Antioxidant and Cytoprotective Properties of Polyphenol-Rich Extracts from Antirhea borbonica and Doratoxylon apetalum against Atherogenic Lipids in Human Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Extraction and Quantification of Polyphenols in Medicinal Plant Extracts
2.3. Polyphenol Compound Identification by UPLC-UV-ESI-MS/MS
2.4. LDL Isolation and Oxidation
2.5. Lipid Hydroperoxide Assay
2.6. Cell Culture
2.7. Thiobarbituric Acid-Reactive Substances Assay
2.8. Quantification of Intracellular Reactive Oxygen Species
2.9. Evaluation of Cytotoxicity, Necrosis and Apoptosis
2.9.1. Cell Viability Test
2.9.2. Apoptotic Versus Necrotic Cell Count by Fluorescence Microscopy
2.9.3. Determination of Phosphatidylserine Exposure by Flow Cytometry
2.9.4. In Situ Detection of Cytochrome C
2.9.5. In Situ Detection of Caspase 3 Activation
2.9.6. In Situ Cell Apoptosis Detection by TUNEL
2.10. Determination of LDL Cellular Uptake
2.11. Cell Protein Extraction and Quantification
2.12. Catalase and Superoxide Dismutase Activity Assays
2.13. Western Blot Analysis
2.14. Statistical Analysis
3. Results
3.1. Polyphenol Content of D. apetalum and A. borbonica Plant Extracts
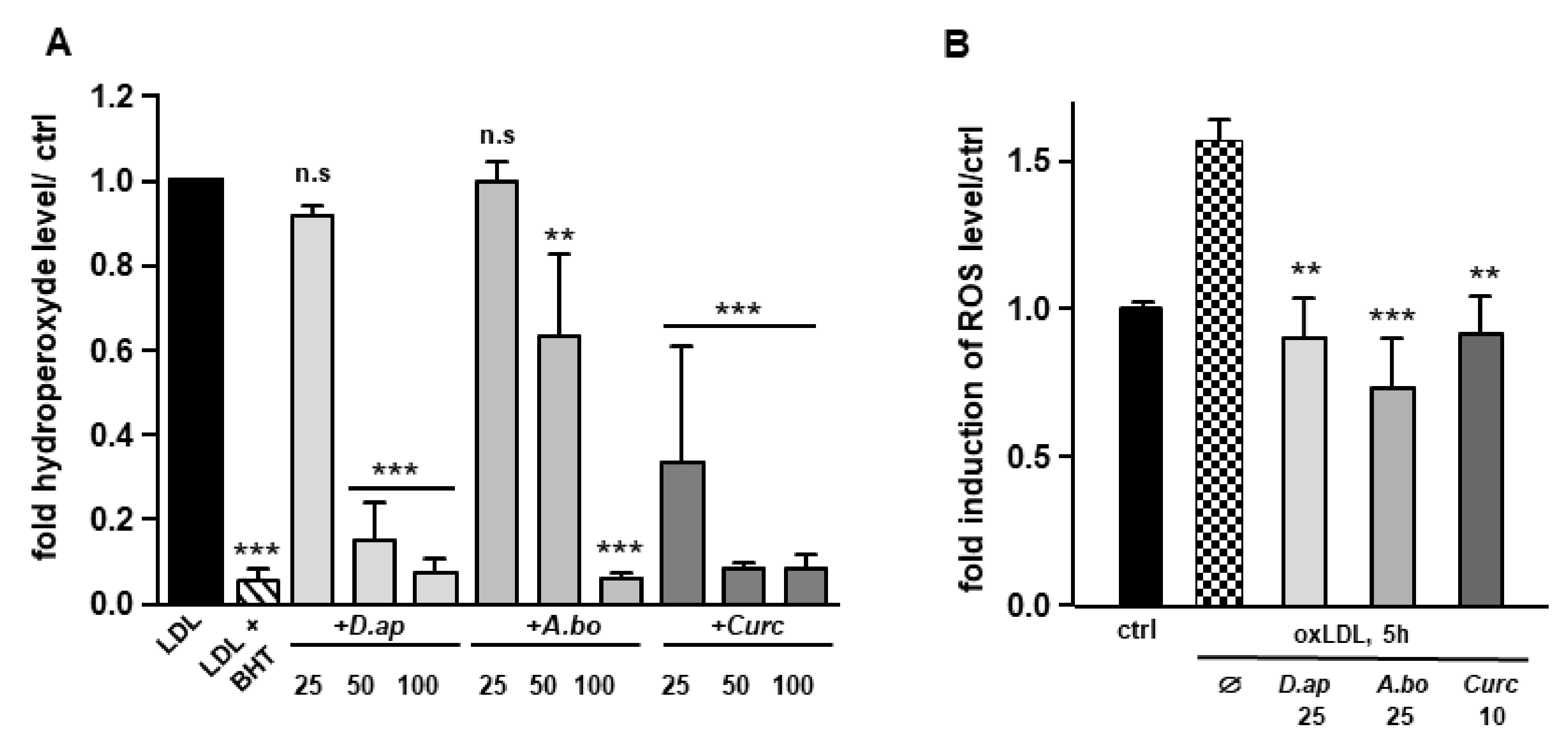
3.2. A. borbonica and D. apetalum Extracts Prevent the Oxidation of LDL
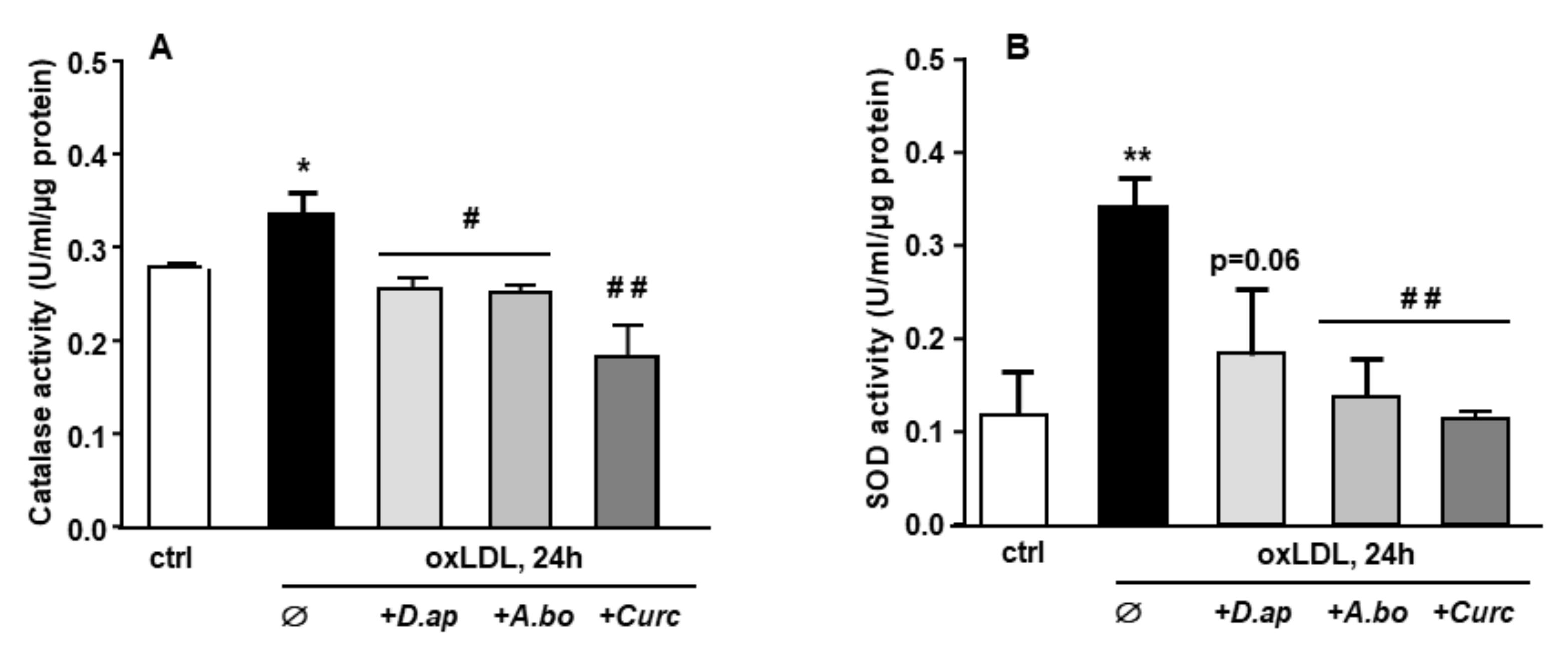
3.3. Oxidative Stress and Antioxidant Response Induced by oxLDLs in Endothelial Cells Are Inhibited by A. borbonica and D. apetalum Extracts
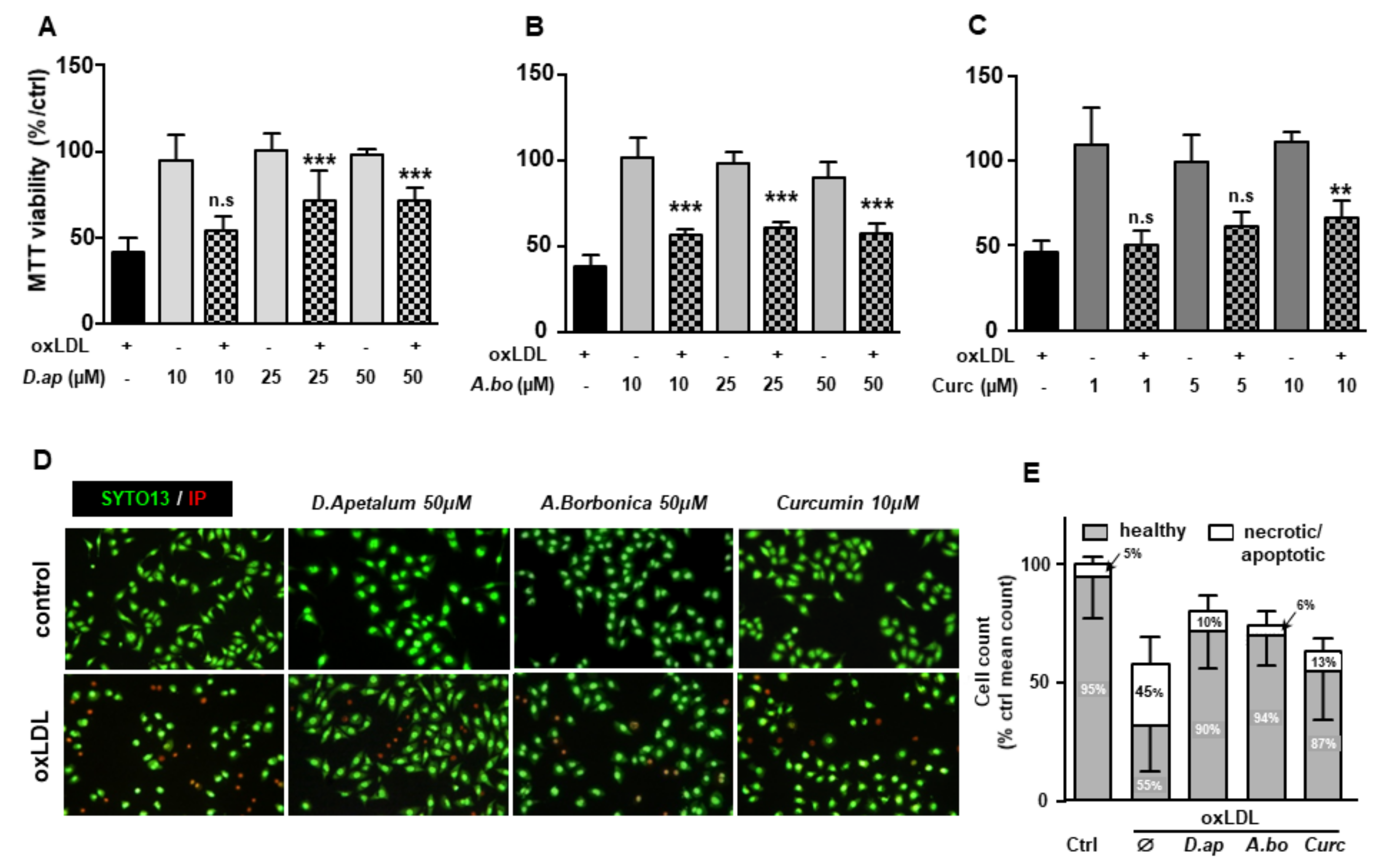
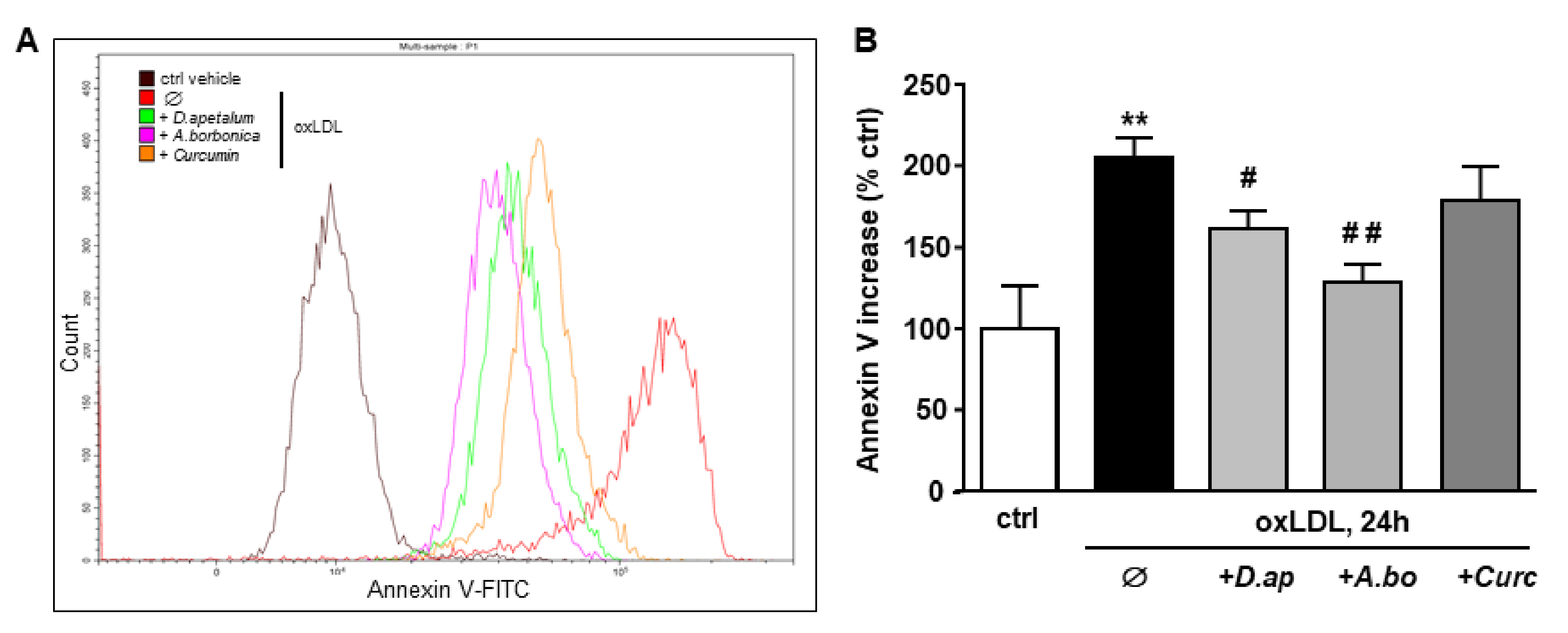
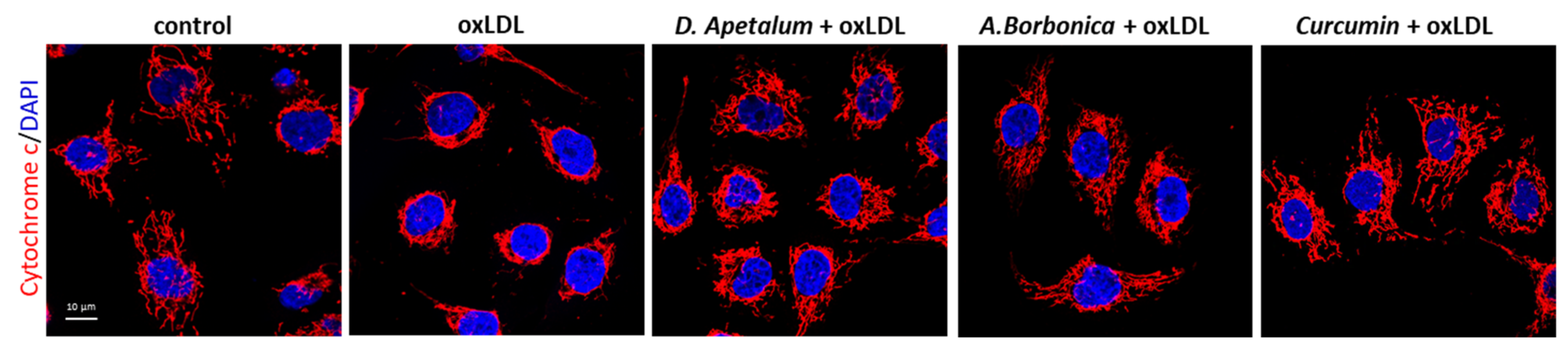
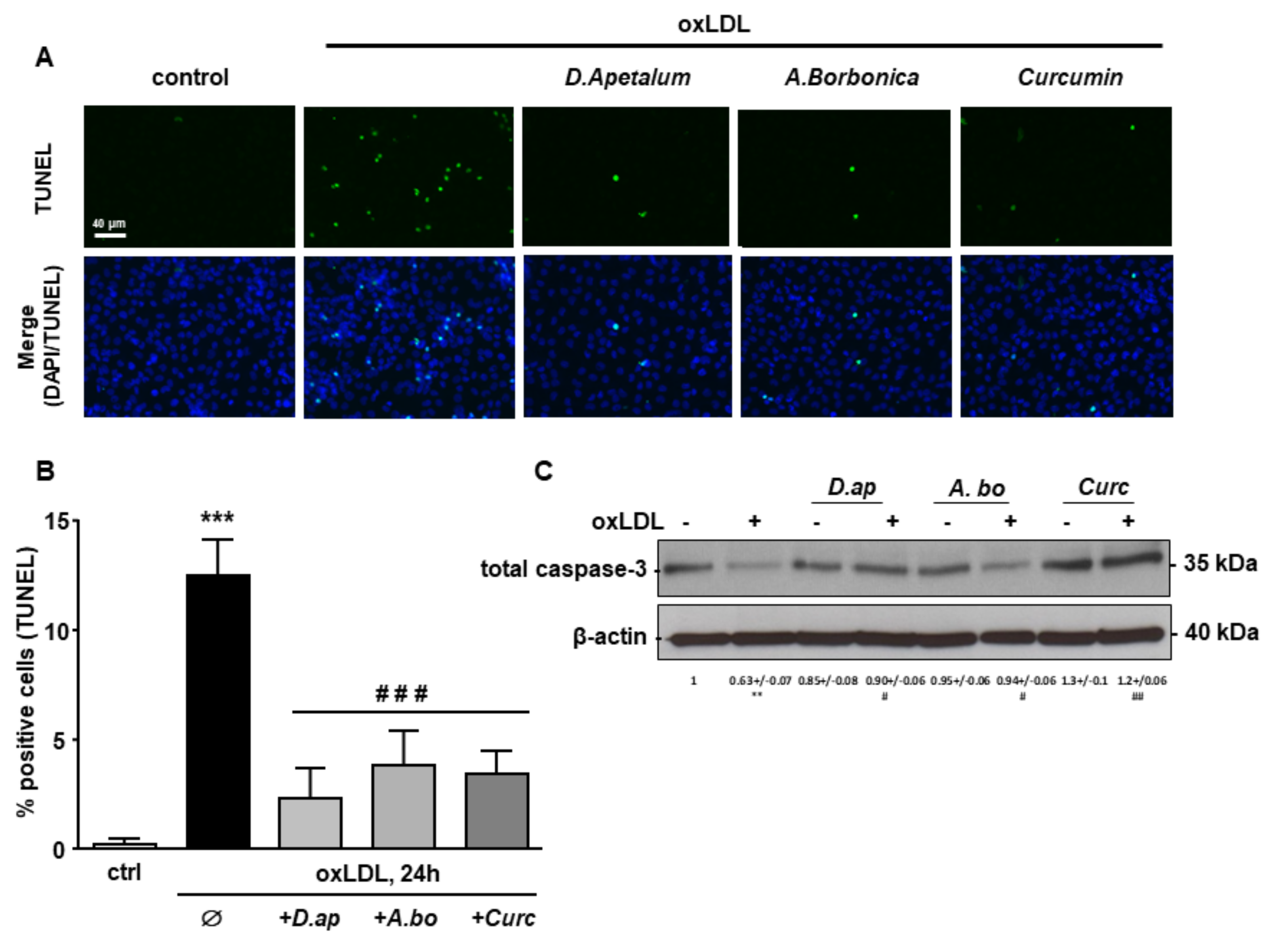
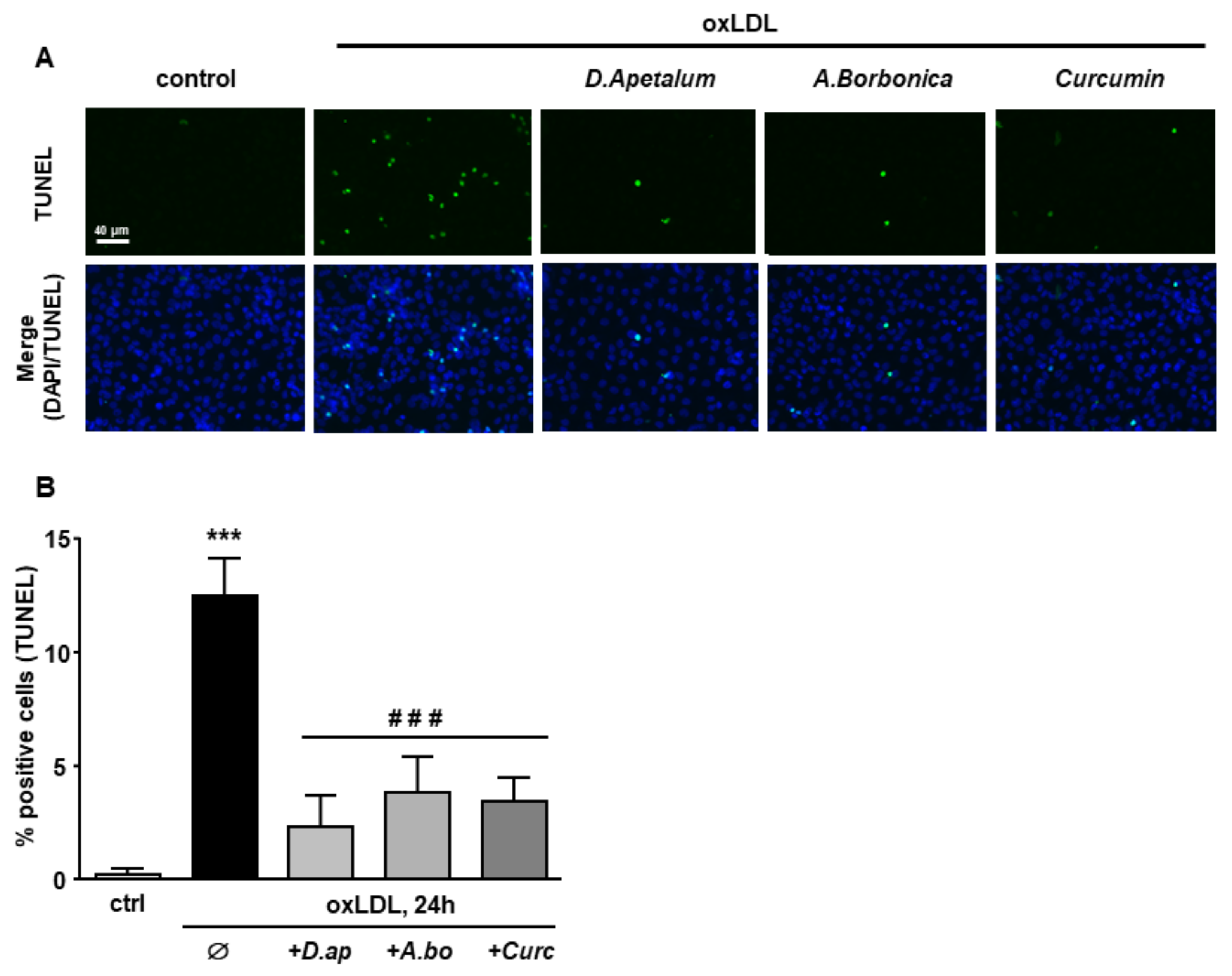
3.4. A. borbonica and D. apetalum Extracts Inhibit the Cytotoxic Effects of oxLDLs on Endothelial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quinn, M.T.; Parthasarathy, S.; Fong, L.G.; Steinberg, D. Oxidatively modified low density lipoproteins: A potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc. Natl. Acad. Sci. USA 1987, 84, 2995–2998. [Google Scholar] [CrossRef] [PubMed]
- Balla, G.; Jacob, H.S.; Eaton, J.W.; Belcher, J.D.; Vercellotti, G.M. Hemin: A possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arter. Thromb. A J. Vasc. Biol. 1991, 11, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.V.; Parthasarathy, S.S.; Alexander, R.W.; Medford, R.M. Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J. Clin. Investig. 1995, 95, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Ho-Tin-Noé, B.; Le Dall, J.; Gomez, D.; Louedec, L.; Vranckx, R.; El-Bouchtaoui, M.; Legrès, L.; Meilhac, O.; Michel, J.B. Early Atheroma-Derived Agonists of Peroxisome Proliferator-Activated Receptor-Gamma Trigger Intramedial Angiogenesis in a Smooth Muscle Cell-Dependent Manner. Circ. Res. 2011, 109, 1003–1014. [Google Scholar] [CrossRef]
