Abstract
We report a study of 3,3-dimethyl substituted acylthioureas. X ray data and quantum mechanical calculations demonstrated that the "S" conformation is the most stable both for the acylthioureas and the corresponding anions. The high regioselectivity towards S-alkylation is explained on the basis of the localization of the HOMO mainly over the sul-fur atom.
Introduction
The acylthioureid group present in acylthioureas [1], contains three heteroatoms of different hard-ness. Thus it is expected that depending on the reaction conditions different series of N, O, or S alkyl-ated derivatives may result [2]. The goal of this work was to study the reasons that favor the experi-mentally observed isothiourea formation (S-alkylation product) [3].
Experimental
Acylthioureas studied: 1-(4'-X-benzoyl)-3,3-dimethylthiourea, with X = H, Me, Br, Cl were ob- tained by a 3 step synthetic sequence as described previously [2]. X-ray difraction studies were carried out on single crystals of the latter 3 compounds.
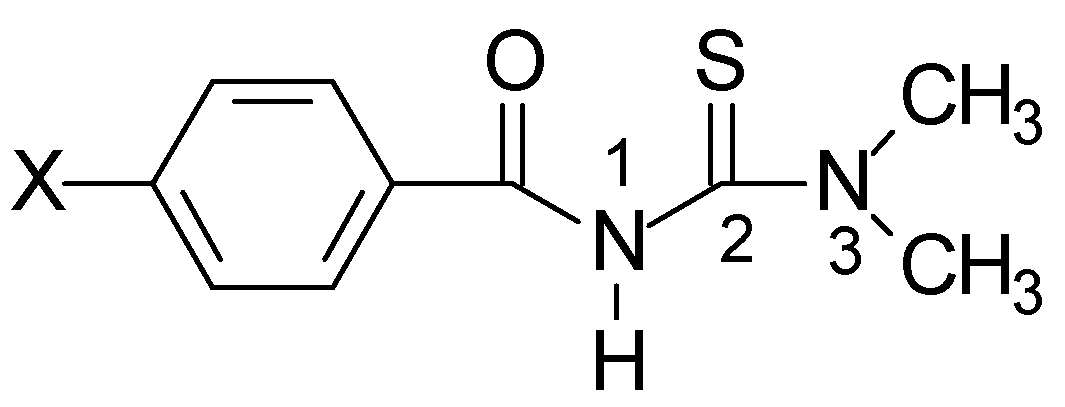
Geometry optimization: Were carried out with the programs Hyperchem 5.02, MOPAC 6.0 and Gaussian 94.
Results and Discussion
The main 4 conformers (S, -S, U and W) of the compounds mentioned in Experimental and their corresponding anions were optimized using semiempirical (AM1 and PM3) and ab initio methods. The calculated structures were compared with single crystal X-ray difraction data when available. Experi-mental and calculated geometries, predict the S conformation as the most stable for the four thioureas. HF calculations also predict the S conformation as the most stable for the corresponding anions, inde-pendently of the electronegativity of the substituent X.
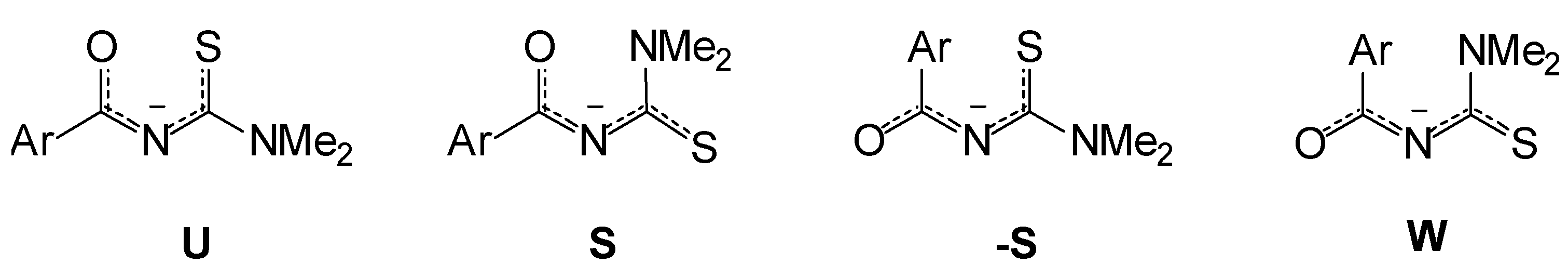
Frontier orbital calculations, show that the HOMO in the anions is localized mainly over the sulfur atom. Larger substituents on N-3 (e.g. 3,3-diethyl substituted analogs), do not show differences re-garding the preferred conformation.
References and Notes
- Rodriguez, Y.; Macias, A. Chem. Het. 1987, 508.
- Plutin, A. Master Thesis. Universidad de la Habana, 1997. [Google Scholar]
- Sosa, M. Master Thesis. Universidad de la Habana, 1999. [Google Scholar]
