Solvent and Substituent Effects on the Thermolysis of Antimalarial Fluorophenyl Substituted 1,2.4-Trioxanes
Abstract
:Introduction

Results and Discussion
| Temp K | Reaction Solvent | 105 ·[I]a M | 105 ·kobs s-1 | 105·[II]a M | 105·kobs s-1 |
| 393.2 | n-hexane | 13.5 | 5.1 ± 0.5 | 82 | 2.7 ± 0.2 |
| 393.2 | methanol | 2.7 | 38.2 ± 0.4 | 1.05 | 27.6 ± 3.0 |
| 403.2 | n-hexane | 13.5 | 14 ± 1.5 | 82 | 4.2 ± 0.4 |
| 403.2 | methanol | 2.7 | 90.3 ± 0.8 | 26 | 73.6 ± 7.0 |
| 413.2 | n-hexane | 13.5 | 37.0 ± 3.0 | 14.5 | 29.4 ± 3.5 |
| 413.2 | methanol | 2.7 | 232 ± 9 | 26 | 180 ± 13 |
| 423.2 | n-hexane | 13.5 | 87 ± 6.5 | 14.5 | 67.3 ±3.5 |
| 423.2 | methanol | 2.7 | 546 ± 21 | 26 | 405 ± 10 |
| 433.2 | n-hexane | 13.5 | 222 ± 18 | 14.5 | 141 ± 4 |
| 433.2 | methanol | 54.0 | 697 ± 8 | 13.0 | 706 ± 47 |
| 443.2 | n-hexane | 13.5 | 532 ± 25 | 14.5 | 277 ± 18 |
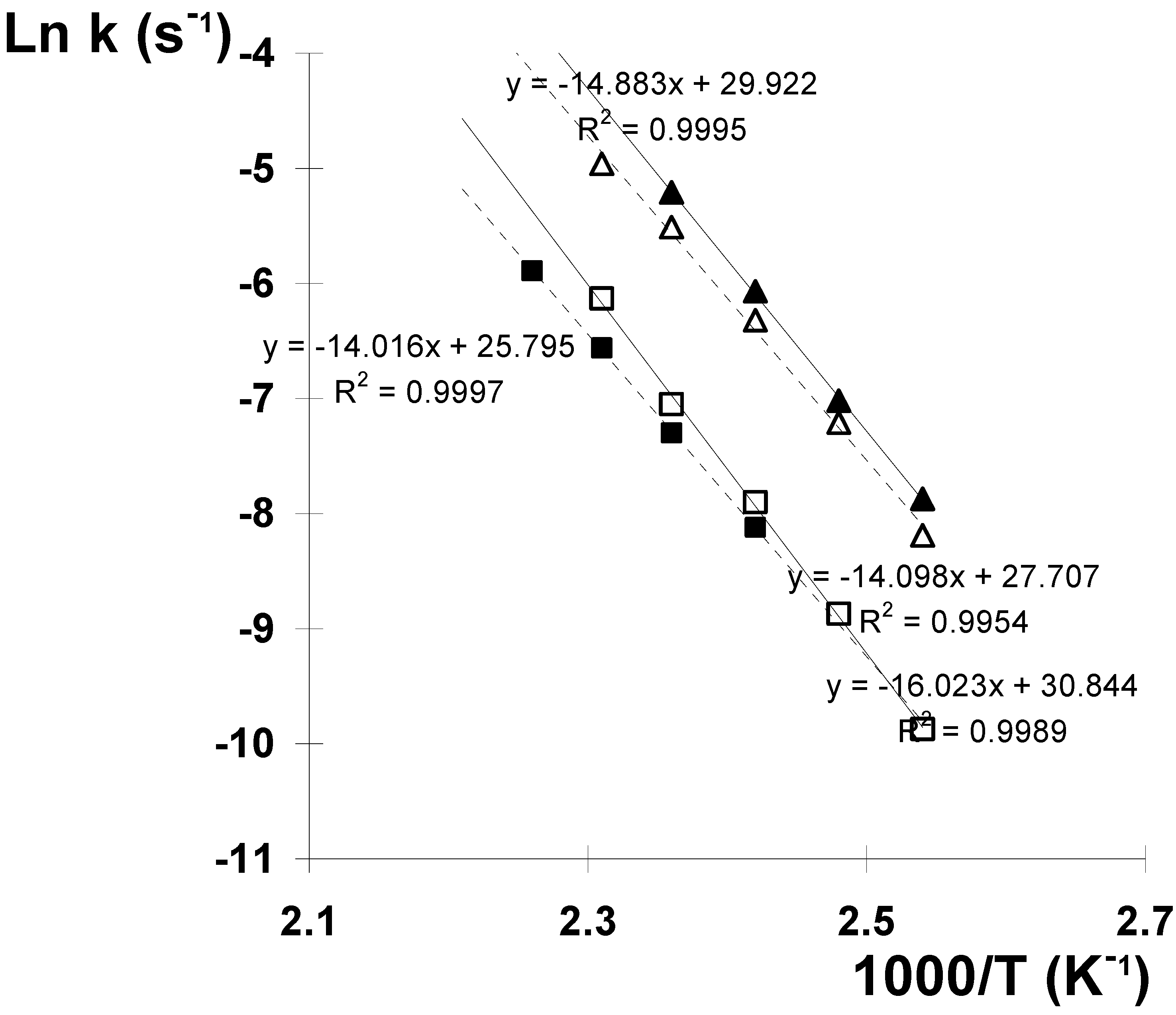
| Trioxane | Reaction Solvent | ΔH# kcal mol-1 | ΔS# cal mol-1 K-1 | ΔG# kcal mol-1 |
| I | methanol | 28.2 ± 0.7 | - 3.0 ± 1.3 | 28.9 ± 0.7 |
| I | n-hexane | 31.1 ± 0.9 | 0.4 ± 2.1 | 30.9 ± 0.9 |
| II | methanol | 29.0 ± 0.7 | - 1.5 ± 1.8 | 29.6 ± 0.7 |
| II | n-hexane | 31.7 ± 0.6 | 0.7 ± 1.7 | 31.4 ± 0.6 |

| Trioxane | Solvent | Reaction Products |
| I | n-hexane | Cyclopentanone (5%); 1,3-di-(4-fluorophenyl)-3-cyclopenten-1-ol (18 %) ; 1,4-di-(4-fluorophenyl)-3-cyclopenten-1,2-diol (39 %). |
| I | methanol | Cyclopentanone (7%); 3-(4-fluorophenyl)-4-(4-fluorobenzoyl)-2-buthenal (6%); 3,5-di-(4-fluorophenyl)-5-methoxy-2-cyclopenten-1-ol (3%); 1,4-di-(4-fluorophenyl)-2-methoxy-3-cyclopenten-1-ol (3%); 1,4-di-(4-fluorophenyl)-3,4-dimethoxy-cyclopentene (2.5%); 1,1-methyl-methoxy-cyclopentane (8%); 4-fluorobenzoic acid (5%) |