- Nègre-Salvayre, A.; Pieraggia, M.T.; Mabile, L.; Salvayrea, R. Protective Effect of 17 Beta-Estradiol against the Cytotoxicity of Minimally Oxidized Ldl to Cultured Bovine Aortic Endothelial Cells. Atherosclerosis 1993, 99, 207–217. [Google Scholar] [CrossRef]
- Reid, V.C.; Mitchinson, M.J.; Skepper, J.N. Cytotoxicity of oxidized low-density lipoprotein to mouse peritoneal macrophages: An ultrastructural study. J. Pathol. 1993, 171, 321–328. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Santanam, N.; Ramachandran, S.; Meilhac, O. Oxidants and antioxidants in atherogenesis. An appraisal. J. Lipid Res. 1999, 40, 2143–2157. [Google Scholar] [CrossRef]
- Saita, E.; Kondo, K.; Momiyama, Y. Anti-Inflammatory Diet for Atherosclerosis and Coronary Artery Disease: Antioxidant Foods. Clin. Med. Insights Cardiol. 2014, 8 (Suppl. 3), CMC-S17071. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Naidu, K.A.; Thippeswamy, N. Inhibition of human low density lipoprotein oxidation by active principles from spices. Mol. Cell. Biochem. 2002, 229, 19–23. [Google Scholar] [CrossRef]
- Marimoutou, M.; Le Sage, F.; Smadja, J.; d’Hellencourt, C.L.; Gonthier, M.P.; Robert-Da Silva, C. Antioxidant Polyphenol-Rich Extracts from the Medicinal Plants Antirhea Borbonica, Doratoxylon Apetalum and Gouania Mauritiana Protect 3t3-L1 Preadipocytes against H2o2, Tnfalpha and Lps Inflammatory Mediators by Regulating the Expression of Superoxide Dismutase and Nf-Kappab Genes. J. Inflamm. 2015, 12, 10. [Google Scholar]
- Le Sage, F.; Meilhac, O.; Gonthier, M.-P. Anti-inflammatory and antioxidant effects of polyphenols extracted from Antirhea borbonica medicinal plant on adipocytes exposed to Porphyromonas gingivalis and Escherichia coli lipopolysaccharides. Pharmacol. Res. 2017, 119, 303–312. [Google Scholar] [CrossRef]
- Delveaux, J.; Turpin, C.; Veeren, B.; Diotel, N.; Bravo, S.B.; Begue, F.; Álvarez, E.; Meilhac, O.; Bourdon, E.; Rondeau, P. Antirhea borbonica Aqueous Extract Protects Albumin and Erythrocytes from Glycoxidative Damages. Antioxidants 2020, 9, 415. [Google Scholar] [CrossRef]
- Vindis, C.; Elbaz, M.; Escargueil-Blanc, I.; Augé, N.; Heniquez, A.; Thiers, J.-C.; Negre-Salvayre, A.; Salvayre, R. Two Distinct Calcium-Dependent Mitochondrial Pathways Are Involved in Oxidized LDL-Induced Apoptosis. Arter. Thromb. Vasc. Biol. 2005, 25, 639–645. [Google Scholar] [CrossRef]
- Yagi, K. Simple Assay for the Level of Total Lipid Peroxides in Serum or Plasma. Free Radic. Antioxid. Protoc. 1998, 108, 101–106. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Woollard, A.C.S.; Wolff, S.P. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 1991, 26, 853–856. [Google Scholar] [CrossRef]
