Receptor-Mediated Vascular Smooth Muscle Migration Induced by LPA Involves p38 Mitogen-Activated Protein Kinase Pathway Activation
Abstract
:1. Introduction
2. Results and Discussion
2.1. RASMCs Migration toward LPA
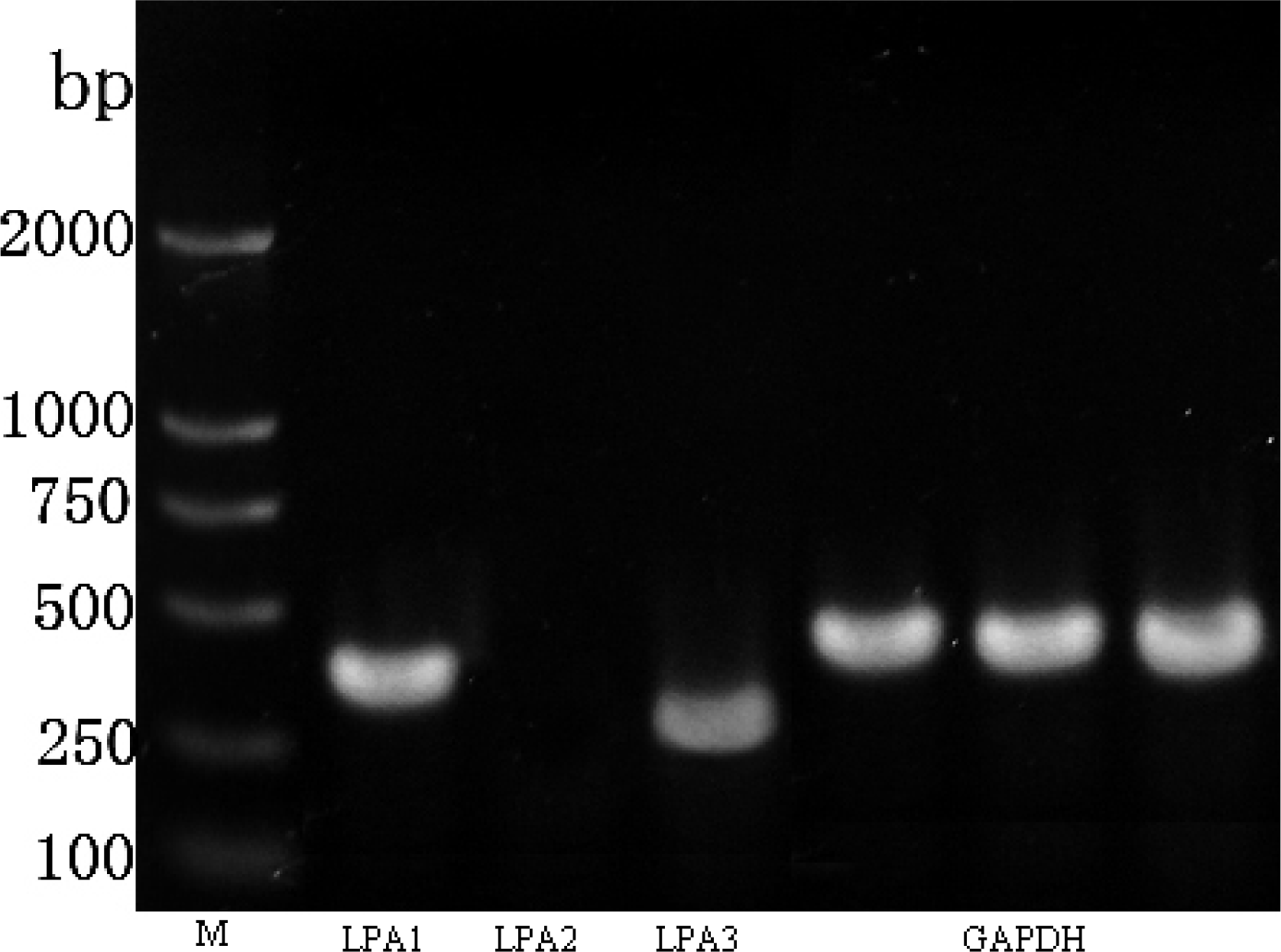
2.2. LPA Receptor Expression
2.3. LPA-Induced Migration in RASMCs Is Mediated by LPA1 Receptor
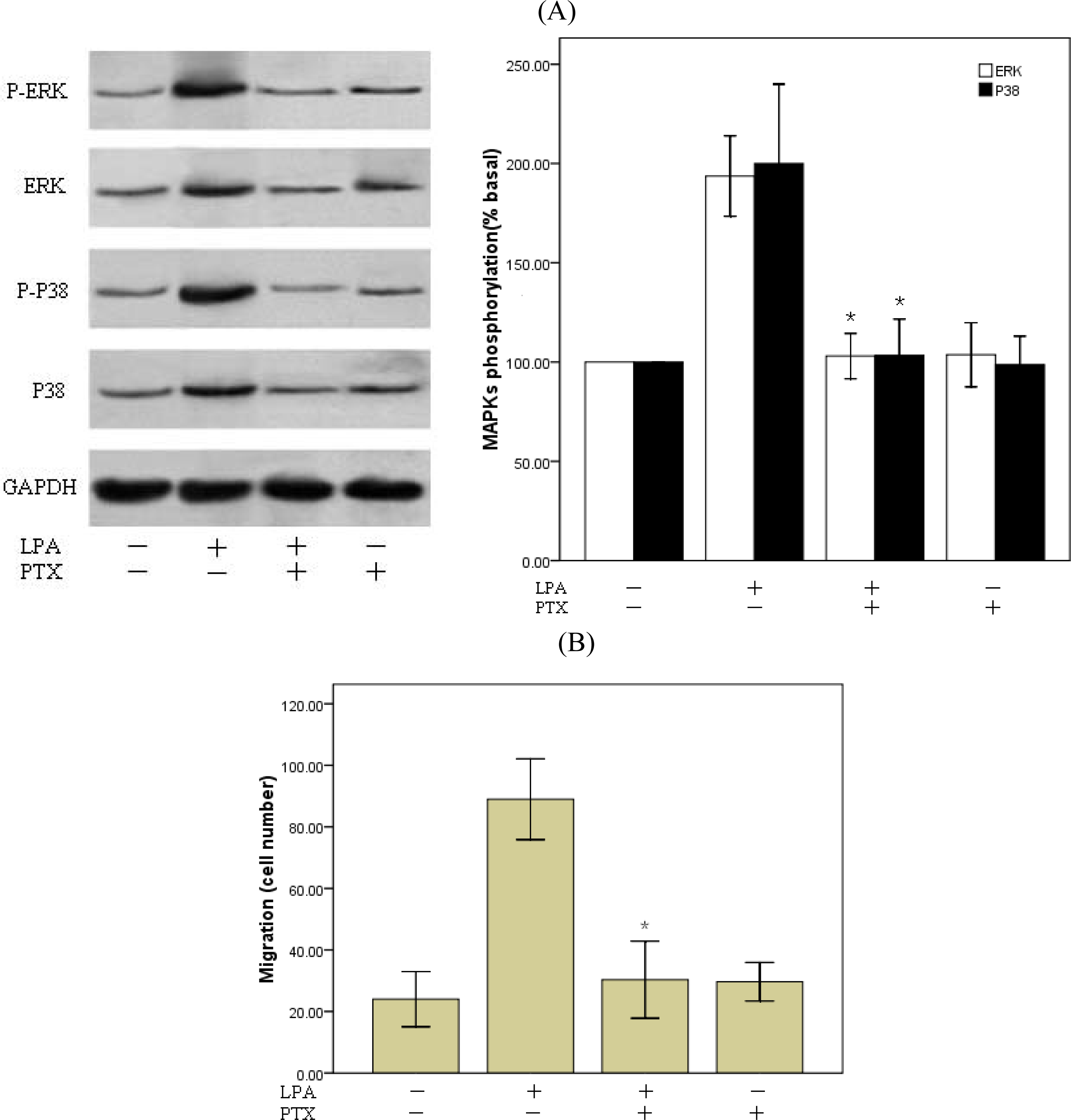
2.4. P38MAPk Pathway Activation Is Required in LPA-Induced Migration
2.5. Involvement of Gi Protein in LPA-Mediated Migration
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Cell Culture
4.3. Determination of LPA Receptor Expression
4.4. Cell Migration Assay
4.5. Measurement of ERK and p38MAPK Activation
4.6. Statistical Analysis
Acknowledgments
References and Notes
- Moolenaar, WH. Development of our current understanding of bioactivelysophospholipids. Ann. NY Acad. Sci 2000, 905, 1–10. [Google Scholar]
- van Corven, EJ; Groenink, A; Jalink, K; Eichholtz, T; Moolenaar, WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell 1989, 59, 45–54. [Google Scholar]
- Moolenaar, WH. Mitogenic action of lysophosphatidic acid. Adv. Cancer Res 1991, 57, 87–102. [Google Scholar]
- Gerrard, JM; Clawson, CC; White, JG. Lysophosphatidic acids: III. Enhancement of neutrophil chemotaxis. Am. J. Pathol 1980, 100, 609–618. [Google Scholar]
- Lümmen, G; Virchow, S; Rümenapp, U; Schmidt, M; Wieland, T; Otto, T; Rübben, H; Jakobs, KH. Identification of G protein-coupled receptors potently stimulating migration of human transitional-cell carcinoma cells. Naunyn Schmiedebergs Arch. Pharmacol 1997, 356, 769–776. [Google Scholar]
- Durieux, ME; Lynch, KR. Signalling properties of lysophosphatidic acid. Trends Pharmacol. Sci 1993, 14, 249–254. [Google Scholar]
- Swarthout, JT; Walling, HW. Lysophosphatidic acid: receptors, signaling and survival. Cell Mol. Life Sci 2000, 57, 1978–1985. [Google Scholar]
- Ye, X; Ishii, I; Kingsbury, MA; Chun, J. Lysophosphatidic acid as a novel cell survival/apoptotic factor. Biochim. Biophys. Acta 2002, 1585, 108–113. [Google Scholar]
- Gerrard, JM; Kindom, SE; Peterson, DA; Peller, J; Krantz, KE; White, JG. Lysophosphatidic acids. Influence on platelet aggregation and intracellular calcium flux. Am. J. Pathol 1979, 96, 423–438. [Google Scholar]
