Fibrinogen and Atherosclerotic Cardiovascular Diseases—Review of the Literature and Clinical Studies
Abstract
:1. Introduction
2. Fibrinogen—Physiological and Pathophysiological Aspects
3. Fibrinogen and Cardiovascular Risk
4. Fibrinogen Molecular Modifications and Cardiovascular Risk
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGill, H.C., Jr.; McMahan, C.A.; Herderick, E.E.; Malcom, G.T.; Tracy, R.E.; Strong, J.P. Origin of atherosclerosis in childhood and adolescence. Am. J. Clin. Nutr. 2000, 72, 1307–1315. [Google Scholar]
- Ji, X.; Leng, X.Y.; Dong, Y.; Ma, Y.H.; Xu, W.; Cao, X.P.; Hou, X.H.; Dong, Q.; Tan, L.; Yu, J.T. Modifiable risk factors for carotid atherosclerosis: A meta-analysis and systematic review. Ann. Transl. Med. 2019, 7, 632. [Google Scholar] [CrossRef] [PubMed]
- Sathiyakumar, V.; Kapoor, K.; Jones, S.R.; Banach, M.; Martin, S.S. Novel Therapeutic Targets for Managing Dyslipidemia. Trends Pharmacol. Sci. 2018, 39, 733–747. [Google Scholar] [CrossRef]
- Roth, G.S.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Ab Khan, M.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AIKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- McDonald, L.; Edgill, M. Coagulability of the blood in ischaemic heart disease. Lancet 1957, 2, 457–460. [Google Scholar] [CrossRef]
- Kannel, W.B.; Wolf, P.A.; Castelli, W.P.; D’Agostino, R.B. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA 1987, 258, 1183–1186. [Google Scholar] [CrossRef]
- Pieters, M.; Wolberg, A.S. Fibrinogen and fibrin: An illustrated review. Res. Pract. Thromb. Haemost. 2019, 3, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Vilar, R.; Fish, R.J.; Casini, A.; Neerman-Arbez, M. Fibrin(ogen) in human disease: Both friend and foe. Haematologica 2020, 105, 284–296. [Google Scholar] [CrossRef] [Green Version]
- Neerman-Arbez, M.; Casini, A. Clinical Consequences and Molecular Bases of Low Fibrinogen Levels. Int. J. Mol. Sci. 2018, 19, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdaca, G.; Spanò, F.; Cagnati, P.; Puppo, F. Free radicals and endothelial dysfunction: Potential positive effects of TNF-α inhibitors. Redox Rep. 2013, 18, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arter. Thromb. Vasc. Biol. 2017, 37, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Simurda, T.; Snahnicanova, Z.; Loderer, D.; Sokol, J.; Stasko, J.; Lasabova, Z.; Kubisz, P. Fibrinogen martin: A novel mutation in FGB (Gln180Stop) causing congenital afibrinogenemia. Semin. Thromb. Hemost. 2016, 42, 455–458. [Google Scholar] [PubMed] [Green Version]
- De Vries, J.J.; Snoek, C.J.M.; Rijken, D.C.; de Maat, M.P.M. Effects of Post-Translational Modifications of Fibrinogen on Clot Formation, Clot Structure, and Fibrinolysis: A Systematic Review. Arter. Thromb. Vasc. Biol. 2020, 40, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Siegerink, B.; Rosendaal, F.R.; Algra, A. Genetic variation in fibrinogen; its relationship to fibrinogen levels and the risk of myocardial infarction and ischemic stroke. J. Thromb. Haemost. 2009, 7, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Lowe, G.D.; Woodward, M.; Tunstall-Pedoe, H. Fibrinogen in relation to personal history of prevalent hypertension, diabetes, stroke, intermittent claudication, coronary heart disease, and family history: The Scottish Heart Health Study. Br. Heart J. 1993, 69, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Kannel, W.B.; D’Agostino, R.B.; Belanger, A.J. Update on fibrinogen as a cardiovascular risk factor. Ann. Epidemiol. 1992, 2, 457–466. [Google Scholar] [CrossRef]
- Kaptoge, S.; White, I.R.; Thompson, S.G.; Wood, A.M.; Lewington, S.; Lowe, G.D.O.; Danesh, J. Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: Individual participant meta-analysis of 154,211 adults in 31 prospective studies: The fibrinogen studies collaboration. Am. J. Epidemiol. 2007, 166, 867–879. [Google Scholar]
- Kryczka, K.E.; Kruk, M.; Demkow, M.; Lubiszewska, B. Fibrinogen and a triad of thrombosis, inflammation, and the renin-angiotensin system in premature coronary artery disease in women: A new insight into sex-related differences in the pathogenesis of the disease. Biomolecules 2021, 11, 1036. [Google Scholar] [CrossRef]
- Lowe, G.D.O.; Rumley, A.; Mackie, I.J. Plasma fibrinogen. Ann. Clin. Biochem. 2004, 41, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Thompson, S.G.; Kienast, J.; Pyke, S.D.; Haverkate, F.; van de Loo, J.C. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N. Engl. J. Med. 1995, 332, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hannekens, C.H.; Ridker, P.M.; Stampfer, M.J. A prospective study of fibrinogen and risk of myocardial infarction in the Physicians’ Health Study. J. Am. Coll. Cardiol. 1999, 33, 1347–1352. [Google Scholar] [CrossRef] [Green Version]
- Yuan, D.; Jiang, P.; Zhu, P.; Jia, S.; Zhang, C.; Liu, Y.; Liu, R.; Xu, J.; Tang, X.; Zhao, X.; et al. Prognostic value of fibrinogen in patients with coronary artery disease and prediabetes or diabetes following percutaneous coronary intervention: 5-year findings from a large cohort study. Cardiovasc. Diabetol. 2021, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Peycheva, M.; Deneva, T.; Zahariev, Z. The role of fibrinogen in acute ischaemic stroke. Neurol. Neurochir. Pol. 2021, 55, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhao, Y.; He, Y. Fibrinogen level predicts outcomes in critically ill patients with acute exacerbation of chronic heart failure. Dis. Markers 2021, 2021, 6639393. [Google Scholar] [CrossRef]