- Muller, C.; Salvayre, R.; Negre-Salvayre, A.; Vindis, C. HDLs inhibit endoplasmic reticulum stress and autophagic response induced by oxidized LDLs. Cell Death Differ. 2010, 18, 817–828. [Google Scholar] [CrossRef]
- Larroque-Cardoso, P.; Swiader, A.; Ingueneau, C.; Nègre-Salvayre, A.; Elbaz, M.; Reyland, M.E.; Salvayre, R.; Vindis, C. Role of Protein Kinase C Delta in Er Stress and Apoptosis Induced by Oxidized Ldl in Human Vascular Smooth Muscle Cells. Cell Death Dis. 2013, 4, e520. [Google Scholar] [CrossRef]
- Salvayre, R.; Vindis, C.; Ingueneau, C.; Athias, A.; Marcheix, B.; Huynh-Do, U.; Gambert, P.; Nègre-Salvayre, A. TRPC1 is regulated by caveolin-1 and is involved in oxidized LDL-induced apoptosis of vascular smooth muscle cells. J. Cell Mol. Med. 2009, 13, 1620–1631. [Google Scholar] [CrossRef]
- Gay, C.; Collins, J.; Gebicki, J.M. Hydroperoxide Assay with the Ferric–Xylenol Orange Complex. Anal. Biochem. 1999, 273, 149–155. [Google Scholar] [CrossRef]
- Mabile, L.; Meilhac, O.; Escargueil-Blanc, I.; Troly, M.; Pieraggi, M.-T.; Salvayre, R.; Nègre-Salvayre, A. Mitochondrial Function Is Involved in LDL Oxidation Mediated by Human Cultured Endothelial Cells. Arter. Thromb. Vasc. Biol. 1997, 17, 1575–1582. [Google Scholar] [CrossRef]
- Cominacini, L.; Pasini, A.F.; Garbin, U.; Davoli, A.; Tosetti, M.L.; Campagnola, M.; Rigoni, A.; Pastorino, A.M.; Cascio, V.L.; Sawamura, T. Oxidized Low Density Lipoprotein (Ox-Ldl) Binding to Ox-Ldl Receptor-1 in Endothelial Cells Induces the Activation of Nf-Kappab through an Increased Production of Intracellular Reactive Oxygen Species. J. Biol. Chem. 2000, 275, 12633–12638. [Google Scholar] [CrossRef]
- Yen, C.H.; Hsieh, C.C.; Chou, S.Y.; Lau, Y.T. 17beta-Estradiol Inhibits Oxidized Low Density Lipoprotein-Induced Generation of Reactive Oxygen Species in Endothelial Cells. Life Sci. 2001, 70, 403–413. [Google Scholar] [CrossRef]
- Escargueil-Blanc, I.; Meilhac, O.; Pieraggi, M.-T.; Arnal, J.-F.; Salvayre, R.; Nègre-Salvayre, A. Oxidized LDLs Induce Massive Apoptosis of Cultured Human Endothelial Cells Through a Calcium-Dependent Pathway. Arter. Thromb. Vasc. Biol. 1997, 17, 331–339. [Google Scholar] [CrossRef]
- Poullain, C.; Girard-Valenciennes, E.; Smadja, J. Plants from reunion island: Evaluation of their free radical scavenging and antioxidant activities. J. Ethnopharmacol. 2004, 95, 19–26. [Google Scholar] [CrossRef]
- Laranjinha, J.A.; Almeida, L.M.; Madeira, V.M. Reactivity of dietary phenolic acids with peroxyl radicals: Antioxidant activity upon low density lipoprotein peroxidation. Biochem. Pharmacol. 1994, 48, 487–494. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Belelli, F.; Scaccini, C. Coffee drinking induces incorporation of phenolic acids into LDL and increases the resistance of LDL to ex vivo oxidation in humans. Am. J. Clin. Nutr. 2007, 86, 604–609. [Google Scholar] [CrossRef]