| II | n-hexane | Cyclopentanone (6%); 4-(4-fluorobenzoyl)-3-(4-fluorophenyl)-2-hydroxy-3-butenal (13%); 1,3-di-(4-fluorophenyl)-2-cyclo-penten-1,4-diol (10 %). |
| II | methanol | Cyclopentanone (10%); 4-(4-fluorobenzoyl)-3-(4-fluorophenyl)-2-hydroxy-3-butenal (10%); 2,4-di-(4-fluorophenyl)-5-methyl-4-methoxy-2-cyclopeten-1-ol (3%); 2,4-di-(4-fluorophenyl)-4-methoxy-2-cyclopenten-1-ol (5%); 2,4-(4-fluorophenyl)-4,5-dimethoxy-2-cyclopenten-2-ol (3%); 1,1-methyl-methoxy-cyclopentane (10%); 1,3-di-(4-fluorophenyl)-5-methyl-2-cyclopenten-1,4-diol (2%). |
Conclusions
Experimental
General
Kinetics and analytical methods
References
- Cafferata, L. F. R.; Furlong, J. J. Thermolysis of Tetroxanes. In Advances in Oxygenated Processes; Baumstark, A. L., Ed.; JAI Press Inc.: USA, 1995; Volume IV, pp. 81–105. [Google Scholar]
- Cafferata, L. F. R.; Nojima, M.; Yamakoshi, H. Kinetics and Mechanism of the Thermal Decomposition Reaction of Dyhydro-3,6-Diphenyl-5-Benzyl-1,2,4,5-Trioxazine. Int. J. Chem. Kin. 1995, 28, 21–25. [Google Scholar]
- Jefford, C. W.; Rossier, J. C.; Boukouvalas, J. C. J. Chem. Soc., Chem. Commun. 1987, 713–716.
- Cafferata, L. F. R.; Mirífico, M. V. Efectos de Solventes en la Cinética de la Descomposición Unimolecular del Peróxido de Benzoílo. An. Asoc. Quím. Argentina 1982, 70, 29–42. [Google Scholar]
- Cafferata, L. F. R.; Quintáns, M. T. An. Asoc. Quím. Argentina 1987, 75, 461–474.