- Ceasovschih, A.; Sorodoc, V.; Onofrei Aursulesei, V.; Tesloianu, D.; Tuchilus, C.; Anisie, E.; Petris, A.; Statescu, C.; Jaba, E.; Stoica, A.; et al. Biomarker utility for peripheral artery disease diagnosis in real clinical practice: A prospective study. Diagnostics 2020, 10, 723. [Google Scholar] [CrossRef]
- Samir, G.M.; Khalil, O.A.; Fawzy, M.S.; Sadek, A.M.E.M. Study of fibrinogen level in acute ischemic stroke patients in medical intensive care unit. Egypt. J. Crit. Care Med. 2020, 7, 51–56. [Google Scholar]
- Song, J.; Yu, T.; Sun, Z.; Li, Z.; He, D.; Sun, Z. Comparison of prognostic significance between serum fibrinogen and Global Registry of Acute Coronary Events score for prognosis of patients with non-ST-elevation acute coronary syndromes undergoing percutaneous coronary intervention. Coron. Artery Dis. 2020, 31, 124–129. [Google Scholar] [CrossRef]
- Liu, S.-L.; Wu, N.-Q.; Shi, H.-W.; Dong, Q.; Dong, Q.-T.; Gao, Y.; Guo, Y.-L.; Li, J.-J. Fibrinogen is associated with glucose metabolism and cardiovascular outcomes in patients with coronary artery disease. Cardiovasc. Diabetol. 2020, 19, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, P.; Gao, Z.; Zhao, W.; Song, Y.; Tang, X.-F.; Xu, J.-J.; Wang, H.-H.; Jiang, L.; Chen, J.; Qiao, S.-B.; et al. Relationship between fibrinogen levels and cardiovascular events in patients receiving percutaneous coronary intervention: A large single-center study. Chin. Med. J. 2019, 132, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, C.; Liu, J.; Bai, X.; Li, R.; Wang, L.; Zhou, J.; Wu, Y.; Yuan, Z. Baseline plasma fibrinogen is associated with haemoglobin A1c and 2-year major adverse cardiovascular events following percutaneous coronary intervention in patients with acute coronary syndrome: A single-centre, prospective cohort study. Cardiovasc. Diabetol. 2019, 18, 52. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.-F.; Cao, D.; Ye, T.-T.; Deng, H.-H.; Zhu, H. Peripheral arterial disease in type 2 diabetes is associated with an increase in fibrinogen levels. Int. J. Endocrinol. 2018, 2018, 3709534. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.-Y.; Zhou, B.-Y.; Zhang, M.-Z.; Zhao, X.; Qing, P.; Zhu, C.-G.; Wu, N.-Q.; Guo, Y.-L.; Gao, Y.; Li, X.-L.; et al. Association between fibrinogen level and the severity of coronary stenosis in 418 male patients with myocardial infarction younger than 35 years old. Oncotarget 2017, 8, 81361–81368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabakci, M.M.; Gerin, F.; Sunbul, M.; Toprak, C.; Durmuş, H.I.; Demir, S.; Arslantaş, U.; Cerşit, S.; Batgerel, U.; Kargın, R. Relation of plasma fibrinogen level with the presence, severity, and complexity of coronary artery disease. Clin. Appl. Thromb. 2017, 23, 638–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-H.; Du, Y.; Zhang, Y.; Li, X.-L.; Li, S.; Xu, R.-X.; Zhu, C.-G.; Guo, Y.-L.; Wu, N.-Q.; Qing, P.; et al. Serum fibrinogen and cardiovascular events in Chinese patients with type 2 diabetes and stable coronary artery disease: A prospective observational study. BMJ Open 2017, 7, e015041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Xia, T.-L.; Li, Y.-M.; Huang, F.-Y.; Chai, H.; Wang, P.-J.; Liu, W.; Zhang, C.; Pu, X.-B.; Chen, S.-J.; et al. Fibrinogen is related to long-term mortality in Chinese patients with acute coronary syndrome but failed to enhance the prognostic value of the GRACE score. Oncotarget 2017, 8, 20622–20629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunusor, S.K.; Kurl, S.; Zaccardi, F.; Laukkanen, J.A. Baseline and long-term fibrinogen levels and risk of sudden cardiac death: A new prospective study and meta-analysis. Atherosclerosis 2016, 245, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotbi, S.; Mjabber, A.; Chadli, A.; El Hammiri, A.; El Aziz, S.; Oukkache, B.; Mifdal, H.; Nourichafi, N.; Kamal, N.; Habbal, R.; et al. Correlation between the plasma fibrinogen concentration and coronary heart disease severity in Moroccan patients with type 2 diabetes. Prospective study. Ann. Endocrinol. 2016, 77, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, H.; Li, Y.-M.; Huang, B.-T.; Huang, F.-Y.; Xia, T.-L.; Chai, H.; Wang, P.-J.; Liu, W.; Zhang, C.; et al. Relation between admission plasma fibrinogen levels and mortality in Chinese patients with coronary artery disease. Sci. Rep. 2016, 6, 30506. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Li, Y.-M.; Chai, H.; Zuo, Z.-L.; Wang, P.-J.; Gui, Y.-Y.; Huang, B.-T.; Liao, Y.-B.; Xia, T.-L.; Huang, F.-Y.; et al. Understanding the controversy surrounding the correlation between fibrinogen level and prognosis of coronary artery disease-the role of the subtypes of coronary artery disease. Int. J. Cardiol. 2016, 222, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, C.-G.; Guo, Y.-L.; Xu, R.-X.; Li, S.; Dong, Q.; Li, J.-J. Higher fibrinogen level is independently linked with the presence and severity of new-onset coronary atherosclerosis among Han Chinese population. PLoS ONE 2014, 9, e113460. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.-F.; Li, X.-L.; Luo, S.-H.; Guo, Y.-L.; Zhu, C.-G.; Qing, P.; Wu, N.-Q.; Li, J.-J. Association of fibrinogen with severity of stable coronary artery disease in patients with type 2 diabetic mellitus. Dis. Markers 2014, 2014, 485687. [Google Scholar] [CrossRef] [PubMed]
- Bosevski, M.; Bosevska, G.; Stojanovska, L. Influence of fibrinogen and C-RP on progression of peripheral arterial disease in type 2 diabetes: A preliminary report. Cardiovasc. Diabetol. 2013, 12, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danesh, J.; Lewington, S.; Thompson, S.G.; Lowe, G.D.O.; Collins, R.; Kostis, J.B.; Wilson, A.C.; Folsom, A.R.; Wu, K.; Benderly, M.; et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: An individual participant meta-analysis. JAMA 2005, 294, 1799–1809. [Google Scholar] [PubMed]