- Wu, C.; Luan, H.; Zhang, X.; Wang, S.; Zhang, X.; Sun, X.; Guo, P. Chlorogenic Acid Protects against Atherosclerosis in Apoe-/- Mice and Promotes Cholesterol Efflux from Raw264.7 Macrophages. PLoS ONE 2014, 9, e95452. [Google Scholar] [CrossRef]
- Lotito, S.B.; Actis-Goretta, L.; Renart, M.L.; Caligiuri, M.; Rein, D.; Schmitz, H.H.; Steinberg, F.M.; Keen, C.L.; Fraga, C.G. Influence of Oligomer Chain Length on the Antioxidant Activity of Procyanidins. Biochem. Biophys. Res. Commun. 2000, 276, 945–951. [Google Scholar] [CrossRef]
- Hirano, R.; Sasamoto, W.; Matsumoto, A.; Itakura, H.; Igarashi, O.; Kondo, K. Antioxidant Ability of Various Flavonoids against DPPH Radicals and LDL Oxidation. J. Nutr. Sci. Vitaminol. 2001, 47, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-F.; Deng, S.-L.; Zhou, B.; Yang, L.; Liu, Z.-L. Curcumin and its analogues as potent inhibitors of low density lipoprotein oxidation: H-atom abstraction from the phenolic groups and possible involvement of the 4-hydroxy-3-methoxyphenyl groups. Free. Radic. Biol. Med. 2006, 40, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Ramırez-Tortosa, M.C.; Mesa, M.D.; Aguilera, M.C.; Quiles, J.L.; Baro, L.; Ramirez-Tortosa, C.L.; Martinez-Victoria, E.; Gil, A. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis 1999, 147, 371–378. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Della-Fera, M.A.; Rayalam, S.; Ambati, S.; Hartzell, D.L.; Park, H.J.; Baile, C.A. Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci. 2008, 82, 1032–1039. [Google Scholar] [CrossRef]
- Hatia, S.; Septembre-Malaterre, A.; Le Sage, F.; Badiou-Bénéteau, A.; Baret, P.; Payet, B.; D’Hellencourt, C.L.; Gonthier, M.-P. Evaluation of antioxidant properties of major dietary polyphenols and their protective effect on 3T3-L1 preadipocytes and red blood cells exposed to oxidative stress. Free. Radic. Res. 2014, 48, 387–401. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Le Sage, F.; Hatia, S.; Catan, A.; Janci, L.; Gonthier, M.P. Curcuma Longa Polyphenols Improve Insulin-Mediated Lipid Accumulation and Attenuate Proinflammatory Response of 3t3-L1 Adipose Cells During Oxidative Stress through Regulation of Key Adipokines and Antioxidant Enzymes. Biofactors 2016, 42, 418–430. [Google Scholar] [CrossRef]
- Lou, S.; Wang, Y.; Yu, Z.; Guan, K.; Kan, Q. Curcumin induces apoptosis and inhibits proliferation in infantile hemangioma endothelial cells via downregulation of MCL-1 and HIF-1α. Medicine 2018, 97, e9562. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The Relative Antioxidant Activities of Plant-Derived Polyphenolic Flavonoids. Free. Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Vieira, O.; Escargueil-Blanc, I.; Meilhac, O.; Basile, J.