- Sandmann, G; Siess, W; Essler, M. Lysophosphatidic acid is the unique platelet-activating substance in human malignant ascites. Eur. J. Med. Res 2003, 8, 397–404. [Google Scholar]
- Toews, ML; Ustinova, EE; Schultz, HD. Lysophosphatidic acid enhances contractility of isolated airway smooth muscle. J. Appl. Physiol 1997, 83, 1216–1222. [Google Scholar]
- Mori, M; Tsushima, H. Activation of Rho signaling contributes tolysophosphatidic acid-induced contraction of intact ileal smooth muscle of guinea-pig. Can. J. Physiol. Pharmacol 2000, 78, 729–736. [Google Scholar]
- Jalink, K; Eichholtz, T; Postma, FR; van Corven, EJ; Moolenaar, WH. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ 1993, 4, 247–255. [Google Scholar]
- Tigyi, G; Fischer, DJ; Sebok, A; Yang, C; Dyer, DL; Miledi, R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and Rho. J. Neurochem 1996, 66, 537–548. [Google Scholar]
- Manning, TJ, Jr; Rosenfeld, SS; Sontheimer, H. Lysophosphatidic acid stimulates actomyosin contraction in astrocytes. J. Neurosci. Res 1998, 53, 343–352. [Google Scholar]
- Weiner, JA; Hecht, JH; Chun, J. Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J. Comp. Neurol 1998, 398, 587–598. [Google Scholar]
- Li, Y; González, MI; Meinkoth, JL; Field, J; Kazanietz, MG; Tennekoon, GI. Lysophosphatidic acid promotes survival and differentiation of rat Schwann cells. J. Biol. Chem 2003, 278, 9585–9591. [Google Scholar]
- Hecht, JH; Weiner, JA; Post, SR; Chun, J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell Biol 1996, 135, 1071–1083. [Google Scholar]
- Yoshida, A; Ueda, H. Neurobiology of the Edg2 lysophosphatidic acid receptor. Jpn. J. Pharmacol 2001, 87, 104–109. [Google Scholar]
- Shiono, S; Kawamoto, K; Yoshida, N; Kondo, T; Inagami, T. Neurotransmitter release from lysophosphatidic acid stimulated PC12 cells: involvement of lysophosphatidic acid receptors. Biochem. Biophys. Res. Commun 1993, 193, 667–673. [Google Scholar]
- Chun, J; Goetzl, EJ; Hla, T; Igarashi, Y; Lynch, KR; Moolenaar, W; Pyne, S; Tigyi, G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev 2002, 54, 265–269. [Google Scholar]