- Kaptoge, S.; Di Agelantonio, E.; Pennells, L.; Wood, A.M.; White, I.R.; Gao, P.; Walker, M.; Thompson, A.; Sarwar, N.; Caslake, M.; et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl. J. Med. 2012, 367, 1310–1320. [Google Scholar]
- Song, B.; Shu, Y.; Xu, Y.N.; Fu, P. Plasma fibrinogen lever and risk of coronary heart disease among Chinese population: A systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 13195–13202. [Google Scholar]
- Levenson, J.; Giral, P.; Razavian, M.; Gariepy, J.; Simon, A. Fibrinogen and silent atherosclerosis in subjects with cardiovascular risk factors. Arter. Thromb. Vasc. Biol. 1995, 15, 1263–1268. [Google Scholar] [CrossRef]
- Green, D.; Foiles, N.; Chan, C.; Schreiner, P.J.; Liu, K. Elevated fibrinogen levels and subsequent subclinical atherosclerosis: The CARDIA Study. Atherosclerosis 2009, 202, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.; Chan, C.; Kang, J.; Liu, K.; Schreiner, P.J.; Jenny, N.S.; Tracy, R.P. Longitudinal assessment of fibrinogen in relation to subclinical cardiovascular disease: The CARDIA study. J. Thromb. Haemost. 2010, 8, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menti, E.; Zaffari, D.; Galarraga, T.; da Conceição E Lessa, J.R.; Pontin, B.; Pellanda, L.C.; Portal, V.L. Early markers of atherosclerotic disease in individuals with excess weight and dyslipidemia. Arq. Bras. Cardiol. 2016, 106, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, F.; Peng, R.; Pei, S.; Hou, Z.; Lu, B.; Cong, X.; Chen, X. Sex-related differences in the association between plasma fibrinogen and non-calcified or mixed coronary atherosclerotic plaques. Biol. Sex Differ. 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, L.; Li, X.; Jin, S.; Li, X.; Liu, F.; Shan, C.; Zhang, Y.; Yang, Y. New insights into the association between fibrinogen and coronary atherosclerotic plaque vulnerability: An intravascular optical coherence tomography study. Cardiovasc. Ther. 2019, 2019, 8563717. [Google Scholar] [CrossRef]
- Bai, Y.; Zheng, Y.-Y.; Tang, J.-N.; Yang, Y.-M.; Guo, Q.-Q.; Zhang, J.-C.; Cheng, M.-D.; Song, F.-H.; Wang, K.; Zhang, Z.-L.; et al. D-dimer to fibrinogen ratio as a novel prognostic marker in patients after undergoing percutaneous coronary intervention: A retrospective cohort study. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620948586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-P.; Mao, X.-F.; Wu, T.-T.; Chen, Y.; Hou, X.-G.; Yang, Y.; Ma, X.; Zhang, J.-Y.; Ma, Y.-T.; Xie, X.; et al. The fibrinogen-to-albumin ratio is associated with outcomes in patients with coronary artery disease who underwent percutaneous coronary intervention. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620933008. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Jansen, C.; M’Pembele, R.; Stroda, A.; Boeken, U.; Akhyari, P.; Lichtenberg, A.; Hollmann, M.W.; Huhn, R.; Buse, G.L.; et al. Fibrinogen-albumin-ratio is an independent predictor of thromboembolic complications in patients undergoing VA-ECMO. Sci. Rep. 2021, 11, 16648. [Google Scholar] [CrossRef] [PubMed]
- Karahan, O.; Acet, H.; Ertaş, F.; Tezcan, O.; Çalişkan, A.; Demir, M.; Kaya, A.F.; Demirtaş, S.; Çevik, M.U.; Yavuz, C. The relationship between fibrinogen to albumin ratio and severity of coronary artery disease in patients with STEMI. Am. J. Emerg. Med. 2016, 34, 1037–1042. [Google Scholar] [CrossRef]
- Celebi, S.; Celebi, O.O.; Berkalp, B.; Amasyali, B. The association between the fibrinogen-to-albumin ratio and coronary artery disease severity in patients with stable coronary artery disease. Coron. Artery Dis. 2020, 31, 512–517. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Ji, Y.Y.; Wang, F.Y.; Wang, S.L.; Lai, G.K.; Wang, T.; Tang, J.M. Value of fibrinogen to albumin ratio on predicting spontaneous recanalization of infarct-related artery in patients with acute ST-segment elevation myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi 2019, 47, 123–128, (Article in Chinese, Only Abstract in English). [Google Scholar]
- Erdoğan, G.; Arslan, U.; Yenercağ, M.; Durmuş, G.; Tuğrul, S.; Şahin, İ. Relationship between the fibrinogen-to-albumin ratio and SYNTAX score in patients with non-st-elevation myocardial infarction. Rev. Investig. Clin. 2021, 73, 182–189. [Google Scholar] [CrossRef]
- Li, M.; Tang, C.; Luo, E.; Qin, Y.; Wang, D.; Yan, G. Relation of fibrinogen-to-albumin ratio to severity of coronary artery disease and long-term prognosis in patients with non-ST elevation acute coronary syndrome. BioMed Res. Int. 2020, 2020, 1860268. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, G.; Liang, X.; Qin, C.; Luo, Q.; Song, R.; Chen, W. Predictive value of fibrinogen-to-albumin ratio for post-contrast acute kidney injury in patients undergoing elective percutaneous coronary intervention. Med. Sci. Monit. 2020, 26, e924498. [Google Scholar] [CrossRef]
- Ishihara, K.K.; Kokubo, Y.; Yokota, C.; Hida, E.; Miyata, T.; Toyoda, K.; Matsumoto, M.; Minematsu, K.; Miyamoto, Y. Effect of plasma fibrinogen, high-sensitive C-reactive protein, and cigarette smoking on carotid atherosclerosis: The Suita study. J. Stroke Cerebrovasc. Dis. 2015, 24, 2385–2389. [Google Scholar] [CrossRef] [PubMed]
- Lassé, M.; Pilbrow, A.P.; Kleffmann, T.; Andersson Överström, E.; von Zychlinski, A.; Frampton, C.M.A.; Poppe, K.K.; Troughton, R.W.; Lewis, L.K.; Prickett, T.C.R.; et al. Fibrinogen and hemoglobin predict near future cardiovascular events in asymptomatic individuals. Sci. Rep. 2021, 11, 4605. [Google Scholar] [CrossRef]
