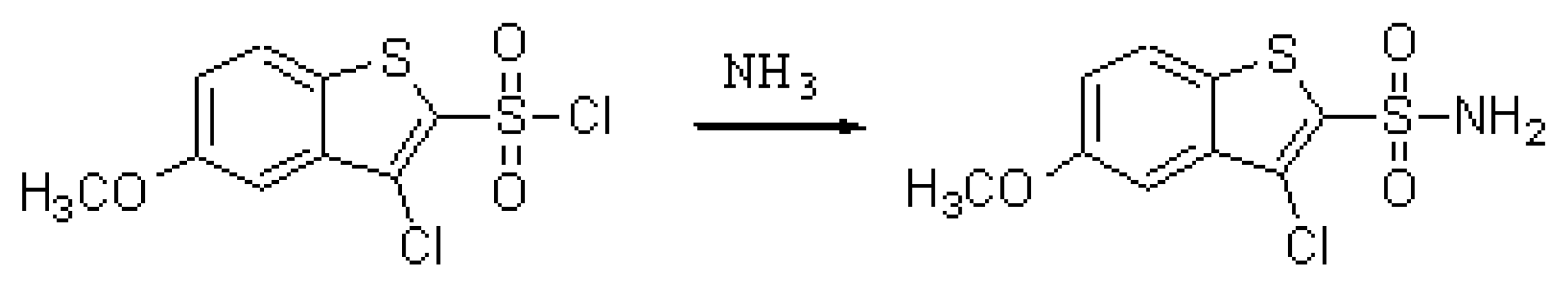
As part of a research programme targeting novel molecules as potential anti-inflammatory agents we synthesised 3-Chloro-5-methoxy-1-benzo[b]thiophene-2-sulphonylamide base on the reported anti-inflammatory activity of the structurally related molecule 3-Isopropoxy-5-methoxy-N-(1H-1,2,3,4-tetraazol-5-yl)-1-benzothiophene-2-carboxamide [1,2].
5-Methoxy-3-chloro-1-benzo[b]thiophene-2-sulphonyl chloride (1.4 g, 4.7 mmol) was dissolved in anhydrous acetone (15.0 mL) and the solution was added dropwise to a mixture of ammonia solution (15.0 mL) dissolved in anhydrous acetone (15.0 mL). The reaction mixture was stirred at room temperature for 30 minutes. The acetone was evaporated under reduced pressure and the precipitated solid was collected by filtration, washed well with water and diethyl ether and dried to afford (1.1 g, 84.0 %) of the desired 3-chloro-5-methoxy-1-benzo[b]thiophene-2-sulphonylamide as a pale yellow solid.
M.p. 189-190 °C.
MS (EI, 70 eV) : 277 (M+.).
1H NMR (300 MHz, MeOH-d4): 3.88 (s, 3H, OCH3), 7.24-7.30 (m, 2H, 2 x ArH), 8.03 (d, 1H, J = 8.79 Hz, ArH), 8.14 (br s, 2H, NH2).
HPLC retention time : 11.30 min. (10 % B/90 % D) to (90 % B/ 10 % D) over 20 minutes (B = 90 % CH3CN 10 % H2O) (D = 0.1N NH4OAc (pH = 4)) using Zorbax 4.6 mm x 250 mm.
Anal. calcd. for C9H8ClNO3S2. C 38.92, H 2.90, N 5.04; Found C 38.96, H 2.75, N 5.00.
Supplementary materials
Supplementary File 1Supplementary File 2References
- Connor, D. T.; Cetenko, M. D.; Mullikan, R. J.; Sorenson, P. C.; Unganst, R. J.; Weikert, R. L.; Adolphson, J. A.; Kennedy, D. O.; Thueson, C. D.; Wright, M. C.; Conroy, J. J. Med. Chem. 1992, 35(5), 958. [CrossRef]
- Wright, C. D.; Stewart, S. F.; Kuipers, P. J.; Hoffman, M. D.; Devall, L. J.; Kennedy, J. A.; Ferin, M. A.; Thueson, D. O.; Conroy, M. C. J. Leukocyte Biol. 1994, 55, 443.
- Sample Availability: available from the authors and MDPI.
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/
