Abstract
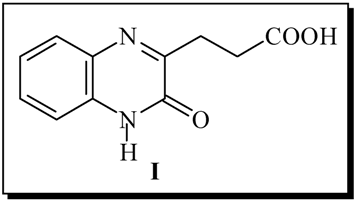
Kinetics of the synthesis of 3-[3-quinoxaline(1H)-one]propionic acid (I) were performed. This compound was achieved from reaction between o-phenylenediamine and α-ketoglutaric acid under different experimental conditions, and it was sent to the National Cancer Institute (USA) for its pharmacological evaluation.
Introduction
Trying to gain more insight into the nature of heterobicyclic chromophores, leading to potential antitumoral compounds [1], we analyzed the influence of an alkylcarboxilic chain in the C-3 of the heterocycle I upon the pharmacological activity [2].
Experimental
Anelation was achieved by reaction of o-phenylenediamine with α-ketoglutaric acid in organic solvents and also in aqueous buffer solutions over a pH range –0.24 -11.5, at room temperature. Kinetics were performed by UV spectrophotometry at 350 nm.
Compound I was insoluble in water so it was transformed into its ammonium salt in order to able the evaluation of its biological activity.
Results and Discussion
Compound I was obtained with good yields (85%) in lab-scale using anhydrous methanol as reaction solvent at room temperature. It was identified by qualitative and quantitative analysis and also by its spectroscopic properties (UV, IR, 1H NMR).
Kinetic studies determined that two competitive reactions take place in acidic buffers (pH –0.24-5.8) with a pseudo first-order rate constant for I formation, kobs = 2.18 ± 0.05 x 10-2 min-1. The reaction is not catalyzed by acids and occurs via formation of several open intermediates.
It is concluded that the presence of the alkylcarboxilic chain in C-3 of the heterocycle is responsible for the lack of reactivity, not only in basic buffer solutions but also in organic non-polar solvents, if compound I is compared with other non-acidic quinoxalinone derivatives descripted previously [3,4].
Acknowledgements
We thank to Karina Piton and Laura Belinque for their technical assistance.
References and Notes
© 2000 MDPI. All rights reserved.