- Cho, H.M.; Kang, D.R.; Kim, H.C.; Oh, S.M.; Kim, B.K.; Suh, I. Association between fibrinogen and carotid atherosclerosis according to smoking status in a Korean male population. Yonsei Med. J. 2015, 56, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Kryczka, K.E.; Płoski, R.; Księżycka, E.; Kruk, M.; Kostrzewa, G.; Kowalik, I.; Demkow, M.; Lubiszewska, B. The association between the insertion/deletion polymorphism of the angiotensin-converting enzyme gene and the plasma fibrinogen level in women and men with premature coronary artery atherosclerosis. Pol. Arch. Intern. Med. 2020, 130, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Lovely, R.S.; Yang, Q.; Massaro, J.M.; Wang, J.; D’Agostino Sr, R.B.; O’Donnell, C.J.; Shannon, J.; Farrell, D.H. Assessment of genetic determinants of the association of γ’ fibrinogen in relation to cardiovascular disease. Arter. Thromb. Vasc. Biol. 2011, 31, 2345–2352. [Google Scholar] [CrossRef] [Green Version]
- De Willige, S.U.; Standeven, K.F.; Philippou, H.; Ariëns, R.A.S. The pleiotropic role of the fibrinogen gamma’ chain in hemostasis. Blood 2009, 114, 3994–4001. [Google Scholar] [CrossRef] [PubMed]
- Farrell, D.H. Primetime for γ′. Blood 2014, 124, 1389–1390. [Google Scholar] [CrossRef] [PubMed]
- Appiah, D.; Schreiner, J.P.; MacLehose, R.F.; Folsom, A.R. Association of plasma γ’ fibrinogen with incident cardiovascular disease: The atherosclerosis risk in communities (ARIC) study. Arter. Thromb. Vasc. Biol. 2015, 35, 2700–2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrae, F.L.; Swieringa, F.; Heemskerk, J.W.M.; Ariëns, R.A.S. High fibrinogen γ’ levels in patient plasma increase clot formation at arterial and venous shear. Blood Adv. 2021, 5, 3468–3477. [Google Scholar] [CrossRef]
- Lovely, R.S.; Kazmierczak, S.C.; Massaro, J.M.; D’Agostino, R.B., Sr.; O’Donnell, C.J.; Farrell, D.H. Gamma’ fibrinogen: Evaluation of a new assay for study of associations with cardiovascular disease. Clin. Chem. 2010, 56, 781–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrell, D.H. γ’ fibrinogen as a novel marker of thrombotic disease. Clin. Chem. Lab. Med. 2012, 50, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Mannila, M.N.; Lovely, R.S.; Kazmierczak, S.C.; Eriksson, P.; Samnegård, A.; Farrell, D.H.; Hamsten, A.; Silveira, A. Elevated plasma fibrinogen gamma’ concentration is associated with myocardial infarction: Effects of variation in fibrinogen genes and environmental factors. J. Thromb. Haemost. 2007, 5, 766–773. [Google Scholar] [CrossRef]
- Drizlionoka, K.; Zariņš, J.; Ozolina, A.; Ņikitina-Zaķe, L.; Mamaja, B. Polymorphism rs2066865 in the fibrinogen gamma chain (FGG) gene increases plasma fibrinogen concentration and is associated with an increased microvascular thrombosis rate. Medicina 2019, 55, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appiah, D.; Heckbert, S.R.; Cushman, M.; Psaty, B.M.; Folsom, A.R. Lack of association of plasma gamma prime (γ’) fibrinogen with incident cardiovascular disease. Thromb. Res. 2016, 143, 50–52. [Google Scholar] [CrossRef] [Green Version]
- Maners, J.; Gill, D.; Pankratz, N.; Laffan, M.A.; Wolberg, A.S.; de Maat, M.P.M.; Ligthart, S.; Tang, W.; Ward-Caviness, C.K.; Fornage, M.; et al. A Mendelian randomization of γ’ and total fibrinogen levels in relation to venous thromboembolism and ischemic stroke. Blood 2020, 136, 3062–3069. [Google Scholar] [CrossRef]
- Kotzé, R.C.M.; Ariëns, R.A.S.; de Lange, Z.; Pieters, M. CVD risk factors are related to plasma fibrin clot properties independent of total and or γ’ fibrinogen concentration. Thromb. Res. 2014, 134, 963–969. [Google Scholar] [CrossRef]
- Pieters, M.; Kotze, R.C.; Jerling, J.C.; Kruger, A.; Ariëns, R.A.S. Evidence that fibrinogen γ’ regulates plasma clot structure and lysis and relationship to cardiovascular risk factors in black Africans. Blood 2013, 121, 3254–3260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rautenbach, P.H.; Nienaber-Rousseau, C.; de Lange-Loots, Z.; Pieters, M. Certain associations between iron biomarkers and total and γ’ fibrinogen and plasma clot properties are mediated by fibrinogen genotypes. Front. Nutr. 2021, 8, 720048. [Google Scholar] [CrossRef]
- Rautenbach, P.H.; Nienaber-Rousseau, C.; Pieters, M. The association of alcohol with circulating total fibrinogen and plasma clot density is mediated by fibrinogen and FXIII genotypes. Thromb. J. 2020, 18, 35. [Google Scholar] [CrossRef]
- Walton, B.L.; Getz, T.M.; Bergmeier, W.; de Willige, S.U.; Wolberg, A.S. Gamma prime fibrinogen does not cause arterial thrombosis. Blood 2013, 122, 1092. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Tousoulis, D.; Siasos, G.; Stefanadis, C. Is fibrinogen a marker of inflammation in coronary artery disease? Hellenic J. Cardiol. 2010, 51, 1–9. [Google Scholar] [PubMed]
- Li, D.; Zhang, X.; Huang, H.; Zhang, H. Association of β-fibrinogen polymorphisms and venous thromboembolism risk: A PRISMA-compliant meta-analysis. Medicine 2019, 98, e18204. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, X.; Jiang, A.; Zhang, B.; Bi, P.; Dong, Y.; Guo, Y. Associations of β-fibrinogen polymorphisms with the risk of ischemic stroke: A meta-analysis. J. Stroke Cerebrovasc. Dis. 2019, 28, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Canseco-Avila, L.M.; Lopez-Roblero, A.; Serrano-Guzman, E.; Aguilar-Fuentes, J.; Jerjes-Sanchez, C.; Rojas-Martinez, A.; Ortiz-Lopez, R. Polymorphisms -455G/A and -148C/T and fibrinogen plasmatic level as risk markers of coronary disease and major adverse cardiovascular events. Dis. Markers 2019, 2019, 5769514. [Google Scholar] [CrossRef]