-P.; Laranjinha, J.; Almeida, L.M.; Salvayre, R.; Negre-Salvayre, A. Effect of dietary phenolic compounds on apoptosis of human cultured endothelial cells induced by oxidized LDL. Br. J. Pharmacol. 1998, 123, 565–573. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lee, M.-S.; Wang, C.-P.; Hsu, C.-C.; Lin, H.-H. Autophagic effects of Hibiscus sabdariffa leaf polyphenols and epicatechin gallate (ECG) against oxidized LDL-induced injury of human endothelial cells. Eur. J. Nutr. 2017, 56, 1963–1981. [Google Scholar] [CrossRef]
- Nègre-Salvayre, A.; Fitoussi, G.; Réaud, V.; Pieraggi, M.-T.; Thiers, J.-C.; Salvayre, R. A delayed and sustained rise of cytosolic calcium is elicited by oxidized LDL in cultured bovine aortic endothelial cells. FEBS Lett. 1992, 299, 60–65. [Google Scholar] [CrossRef]
- Hsiung, G.D.; McTighe, A.H. An animal model for DNA-RNA virus interaction based upon virological and histological findings. Cancer Res. 1976, 36, 674–677. [Google Scholar]
- Meilhac, O.; Escargueil-Blanc, I.; Thiers, J.-C.; Salvayre, R.; Negre-Salvayre, A. Bcl-2 alters the balance between apoptosis and necrosis, but does not prevent cell death induced by oxidized low density lipoproteins. FASEB J. 1999, 13, 485–494. [Google Scholar] [CrossRef]
- Meilhac, O.; Zhou, M.; Santanam, N.; Parthasarathy, S. Lipid peroxides induce expression of catalase in cultured vascular cells. J. Lipid Res. 2000, 41, 1205–1213. [Google Scholar] [CrossRef]
- Su, H.; Li, Y.; Hu, D.; Xie, L.; Ke, H.; Zheng, X.; Chen, W. Procyanidin B2 ameliorates free fatty acids-induced hepatic steatosis through regulating TFEB-mediated lysosomal pathway and redox state. Free. Radic. Biol. Med. 2018, 126, 269–286. [Google Scholar] [CrossRef]
- Arcambal, A.; Taïlé, J.; Couret, D.; Planesse, C.; Veeren, B.; Diotel, N.; Gauvin-Bialecki, A.; Meilhac, O.; Gonthier, M. Protective Effects of Antioxidant Polyphenols against Hyperglycemia-Mediated Alterations in Cerebral Endothelial Cells and a Mouse Stroke Model. Mol. Nutr. Food Res. 2020, 64, e1900779. [Google Scholar] [CrossRef]
- Taïlé, J.; Arcambal, A.; Clerc, P.; Gauvin-Bialecki, A.; Gonthier, M.-P. Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition. Antioxidants 2020, 9, 573. [Google Scholar] [CrossRef]
- Dobi, A.; Bravo, S.B.; Veeren, B.; Paradela-Dobarro, B.; Álvarez, E.; Meilhac, O.; Viranaicken, W.; Baret, P.; Devin, A.; Rondeau, P. Advanced glycation end-products disrupt human endothelial cells redox homeostasis: New insights into reactive oxygen species production. Free. Radic. Res. 2019, 53, 150–169. [Google Scholar] [CrossRef]
- Taïlé, J.; Patché, J.; Veeren, B.; Gonthier, M.P. Hyperglycemic Condition Causes Pro-Inflammatory and Permeability Alterations Associated with Monocyte Recruitment and Deregulated Nfkappab/Ppargamma Pathways on Cerebral Endothelial Cells: Evidence for Polyphenols Uptake and Protective Effect. Int. J. Mol. Sci. 2021, 22, 1385. [Google Scholar] [CrossRef]