- Contos, JJ; Ishii, I; Chun, J. Lysophosphatidic acid receptors. Mol. Pharmacol 2000, 58, 1188–1196. [Google Scholar]
- Fukushima, N; Ishii, I; Contos, JJ; Weiner, JA; Chun, J. Lysophospholipid receptors. Annu. Rev. Pharmacol. Toxicol 2001, 41, 507–534. [Google Scholar]
- Noguchi, K; Ishii, S; Shimizu, T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J. Biol. Chem 2003, 278, 25600–25606. [Google Scholar]
- Kotarsky, K; Boketoft, A; Bristulf, J; Nilsson, NE; Norberg, A; Hansson, S; Owman, C; Sillard, R; Leeb-Lundberg, LM; Olde, B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J. Pharmacol. Exp. Ther 2006, 318, 619–628. [Google Scholar]
- Lee, CW; Rivera, R; Gardell, S; Dubin, AE; Chun, J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J. Biol. Chem 2006, 281, 23589–23597. [Google Scholar]
- Tabata, K; Baba, K; Shiraishi, A; Ito, M; Fujita, N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun 2007, 363, 861–866. [Google Scholar]
- Yang, AH; Ishii, I; Chun, J. In vivo roles of lysophospholipid receptors revealed by gene targeting studies in mice. Biochim. Biophys. Acta 2002, 1582, 197–203. [Google Scholar]
- Hilal-Dandan, R; Means, CK; Gustafsson, AB; Morissette, MR; Adams, JW; Brunton, LL; Heller-Brown, J. Lysophosphatidic acid induces hypertrophy of neonatal cardiac myocytes via activation of Gi and Rho. J. Mol. Cell Cardiol 2004, 36, 481–93. [Google Scholar]
- Ross, R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar]
- Ai, S; Kuzuya, M; Koike, T; Asai, T; Kanda, S; Maeda, K; Shibata, T; Iguchi, A; Satake, S; Ramos, MA; Miura, H; Ueda, M. Rho-Rho kinase is involved in smooth muscle cell migration through myosin light chain phosphorylation-dependent and independent pathways. Inhibition of angiogenesis on glycated collagen lattices. Atherosclerosis 2001, 155, 321–327. [Google Scholar]
- Yoshida, K; Nishida, W; Hayashi, K; Ohkawa, Y; Ogawa, A; Aoki, J; Arai, H; Sobue, K. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation 2003, 108, 1746–1752. [Google Scholar]
- Panchatcharam, M; Miriyala, S; Yang, F; Rojas, M; End, C; Vallant, C; Dong, A; Lynch, K; Chun, J; Morris, AJ; Smyth, SS. Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ. Res 2008, 103, 662–670. [Google Scholar]