- Gu, L.; Wu, G.; Su, L.; Yan, Y.; Long, J.; Tan, J.; Liang, B.; Guo, X.; Huang, G. Genetic polymorphism of β-fibrinogen gene-455G/A can contribute to the risk of ischemic stroke. Neurol. Sci. 2014, 35, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Liu, W.; Yan, Y.; Su, L.; Wu, G.; Liang, B.; Tan, J.; Huang, G. Influence of the β-fibrinogen-455G/A polymorphism on development of ischemic stroke and coronary heart disease. Thromb. Res. 2014, 133, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, J.; Li, Y.; Wu, J.; Qiao, S.; Xu, S.; Huang, J.; Chen, L. The β-fibrinogen gene 455G/A polymorphism associated with cardioembolic stroke in atrial fibrillation with low CHA2DS2-VaSc score. Sci. Rep. 2017, 7, 17517. [Google Scholar] [CrossRef]
- Golenia, A.; Chrzanowska-Wasko, J.; Jagiella, J.; Wnuk, M.; Ferens, A.; Klimkowicz-Mrowiec, A.; Adamski, M.; Ciecko-Michalska, I.; Słowik, A. The β-fibrinogen -455G/A gene polymorphism and the risk of ischaemic stroke in a Polish population. Neurol. Neurochir. Pol. 2013, 47, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Durmus, G.; Karakus, N.; Yuksel, S.; Kara, N. Analysis of twelve cardiovascular disease related gene mutations among Turkish patients with coronary artery disease. Int. J. Blood Res. Disord. 2020, 7, 047. [Google Scholar]
- Alkhiary, W.; Azzam, H.; Yossof, M.M.A.; Aref, S.; Othman, M.; El-Sharawy, S. Association of hemostatic gene polymorphisms with early-onset ischemic heart disease in Egyptian patients. Clin. Appl. Thromb. Hemost. 2016, 22, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Sewelam, M.I.; Ahmed, A.A.; Alwakeel, H.A.; Khaled, M.Y. Fibrinogen -455G/A promoter polymorphism in acute ST elevation myocardial infarction in Egyptian patients. Egypt J. Haematol. 2014, 39, 98–102. [Google Scholar]
- Martiskainen, M.; Oksala, N.; Pohjasvaara, T.; Kaste, M.; Oksala, A.; Karhunen, P.J.; Erkinjuntti, T. Βeta-fibrinogen gene promoter A -455 allele associated with poor longterm survival among 55–71 years old Caucasian women in Finnish stroke cohort. BMC Neurol. 2014, 14, 137. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Yang, B.; Feng, Y.; Zhao, L.; Feng, Q.; Guan, H.; Song, D.; Yin, F.; Zhuang, L. The correlation between FGB promoter polymorphism and clotting function in patients with idiopathic lower extremity deep venous thrombosis. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029620967108. [Google Scholar] [CrossRef] [PubMed]
- Simurda, T.; Brunclikova, M.; Asselta, R.; Caccia, S.; Zolkova, J.; Kolkova, Z.; Loderer, D.; Skornova, I.; Hudecek, J.; Lasabova, Z.; et al. Genetic variants in the FGB and FGG genes mapping in the beta and gamma nodules of the fibrinogen molecule in congenital quantitative fibrinogen disorders associated with a thrombotic phenotype. Int. J. Mol. Sci. 2020, 21, 4616. [Google Scholar] [CrossRef] [PubMed]
- Simurda, T.; Caccia, S.; Asselta, R.; Zolkova, J.; Stasko, J.; Skornova, I.; Snahnicanova, Z.; Loderer, D.; Lasabova, Z.; Kubisz, P. Congenital hypofibrinogenemia associated with a novel heterozygous nonsense mutation in the globular C-terminal domain of the γ-chain (p.Glu275Stop). J. Thromb. Thromb. 2020, 50, 233–236. [Google Scholar] [CrossRef]
- Simurda, T.; Casini, A.; Stasko, J.; Hudecek, J.; Skornova, I.; Vilar, R.; Neerman-Arbez, M.; Kubisz, P. Perioperative management of a severe congenital hypofibrinogenemia with thrombotic phenotype. Thromb. Res. 2020, 188, 1–4. [Google Scholar] [CrossRef]
- Cronjé, H.T.; Nienaber-Rousseau, C.; Zandberg, L.; Chikowore, T.; de Lange, Z.; van Zyl, T.; Pieters, M. Candidate gene analysis of the fibrinogen phenotype reveals the importance of polygenic co-regulation. Matrix Biol. 2017, 60–61, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Cronjé, H.T.; Nienaber-Rousseau, C.; Zandberg, L.; de Lange, Z.; Green, F.R.; Pieters, M. Fibrinogen and clot-related phenotypes determined by fibrinogen polymorphisms: Independent and IL-6-interactive associations. PLoS ONE 2017, 12, e0187712. [Google Scholar]
- Titov, B.V.; Barsova, R.M.; Martynov, M.Y.; Nikonova, A.A.; Favorov, A.V.; Gusev, E.I.; Favorova, O.O. Polymorphic variants of genes encoding interleukin-6 and fibrinogen, the risk of ischemic stroke and fibrinogen levels. Mol. Biol. 2012, 46, 93–102, (Article in Russian, Only Abstract in English). [Google Scholar] [CrossRef]
- Ward-Caviness, C.K.; de Vries, P.S.; Wiggins, K.L.; Huffman, J.E.; Yanek, L.R.; Bielak, L.F.; Franco, G.; Xiuqing, G.; Marcus, E.K.; Tim, K.; et al. Mendelian randomization evaluation of causal effects of fibrinogen on incident coronary heart disease. PLoS ONE 2019, 14, e0216222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, A.I.; Boerwinkle, E.; Davis, B.R.; Ford, C.E.; Eckfeldt, J.H.; Leiendecker-Foster, C.; Arnett, D.K. Antihypertensive pharmacogenetic effect of fibrinogen-beta variant -455G>A on cardiovascular disease, end-stage renal disease, and mortality: The GenHAT study. Pharm. Genom. 2009, 19, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahebkar, A.; Serban, M.-C.; Mikhailidis, D.P.; Toth, P.P.; Muntner, P.; Ursoniu, S.; Mosterou, S.; Glasser, S.; Martin, S.S.; Jones, S.R.; et al. Head-to-head comparison of statins versus fibrates in reducing plasma fibrinogen concentrations: A systematic review and meta-analysis. Pharmacol. Res. 2016, 103, 236–252. [Google Scholar] [CrossRef]
- Lovely, R.; Hossain, J.; Ramsey, J.P.; Komakula, V.; George, D.; Farrell, D.H.; Balagopal, P.B. Obesity-related increased γ’ fibrinogen concentration in children and its reduction by a physical activity-based lifestyle intervention: A randomized controlled study. J. Pediatr. 2013, 163, 333–338. [Google Scholar] [CrossRef]