- Vinson, J.A. Intracellular Polyphenols: How Little We Know. J. Agric. Food Chem. 2019, 67, 3865–3870. [Google Scholar] [CrossRef]
- Choi, J.S.; Bae, J.Y.; Kim, D.S.; Li, J.; Kim, J.L.; Lee, Y.J.; Kang, Y.H. Dietary Compound Quercitrin Dampens Vegf Induction and Ppargamma Activation in Oxidized Ldl-Exposed Murine Macrophages: Association with Scavenger Receptor Cd36. J. Agric. Food Chem. 2010, 58, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Nishizuka, T.; Fujita, Y.; Sato, Y.; Nakano, A.; Kakino, A.; Ohshima, S.; Kanda, T.; Yoshimoto, R.; Sawamura, T. Procyanidins are potent inhibitors of LOX-1: A new player in the French Paradox. Proc. Jpn. Acad. Ser. B 2011, 87, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Tavares, L.; Jardim, C.; Costa, I.; Terrasso, A.P.; Almeida, A.F.; Govers, C.; Mes, J.J.; Gardner, R.; Becker, J.D.; et al. Blood–brain barrier transport and neuroprotective potential of blackberry-digested polyphenols: An in vitro study. Eur. J. Nutr. 2017, 58, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Yi, L.; Jin, X.; Xie, Q.; Zhang, T.; Zhou, X.; Chang, H.; Fu, Y.-J.; Zhu, J.-D.; Zhang, Q.-Y.; et al. Absorption of resveratrol by vascular endothelial cells through passive diffusion and an SGLT1-mediated pathway. J. Nutr. Biochem. 2013, 24, 1823–1829. [Google Scholar] [CrossRef]
- Holvoet, P. Endothelial dysfunction, oxidation of low-density lipoprotein, and cardiovascular disease. Ther. Apher. 1999, 3, 287–293. [Google Scholar] [CrossRef]
- Chang, H.-C.; Chen, T.-G.; Tai, Y.-T.; Chen, T.-L.; Chiu, W.-T.; Chen, R.-M. Resveratrol Attenuates Oxidized LDL-Evoked Lox-1 Signaling and Consequently Protects against Apoptotic Insults to Cerebrovascular Endothelial Cells. Br. J. Pharmacol. 2010, 31, 842–854. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Li, J. Resveratrol protects against oxidized low-density lipoprotein-induced human umbilical vein endothelial cell apoptosis via inhibition of mitochondrial-derived oxidative stress. Mol. Med. Rep. 2017, 15, 2457–2464. [Google Scholar] [CrossRef]
- Ou, H.-C.; Chou, F.-P.; Sheen, H.-M.; Lin, T.-M.; Yang, C.-H.; Sheu, W.H.-H. Resveratrol, a polyphenolic compound in red wine, protects against oxidized LDL-induced cytotoxicity in endothelial cells. Clin. Chim. Acta 2006, 364, 196–204. [Google Scholar] [CrossRef]
- Ou, H.-C.; Lee, W.-J.; Lee, S.-D.; Huang, C.-Y.; Chiu, T.-H.; Tsai, K.-L.; Hsu, W.-C.; Sheu, W.H.-H. Ellagic acid protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol. Appl. Pharmacol. 2010, 248, 134–143. [Google Scholar] [CrossRef]