- Cafferata, L. F. R.; Eyler, G. N.; Svartman, E. L.; Cañizo, A.; Alvarez, E. Solvent effects in the Thermal Decomposition Reactions of Cyclic Ketone Diperoxides. J. Org. Chem. 1991, 56, 411–414. [Google Scholar]
- Eyler, G. N.; Cañizo, A.; Alvarez, E.; Mateo, C.; Cafferata, L. F. R. Induced Decomposition Reaction in Solution of Acetone Derived Cyclic Peroxides. Afinidad 2002, 59(502), 684–687. [Google Scholar]
- Leiva, L.; Castellanos, M. G.; Gómez Vara, M.; Cafferata, L. F. R. Thermolysis of Benzaldehyde and Acetone Cyclic Diperoxide in Tetrahydrofurane Solution. Afinidad 2002, 59(502), 676–683. [Google Scholar]
- Allegretti, P.; Rimada, R. S.; Furlong, J. J.; Cafferata, L. F. R. Mass Spectrometric Evidence for the Reaction Mechanism of Thermolysis of 1,2,4-trioxanes in Solution. Asian J. Spectrosc. 1998, 2, 165–172. [Google Scholar]
- Cafferata, L. F. R.; Jefford, C. W.; Rimada, R. S. Kinetics and Mechanism of Thermolysis of Fluorine Substituted 1,2,4-trioxanes in Methanol Solution. Int. J. Chem. Kin. 2000, 32, 523–528. [Google Scholar]
- Cafferata, L. F. R.; Jefford, C. W.; Rimada, R. S. Kinetics and Mechanism of Thermolysis of 1,2,4-trioxanes in n-hexane Solution. Afinidad 2002, 59(502), 670–675. [Google Scholar]
- Cafferata, L. F. R.; Jefford, C. W. Solvent and Substituent Effects on the Kinetics of Thermolysis of cis-fused 1,2,4-Trioxanes. Molecules 2001, 6, 699–709. [Google Scholar]
- Jefford, C. W.; Kohmoto, S.; Joggi, D.; Rossier, J. C.; Timarí, G.; Ruday, M.; Barbuzzi, O.; Gérard, D.; Burger, U.; Kamalaprija, P.; Bernardinelli, G.; Canfield, C.; Fleck, B. I.; Robinson, R.; Peters, W. Synthesis, Structure and Antimalarial Activity of Some Enantiomerically Pure cis-fused Cyclopentene-1,2,4-trioxanes. Helv., Chim. Acta 1995, 78, 647–655. [Google Scholar]
- Cafferata, L. F. R.; Rimada, R. S.; Furlong, J. J.; Allegretti, P. RP-HPLC and GC-MS Techniques for the Quantitative Analysis of Substituted 1,2,4-trioxanes and their Thermolysis Products in Solution. J. High Resol. Chrom. 1999, 22, 67–70. [Google Scholar]
- Bunnet, J. Investigation of Rates and Mechanism of Reaction. In Techniques of Chemistry; Weissberger, A., Ed.; Wiley: New York, 1974; Part I; Vol. VI, Chapter VIII. [Google Scholar]
- Huyberechts, S.; Halleux, A.; Kruys, P. Une Aplication de Calcule Statistique a la Cinetique Chimique. Bull. Chim. Belg. 1955, 64, 203–209. [Google Scholar]
- Sample availability: Samples of compounds I and II are available from the authors.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Cafferata, L.F.R.; Rimada, R.S. Solvent and Substituent Effects on the Thermolysis of Antimalarial Fluorophenyl Substituted 1,2.4-Trioxanes. Molecules 2003, 8, 655-662. https://doi.org/10.3390/80900655
Cafferata LFR, Rimada RS. Solvent and Substituent Effects on the Thermolysis of Antimalarial Fluorophenyl Substituted 1,2.4-Trioxanes. Molecules. 2003; 8(9):655-662. https://doi.org/10.3390/80900655
Chicago/Turabian StyleCafferata, Lazaro F. R., and Ruben S. Rimada. 2003. "Solvent and Substituent Effects on the Thermolysis of Antimalarial Fluorophenyl Substituted 1,2.4-Trioxanes" Molecules 8, no. 9: 655-662. https://doi.org/10.3390/80900655
APA StyleCafferata, L. F. R., & Rimada, R. S. (2003). Solvent and Substituent Effects on the Thermolysis of Antimalarial Fluorophenyl Substituted 1,2.4-Trioxanes. Molecules, 8(9), 655-662. https://doi.org/10.3390/80900655