- Grobler, C.; Maphumulo, S.C.; Grobbelaar, L.M.; Bredenkamp, J.C.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B.; Pretorius, E. Covid-19: The rollercoaster of gibrin(ogen), D-dimer, von Willebrand factor, P-selectin and their interactions with endothelial cells, platelets and erythrocytes. Int. J. Mol. Sci. 2020, 21, 5168. [Google Scholar] [CrossRef]
- Leibovitz, E.; Hazanov, N.; Frieman, A.; Elly, I.; Gavish, D. Atorvastatin reduces fibrinogen levels in patients with severe hypercholesterolemia: Additional evidence to support the anti-inflammatory effects of statins. Isr. Med. Assoc. J. 2004, 6, 456–459. [Google Scholar]
- Surma, S.; Banach, M.; Lewek, J. COVID-19 and lipids. The role of lipid disorders and statin use in the prognosis of patients with SARS-CoV-2 infection. Lipids Health Dis. 2021, 20, 141. [Google Scholar] [CrossRef]
- Bi, X.; Su, Z.; Yan, H.; Du, J.; Wang, J.; Chen, L.; Peng, M.; Chen, S.; Shen, B.; Li, J. Prediction of severe illness due to COVID-19 based on an analysis of initial Fibrinogen to Albumin Ratio and Platelet count. Platelets 2020, 31, 674–679. [Google Scholar] [CrossRef]
- Di Micco, P.; Russo, V.; Carannante, N.; Imparato, M.; Cardillo, G.; Lodigiani, C. Prognostic value of fibrinogen among COVID-19 patients admitted to an emergency department: An Italian cohort study. J. Clin. Med. 2020, 9, 4134. [Google Scholar] [CrossRef]
- Küçükceran, K.; Ayranci, M.K.; Girişgin, A.S.; Koçak, S. Predictive value of D-dimer/albumin ratio and fibrinogen/albumin ratio for in-hospital mortality in patients with COVID-19. Int. J. Clin. Pract. 2021, 75, e14263. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Noubouossie, D.F.; Gandotra, S.; Cao, L. Elevated plasma fibrinogen is associated with excessive inflammation and disease severity in COVID-19 patients. Front. Cell. Infect. Microbiol. 2021, 11, 734005. [Google Scholar] [CrossRef] [PubMed]

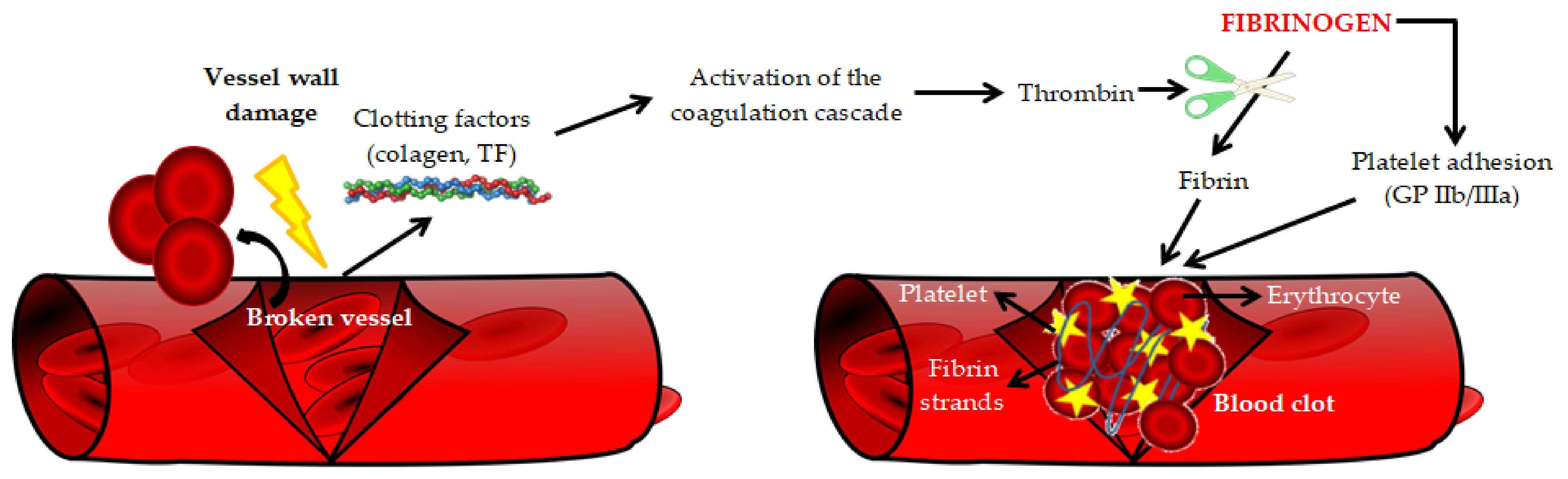
| Main Pro-Atherogenic Properties of Fibrinogen |
|---|
|
| Author; Year | Type of Study | Characteristics and Size of the Sample | Results | Conclusions |
|---|---|---|---|---|
| Yuan et al.; 2021 [24] | Prospective, observational | 6140 patients with CAD undergoing PCI | Fibrinogen plasma concentrations were positively associated with HbA1C and FBG in CAD patients with and without DM (p < 0.001). Elevated fibrinogen plasma concentrations were significantly associated with long-term, all-cause mortality (HR = 1.86; 95% CI: 1.28–2.69; p = 0.001) and cardiac mortality (HR = 1.82; 95% CI: 1.15–2.89; p = 0.011). | Plasma fibrinogen concentrations in patients with CAD after PCI (especially in patients with DM and pre-DM) were independently associated with the long-term risk of death from all causes and cardiac causes. |
| Peycheva et al.; 2021 [25] | Observational | 153 patients categorised into two groups: with acute ischaemic stroke, and with risk factors but no stroke | Patients with ischaemic stroke had a significantly increased mean plasma fibrinogen concentration (>4 g/L). A significant association between fibrinogen plasma concentrations and the presence of ischaemic lesions on cerebral computed tomography was observed: patients with a fibrinogen concentration > 3.41 g/L showed a 3.29-times increased risk of ischaemic lesions. Analysis of stroke subtypes shows that subjects with undetermined cause of stroke, and subject to atherosclerotic stroke, had significantly higher median fibrinogen plasma concentrations compared to subjects with some other types of strokes. A negative association was established between the clinical evolution of ischaemic stroke subjects and fibrinogen plasma concentrations. | Fibrinogen plasma concentration is a clinically useful biomarker that could characterise acute ischaemic stroke. |
| Meng et al.; 2021 [26] | Prospective, observational | 554 critically ill patients with acute exacerbation of chronic HF | Subjects with plasma fibrinogen concentrations ≥284 mg/mL had a significantly higher risk of death by 185% in the 90-day follow-up period (HR = 2.85; 95% CI: 1.65–4.92, p <0.0001). | High-fibrinogen plasma concentrations independently predict mortality in critically ill subjects with acute exacerbation of chronic HF. |
| Ceasovschih et al.; 2020 [27] | Observational | 216 subjects with PAD and 80 subjects without PAD as a control | In subjects with PAD, a significantly higher fibrinogen plasma concentration was demonstrated (417 mg/dL (367–467 mg/dL) vs. 355.5 mg/dL (302.25–362 mg/dL), p < 0.0001). Plasma fibrinogen concentration was significantly associated with the risk of PAD (OR = 1.034; 95% CI: 1.005–1.063, p = 0.019). | Fibrinogen plasma concentration have a significant value for the presence of PAD. |
| Samir et al.; 2020 [28] | Observational | 64 ICU patients were divided into acute ischemic stroke patients (group I; n = 32) and non-stroke patients (group II; n = 32) | A significant increase in serum fibrinogen concentrations was noticed in group I (p < 0.001) with a cutoff value ≥439 mg/dL showing sensitivity of 92.31%, specificity of 75.36%, and accuracy of 84.34% for stroke occurrence. A cutoff value ≥557 mg/dL showed sensitivity of 85.71%, specificity of 96%, and an accuracy of 93.75% for mortality in this group. | High-serum fibrinogen concentrations in high-risk individuals may be used as a predictor for the occurrence of acute ischemic stroke and mortality from stroke. |
| Song et al.; 2020 [29] | Prospective, observational | 1211 subjects with NSTEMI acute coronary syndromes undergoing PCI | Showed that increased baseline fibrinogen plasma concentrations were an independent predictor of death/nonfatal reinfarction (HR = 1.498; 95% CI: 1.030–2.181, p = 0.035). | Fibrinogen plasma concentrations is an independent predictor of death/nonfatal reinfarction in NSTEMI subjects undergoing PCI, and its accuracy is similar to that of the GRACE system. |