- Li, P.; Zhang, L.; Zhou, C.; Lin, N.; Liu, A. Sirt 1 activator inhibits the AGE-induced apoptosis and p53 acetylation in human vascular endothelial cells. J. Toxicol. Sci. 2015, 40, 615–624. [Google Scholar] [CrossRef]
| Botanical Name | Family | Voucher Number | Parts Used |
|---|---|---|---|
| Doratoxylon apetalum a | Sapindaceae | RUN-055E | Leaf |
| (Poir.) Radlk | |||
| Antirhea borbonica b | Rubiaceae | RUN-052F | Leaf, stem |
| J.F Gmelin |
| Peak Number | RT (min) | Compound | Molecular Formula | Mass Error (ppm) | [M-H]− | MS/MS Fragments |
|---|---|---|---|---|---|---|
| 1 | 2.1 | Protocatechuic acid | C7H6O45 | 0 | 153.0182 | 109.0282 |
| 2 | 2.3 | Gallic acid 4-O-glucoside | C13H15O10 | 2.4 | 331.0668 | 168.0053, 125.0231, 211.0238 |
| 3 | 2.7 | Unknown | C15H18O8 | 2.6 | 325.0927 | |
| 4 | 3.1 | Unknown | C13H12O8 | 2.5 | 295.0456 | 163.0389, 112.9867 |
| 5 | 3.3 | Procyanidin dimer type B | C30H26O12 | 5.1 | 577.1371 | 289.0714, 407.0766, 125.0231, 109.0282 |
| 6 | 3.7 | Epicatechin | C15H15O6 | 2.7 | 289.0715 | 245.0814, 179.0340, 125.0232, 109.0282 |
| 7 | 3.9 | Procyanidin trimer type C | C45H38O18 | 1.2 | 865.1985 | 289.0714, 411.0718, 125.0232, 109.0283, 560.0907 |
| 8 | 4.4 | Coumaric acid | C9H8O3 | 0 | 163.039 | 119.049 |
| 9 | 4.7 | Quercetin 3-O-rutinoside (Rutin) | C27H30O16 | 1.7 | 609.1461 | 301.0348 |
| 10 | 4.7 | Unknown | C13H12O7 | 2.7 | 279.0507 | 133.0130, 163.0390 |
| 11 | 4.9 | Kaempferol 3-O-rutinoside | C27H30O19 | 1.3 | 593.1509 | 285.04 |
| 12 | 4.9 | Quercetin 3-O-hexoside | C21H19O12 | 0.5 | 468.0881 | 301.0347 |
| 13 | 5.1 | Kaempferol 3-O-hexoside | C21H20O11 | 2 | 447.0931 | 285.0402 |
| 14 | 5.5 | Apigenin-7-O-rutinoside (Isorhoifolin) | C27H30O14 | 1.8 | 577.1563 | 269.0453 |
| 15 | 5.7 | Apigenin hexoside | C21H20O10 | 1.6 | 431.0984 | 269.0453 |
| 16 | 5.9 | Kaempferol 3-O-(6-malonyl-hexoside) | C24H22O14 | 1.5 | 533.0934 | 489.1035, 285.0401 |
| 17 | 6.5 | Apigenin | C15H10O5 | 3.1 | 269.0454 |
| Peak Number | RT (min) | Compound | Molecular Formula | Mass Error (ppm) | [M-H]− | MS/MS Fragments |
|---|---|---|---|---|---|---|
| 1 | 0.6 | Quinic acid | C6H12O7 | −0.3 | 195.0499 | 127.0388, 111.0438 |
| 2 | 2.1 | Protocatechuic acid | C7H6O4 | −0.2 | 153.018 | 109.0281 |
| 3 | 2.4 | 5-Caffeoylquinic acid | C16H18O10 | 0.23 | 353.0869 | 191.0549, 179.0337, 173.0442, 135.0437 |
| 4 | 2.6 | hydroxybenzoic acid isomer | C7H6O3 | −0.29 | 137.023 | 93.0331 |
| 5 | 3.3 | 5-Caffeoylquinic acid | C16H18O9 | 0.23 | 353.0869 | 191.0549, 179.0337, 173.0443, 135.0437 |
| 6 | 3.5 | Caffeic acid | C9H8O4 | −0.14 | 179.0337 | 135.0438 |
| 7 | 4 | 5-p-Coumaroylquinic acid | C16H17O8 | 0.2 | 337.092 | 191.0549, 173.0442, 93.0331 |
| 8 | 4.1 | o/m-Coumaric acid | C15H10O4 | −0.2 | 163.0387 | 119.0488 |
| 9 | 4.4 | p-Coumaric acid | C15H10O4 | −0.2 | 163.0387 | 119,30,488 |