- Damirin, A; Tomura, H; Komachi, M; Liu, JP; Mogi, C; Tobo, M; Wang, JQ; Kimura, T; Kuwabara, A; Yamazaki, Y; Ohta, H; Im, DS; Sato, K; Okajima, F. Role of lipoprotein-associated lysophospholipids in migratory activity of coronary artery smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol 2007, 292, H2513–2522. [Google Scholar]
- Liu, B; Ryer, EJ; Kundi, R; Kamiya, K; Itoh, H; Faries, PL; Sakakibara, K; Kent, KC. Protein kinase C-δ regulates migration and proliferation of vascular smooth muscle cells through the extracellular signal regulated kinase 1/2. J. Vasc. Surg 2007, 45, 160–168. [Google Scholar]
- Wei, CY; Chou, YH; Ho, FM; Hsieh, SL; Lin, WW. Signaling pathways of LIGHT induced macrophage migration and vascular smooth muscle cell proliferation. J. Cell Physiol 2006, 209, 735–743. [Google Scholar]
- Hayashi, K; Takahashi, M; Nishida, W; Yoshida, K; Ohkawa, Y; Kitabatake, A; Aoki, J; Arai, H; Sobue, K. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ. Res 2001, 89, 251–258. [Google Scholar]
- Fischer, DJ; Nusser, N; Virag, T; Yokoyama, K; Wang, DA; Baker, DL; Bautista, D; Parrill, AL; Tigyi, G. Short-chain phosphatidates are subtype-selective antagonists of lysophosphatidic acid receptors. Mol. Pharmacol 2001, 60, 776–784. [Google Scholar]
- Moolenaar, WH; Kranenburg, O; Postma, FR; Zondag, GC. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr. Opin. Cell Biol 1997, 9, 168–173. [Google Scholar]
- Siess, W. Athero-and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim. Biophys. Acta 2002, 1582, 204–215. [Google Scholar]
- Rother, E; Brandl, R; Baker, DL; Goyal, P; Gebhard, H; Tigyi, G; Siess, W. Subtype-selective antagonists of lysophosphatidic Acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation 2003, 108, 741–747. [Google Scholar]
- Anliker, B; Chun, J. Lysophospholipid G Protein-coupled Receptors. J. Biol. Chem 2004, 279, 20555–20558. [Google Scholar]
- Hirshman, CA; Emala, CW. Actin reorganization in airway smooth muscle cells involves Gq and Gi-2 activation of Rho. Am. J. Physiol 1999, 277, L653–661. [Google Scholar]