| Liu et al.; 2020 [30] | Retrospective, observational | 5237 patients with stable CAD | FBG and HbA1c were positively associated with fibrinogen plasma concentrations in overall CAD subjects, either with or without DM (all p < 0.001). High fibrinogen plasma concentrations were independently associated with MACEs after adjusting for confounding factors (HR = 1.57; 95% CI: 1.26–1.97, p < 0.001). | Fibrinogen plasma concentrations were associated with FBG and HbA1c in stable CAD subjects. Moreover, increased fibrinogen plasma concentrations were independently associated with a risk of MACEs in CAD subjects, especially among those with DM and pre-DM. |
| Jiang et al.; 2019 [31] | Prospective, observational | 6293 patients undergoing PCI | The 2-year all-cause mortality rate was 1.2%. Patients with higher plasma fibrinogen concentrations died more frequently than those with low or moderate levels (1.7% vs. 0.9% and 1.7% vs. 1.0%, respectively; p = 0.022). Fibrinogen was significantly associated with risk of all-cause mortality (HR = 1.339; 95% CI: 1.109–1.763, p = 0.005). | High-fibrinogen plasma concentrations were associated with a worse prognosis in subjects after PCI. |
| Zhang et al.; 2019 [32] | Prospective, observational | 411 ACS patients undergoing PCI (103 subjects with DM and 308 subjects with non-DM) | Patients with DM had higher plasma concentrations of fibrinogen than patients without DM (3.56 ± 0.99 mg/dL vs. 3.34 ± 0.80 mg/dL, p < 0.05). HbA1c and FBG were significantly positively correlated with fibrinogen in patients with DM, but not in subjects without DM (all p < 0.05). Increased plasma fibrinogen concentration was significantly associated with a higher risk of MACE only in patients with DM (HR = 7.783; 95% CI: 1.012–59.854, p = 0.049). | Fibrinogen was positively associated with glucose metabolism in DM populations with ACS. Moreover, elevated baseline fibrinogen plasma concentrations may be an important and independent predictor of MACEs following PCI, especially amongst those with DM. |
| Chen et al.; 2018 [33] | Cross-sectional | 1096 T2DM patients | Patients with PAD had higher serum fibrinogen concentrations than non-PAD group (p < 0.001). Higher fibrinogen quartiles were positively related with the development of PAD— Tercile 2 (3.02–3.65 g/L): OR = 1.993; 95% CI: 1.322–3.005, p < 0.001; Tercile 3 (3.66–4.55 g/L): OR = 2.469; 95% CI: 1.591–3.831, p < 0.001; Tercile 4 (≥4.56 g/L): OR = 2.942; 95% CI: 1.838–4.711, p < 0.001. | Serum fibrinogen concentrations were an independent risk factor for PAD in patients with T2DM. |
| Gao et al.; 2017 [34] | Observational | 418 males with myocardial infraction who were under 35 years old | Positive correlation between plasma fibrinogen concentration and GS was found (p < 0.001). The best cut-off level for plasma fibrinogen concentration predicting the severity of coronary stenosis was 3.475 g/L (sensitivity 64%; specificity 70%). Plasma fibrinogen concentration was also independently associated with high GS (OR = 2.173; 95% CI: 1.011–4.670, p = 0.047). | Plasma fibrinogen concentration is significantly associated with the presence and severity of coronary artery stenosis in men under 35 years of age with MI. |
| Tabakci et al.; 2017 [35] | Observational | 134 subjects with stable CAD | Strong correlation between fibrinogen plasma concentrations and the SS (r = 0.535, p < 0.001). Fibrinogen plasma concentrations higher than 411 mg/dL had a sensitivity of 75% and a specificity of 64% in the prediction of high SS. Plasma fibrinogen concentrations were an independent predictor for high SS in subjects with stable CAD (OR = 1.01; 95% CI: 1.01–1.02, p < 0.001). | Plasma fibrinogen concentrations were independently associated with severity and complexity of CAD. |
| Yang et al.; 2017 [36] | Prospective, observational | 1466 subjects with T2DM and angiographically proven stable CAD | Patients who had high plasma fibrinogen concentration (≥3.51 g/L) had a significantly higher risk of CVD by 102% (HR = 2.02; 95% CI: 1.11–3.68, p = 0.049). | Elevated fibrinogen plasma concentrations were independently associated with higher risk of CVD. |
| Peng et al.; 2017 [37] | Retrospective, observational | 2253 patients with acute coronary syndrome confirmed by coronary angiography | Cumulative survival curves indicated that the risk of all-cause death increased with increasing plasma fibrinogen concentration (mortality rates for Tercile 1 vs. Tercile 2 vs. Tercile 3 = 6.6% vs. 10.8% vs. 12.3%, p < 0.001). Similar trends were observed for CVD death, although the differences between terciles were not statistically significant (cardiac mortality rates for Tercile 1 vs. Tercile 2 vs. Tercile 3 = 4.6% vs. 6.3% vs. 6.4%, p = 0.206). HR for all-cause mortality and cardiac mortality across terciles (3 vs. 1) of fibrinogen: 1.96; 95% CI: 1.39–2.77 and 1.47; 95% CI: 1.03–2.10. | Plasma fibrinogen concentrations at admission were independently associated with risk of death among subjects with acute MI. |
| Kunutsor et al.; 2016 [38] | Prospective with meta-analysis | 1773 men free of HF or cardiac arrhythmias who recorded 131 SCD for 22 years of follow-up | Men who experienced SCD had a higher plasma fibrinogen concentration (2.93 g/L (92.61–3.30) vs. 3.19 g/L (2.87–3.57), p < 0.0001). Fibrinogen was log-linearly associated with risk of SCD. Hazard ratio for SCD per 1 standard deviation higher baseline loge fibrinogen was 1.32 (95% CI: 1.11–1.57). Meta-analysis of three cohort studies was showed that fully adjusted the relative risks for SCD per 1 standard deviation higher baseline and long-term fibrinogen plasma concentrations were 1.42 (95% CI: 1.25–1.61) and 2.07 (95% CI: 1.59–2.69), respectively. | Fibrinogen plasma concentrations were positively, log-linearly, and independently associated with the risk of SCD. |