| 10 | 4.4 | 5-Feruloylquinic acid | C17H20O9 | 0.2 | 367.1026 | |
| 11 | 4.8 | Quercetin 3-O-rutinoside | C27H30O16 | 0.1 | 609.1448 | 301.0341 |
| 12 | 5 | Quercetin 3-O-hexoside | C21H20O12 | 0.1 | 463.087 | 300.0268 |
| 13 | 5.1 | Kaempferol 3-O-galactoside 7-O-rhamnoside | C27H30O15 | 0.1 | 593.15 | 284.032 |
| 14 | 5.5 | 3,5-Dicaffeoylquinic acid | C25H24O12 | −0.2 | 515.1182 | 191.0550, 179.0338, 353.0870, 135.0438 |
| 15 | 6 | Unknown | C26H32O14 | −0.01 | 567.1719 | 163.0387, 195.0650, 315.1231, 359.1124, 521.1653 |
| 16 | 6 | hydroxybenzoic acid isomer | C7H6O3 | −0.29 | 137.023 | 93.0331 |
| 17 | 6.1 | 3,4-Dicaffeoylquinic acid | C25H24O12 | −0.2 | 515.1182 | 353.0870, 173.0443, 191.0549, 135.0437 |
| 18 | 6.2 | 5-Caffeoylquinic acid | C16H18O11 | 0.05 | 353.0868 | |
| 19 | 6.5 | 1,4/4,5-Dicaffeoylquinic acid | C25H24O12 | −0.1 | 515.1183 | 173.0443, 353.0869, 191.0549, 135.0436 |
| 20 | 7.3 | Quercetin | C15H10O7 | 0.9 | 301.0345 | |
| 21 | 8.2 | Kaempferol | C15H10O6 | 1.3 | 285.0405 |
| Total Polyphenol Content (mg GAE/g Plant) | |
|---|---|
| Doratoxylon apetalum | 3.79 ± 0.14 |
| Antirhea borbonica | 1.98 ± 0.09 |
| TBARS (µM) | TBARS (µM) | p | TBARS (µM) | p | TBARS (µM) | p | |
|---|---|---|---|---|---|---|---|
| control | 10 µM | 25 µM | 50 µM | ||||
| D. apetalum | 4.210 | 0.046 | <0.001 | 0.023 | <0.001 | 0.027 | <0.001 |
| ±0.710 | ±0.011 | ±0.012 | ±0.014 | ||||
| A. borbonica | 3.578 | 0.042 | <0.001 | 0.019 | <0.001 | 0.025 | <0.001 |
| ±0.188 | ±0.003 | ±0.007 | ±0.011 | ||||
| control | 1 µM | 5 µM | 10 µM | ||||
| Curcumin | 3.742 | 3.141 | n.s | 0.130 | <0.001 | 0.031 | <0.001 |
| ±0.572 | ±0.621 | ±0.031 | ±0.011 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonneville, J.; Rondeau, P.; Veeren, B.; Faccini, J.; Gonthier, M.-P.; Meilhac, O.; Vindis, C. Antioxidant and Cytoprotective Properties of Polyphenol-Rich Extracts from Antirhea borbonica and Doratoxylon apetalum against Atherogenic Lipids in Human Endothelial Cells. Antioxidants 2022, 11, 34. https://doi.org/10.3390/antiox11010034
Bonneville J, Rondeau P, Veeren B, Faccini J, Gonthier M-P, Meilhac O, Vindis C. Antioxidant and Cytoprotective Properties of Polyphenol-Rich Extracts from Antirhea borbonica and Doratoxylon apetalum against Atherogenic Lipids in Human Endothelial Cells. Antioxidants. 2022; 11(1):34. https://doi.org/10.3390/antiox11010034
Chicago/Turabian StyleBonneville, Jonathan, Philippe Rondeau, Bryan Veeren, Julien Faccini, Marie-Paule Gonthier, Olivier Meilhac, and Cécile Vindis. 2022. "Antioxidant and Cytoprotective Properties of Polyphenol-Rich Extracts from Antirhea borbonica and Doratoxylon apetalum against Atherogenic Lipids in Human Endothelial Cells" Antioxidants 11, no. 1: 34. https://doi.org/10.3390/antiox11010034