- Blanc, A; Pandey, NR; Srivastava, AK. Synchronous activation of ERK 1/2, p38 MAPK and PKB/Akt signaling by H2O2 in vascular smooth muscle cells: potential involvement in vascular disease. Int. J. Mol. Med 2003, 11, 229–234. [Google Scholar]
- Hauck, CR; Hsia, DA; Schlaepfer, DD. Focal adhesion kinase facilitates platelet-derived growth factor-BB-stimulated ERK2 activation required for chemotaxis migration of vascular smooth muscle cells. J. Biol. Chem 2000, 275, 41092–41099. [Google Scholar]
- Krymskaya, VP; Goncharova, EA; Ammit, AJ; Lim, PN; Goncharov, DA; Eszterhas, A; Panettieri, RA, Jr. Src is necessary and sufficient for human airway smooth muscle cell proliferation and migration. FASEB J 2005, 19, 428–430. [Google Scholar]
- Matsumoto, T; Yokote, K; Tamura, K; Takemoto, M; Ueno, H; Saito, Y; Mori, S. Platelet-derived growth factor activates p38 mitogen-activated protein kinase through a Ras-dependent pathway that is important for actin reorganization and cell migration. J. Biol. Chem 1999, 274, 13954–13960. [Google Scholar]
- Bakin, AV; Rinehart, C; Tomlinson, AK; Arteaga, CL. p38 mitogenactivated protein kinase is required for TGF beta-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci 2002, 115, 3193–3206. [Google Scholar]
- Hedges, JC; Dechert, MA; Yamboliev, IA; Martin, JL; Hickey, E; Weber, LA; Gerthoffer, WT. A role for p38 MAPK/HSP27 pathway in smooth muscle cell migration. J. Biol. Chem 1999, 274, 24211–24219. [Google Scholar]
- Kozawa, O; Tanabe, K; Ito, H; Matsuno, H; Niwa, M; Kato, K; Uematsu, T. Sphingosine 1-phosphate regulates heat shock protein 27 induction by a p38 MAP kinase-dependent mechanism in aortic smooth muscle cells. Exp. Cell Res 1999, 250, 376–380. [Google Scholar]
- Wang, XQ; Lindberg, FP; Frazier, WA. Integrin-associated protein stimulates alpha2beta1-dependent chemotaxis via Gi-mediated inhibition of adenylate cyclase and extracellular-regulated kinases. J. Cell Biol 1999, 147, 389–400. [Google Scholar]
- Rolfe, BE; Worth, NF; World, CJ; Campbell, JH; Campbell, GR. Rho and vascular disease. Atherosclerosis 2005, 183, 1–16. [Google Scholar]