| Kotbi et al.; 2016 [39] | Prospective, observational | 120 subjects: 30 non-DM and with CAD, 30 with DM and CAD, 30 non-CAD with DM, and 30 healthy subjects | The plasma fibrinogen concentration increased in parallel with the CVD risk (p = 0.0001); there was also a significant correlation between the plasma fibrinogen concentration and the clinical and para-clinical CAD severity (respectively p = 0.005 and p = 0.0001). | Plasma fibrinogen concentrations were positively and significantly associated with the CAD severity. |
| Peng et al., 2016 [40] | Observational | 3020 subjects with CAD confirmed by coronary angiography | Cumulative survival curves showed that the risk of all-cause mortality was significantly higher in subjects with plasma fibrinogen concentrations ≥3.17 g/L vs. those with < 3.17 g/L (mortality rate, 11.5% vs. 5.7%, p < 0.001); and cardiac mortality rate—5.9% vs. 3.6%, p = 0.002). Plasma fibrinogen concentrations remained independently associated with all-cause mortality after adjustment for multiple CVD risk factors (HR = 2.01; 95% CI 1.51–2.68, p < 0.001). | Plasma fibrinogen concentrations were independently associated with the mortality risk in CAD patients. |
| Peng et al.; 2016 [41] | Observational | 3020 patients with CAD confirmed by coronary angiography | Mortality rates for subjects with CAD and those in the stable CAD and unstable CAD groups exhibited an overall rising trend as fibrinogen plasma concentrations increased (all p < 0.05). Fibrinogen plasma concentrations were independently associated with the risk of death in CAD subjects, as well as those in the stable CAD and unstable CAD groups (CAD, HR = 1.40; 95% CI: 1.16–1.68; stable CAD, HR = 1.86; 95% CI: 1.24–2.79 and unstable CAD, HR = 1.42; 95% CI: 1.06–1.90). In the acute MI group, however, no independent correlation was observed between fibrinogen plasma concentrations and mortality. | The different proportions of subtypes of CAD affected the correlation between fibrinogen plasma concentrations and the clinical prognosis of subjects with CAD. |
| Zhang et al., 2014 [42] | Observational | 2288 new-onset subjects undergoing coronary angiography with angina pain | Subjects with high GS had significantly increased fibrinogen plasma concentrations (p < 0.001). Plasma fibrinogen concentrations were independently associated with high GS (OR = 1.275; 95% CI: 1.082–1.502, p = 0.004) after adjusting for potential confounders. The risk of stenosis (≥75%) was increased with the elevated plasma fibrinogen concentrations: Tercile 2 (2.83–3.38 g/L)—OR = 1.112; 95% CI: 0.887–1.395, p = 0.365. Tercile 3 (> 3.38 g/L)—OR = 1.939; 95% CI: 1.484–2.533, p < 0.001. | Higher fibrinogen plasma concentrations were independently associated with new-onset atherosclerosis in the coronary arteries |
| Hong et al.; 2014 [43] | Observational | 373 subjects with DM and angina pectoris | Plasma fibrinogen concentration was an independent predictor of a high GS for DM subjects (OR = 1.40; 95% CI: 1.04–1.88, p = 0.026) after adjusting for traditional risk factors of CAD. | Plasma fibrinogen concentrations appeared to be an independent predictor for the severity of CAD in DM subjects |
| Bosevski et al.; 2013 [44] | Prospective, observational | 62 patients with T2DM and PAD Follow-up: 36 months | Linear regression analysis defined plasma fibrinogen concentrations as a predictor for endpoint value of ankle-brachial index (β = 0.469, p = 0.007). | Plasma fibrinogen concentrations can be used to evaluate the progression of PAD in subjects with T2DM. |
| Gene | Polymorphism/Mutation | Effect on Cardiovascular Risk | Bibliography |
|---|---|---|---|
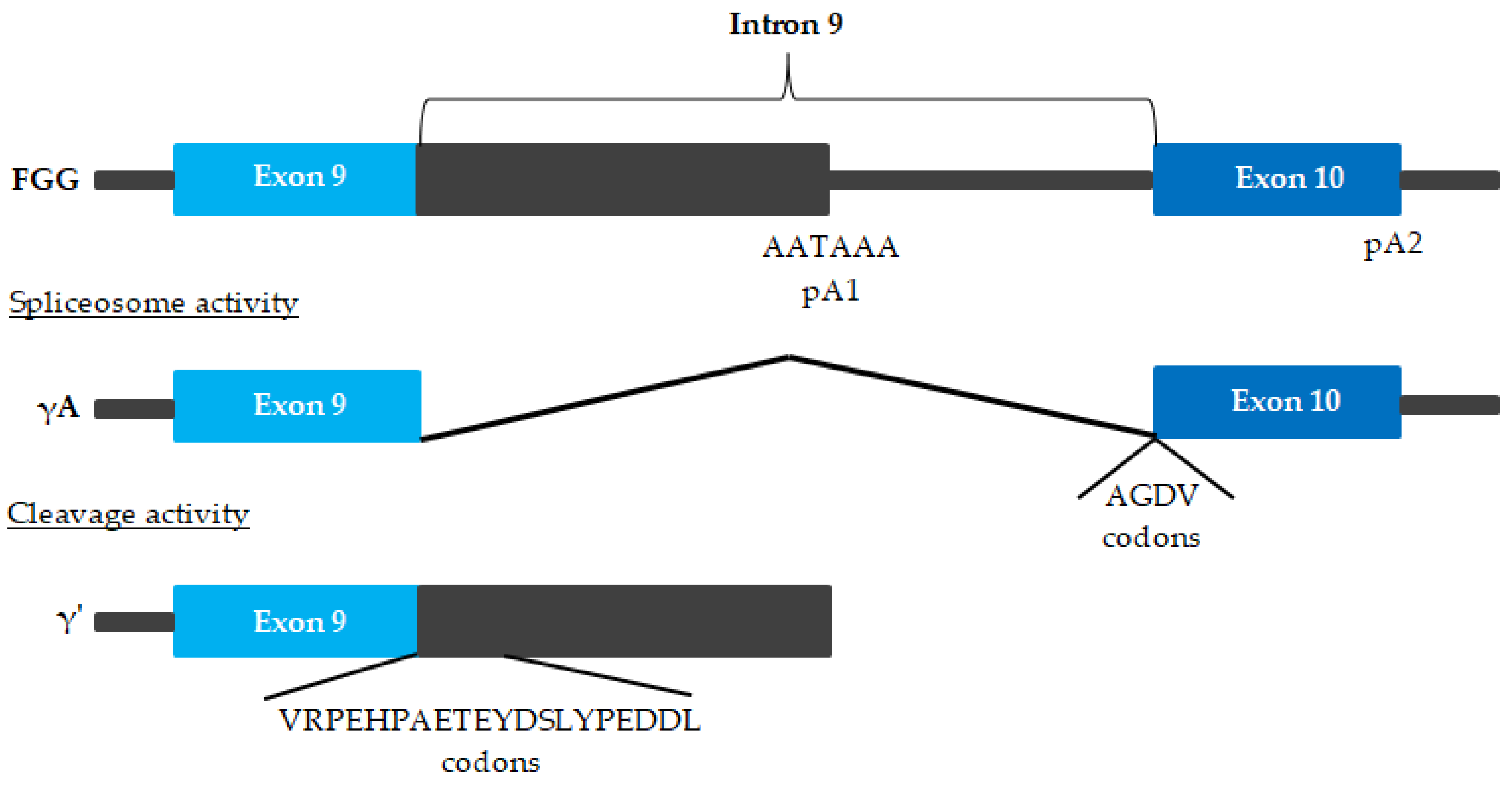
| FGG | γ′ fibrinogen | ↑ myocardial infraction ↑/↔ CVD ↓ venous thromboembolism and ischemic stroke | [67,74,76,77] |
| rs7681423 and rs1049636 | ↔ CVD | [67] | |
| rs2066865 | ↑ microvascular thrombosis | [75] | |
| FGB | -455 G/A | ↔ venous thromboembolism ↓ venous thromboembolism (Caucasians) ↑ ischemic stroke (Asian) ↔ ischemic stroke (Caucasians and children) ↑ cerebral infarction ↑ CAD ↑ cardioembolic stroke | [84,85,86,87,88,89] |
| -148 C/T | ↔ venous thromboembolism ↑ ischemic stroke (Asians and Caucasians) ↑ cerebral infarction ↑ CAD ↑ MACE | [84,85,86] | |
| -1420 (AG + AA) and -148 (CT + TT) | ↑ lower extremity deep venous thrombosis | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surma, S.; Banach, M. Fibrinogen and Atherosclerotic Cardiovascular Diseases—Review of the Literature and Clinical Studies. Int. J. Mol. Sci. 2022, 23, 193. https://doi.org/10.3390/ijms23010193
Surma S, Banach M. Fibrinogen and Atherosclerotic Cardiovascular Diseases—Review of the Literature and Clinical Studies. International Journal of Molecular Sciences. 2022; 23(1):193. https://doi.org/10.3390/ijms23010193
Chicago/Turabian StyleSurma, Stanisław, and Maciej Banach. 2022. "Fibrinogen and Atherosclerotic Cardiovascular Diseases—Review of the Literature and Clinical Studies" International Journal of Molecular Sciences 23, no. 1: 193. https://doi.org/10.3390/ijms23010193
APA StyleSurma, S., & Banach, M. (2022). Fibrinogen and Atherosclerotic Cardiovascular Diseases—Review of the Literature and Clinical Studies. International Journal of Molecular Sciences, 23(1), 193. https://doi.org/10.3390/ijms23010193