- Ryu, Y; Takuwa, N; Sugimoto, N; Sakurada, S; Usui, S; Okamoto, H; Matsui, O; Takuwa, Y. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ. Res 2002, 90, 325–332. [Google Scholar]
- Arikawa, K; Takuwa, N; Yamaguchi, H; Sugimoto, N; Kitayama, J; Nagawa, H; Takehara, K; Takuwa, Y. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor. Requirement of inhibition of cellular RAC activity. J. Biol. Chem 2003, 278, 32841–32851. [Google Scholar]
- Lepley, D; Paik, JH; Hla, T; Ferrer, F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res 2005, 65, 3788–3795. [Google Scholar]
- Eguchi, S; Hirata, Y; Imai, T; Kanno, K; Marumo, F. Phenotypic change of endothelin receptor subtype in cultured rat vascular smooth muscle cells. Endocrinology 1994, 134, 222–228. [Google Scholar]
- Saito, S; Frank, GD; Motley, ED; Dempsey, PJ; Utsunomiya, H; Inagami, T; Eguchi, S. Metalloprotease inhibitor blocks angiotensin II-induced migration through inhibition of epidermal growth factor receptor transactivation. Biochem. Biophys. Res. Commun 2002, 294, 1023–1029. [Google Scholar]






© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhou, Z.-B.; Niu, J.-P.; Zhang, Z.-J. Receptor-Mediated Vascular Smooth Muscle Migration Induced by LPA Involves p38 Mitogen-Activated Protein Kinase Pathway Activation. Int. J. Mol. Sci. 2009, 10, 3194-3208. https://doi.org/10.3390/ijms10073194
Zhou Z-B, Niu J-P, Zhang Z-J. Receptor-Mediated Vascular Smooth Muscle Migration Induced by LPA Involves p38 Mitogen-Activated Protein Kinase Pathway Activation. International Journal of Molecular Sciences. 2009; 10(7):3194-3208. https://doi.org/10.3390/ijms10073194
Chicago/Turabian StyleZhou, Zhi-Bin, Jian-Ping Niu, and Zhi-Jun Zhang. 2009. "Receptor-Mediated Vascular Smooth Muscle Migration Induced by LPA Involves p38 Mitogen-Activated Protein Kinase Pathway Activation" International Journal of Molecular Sciences 10, no. 7: 3194-3208. https://doi.org/10.3390/ijms10073194
APA StyleZhou, Z.-B., Niu, J.-P., & Zhang, Z.-J. (2009). Receptor-Mediated Vascular Smooth Muscle Migration Induced by LPA Involves p38 Mitogen-Activated Protein Kinase Pathway Activation. International Journal of Molecular Sciences, 10(7), 3194-3208. https://doi.org/10.3390/ijms10073194



