Effect of ?-Cyclodextrin Complexation on Solubility and Enzymatic Conversion of Naringin
Abstract
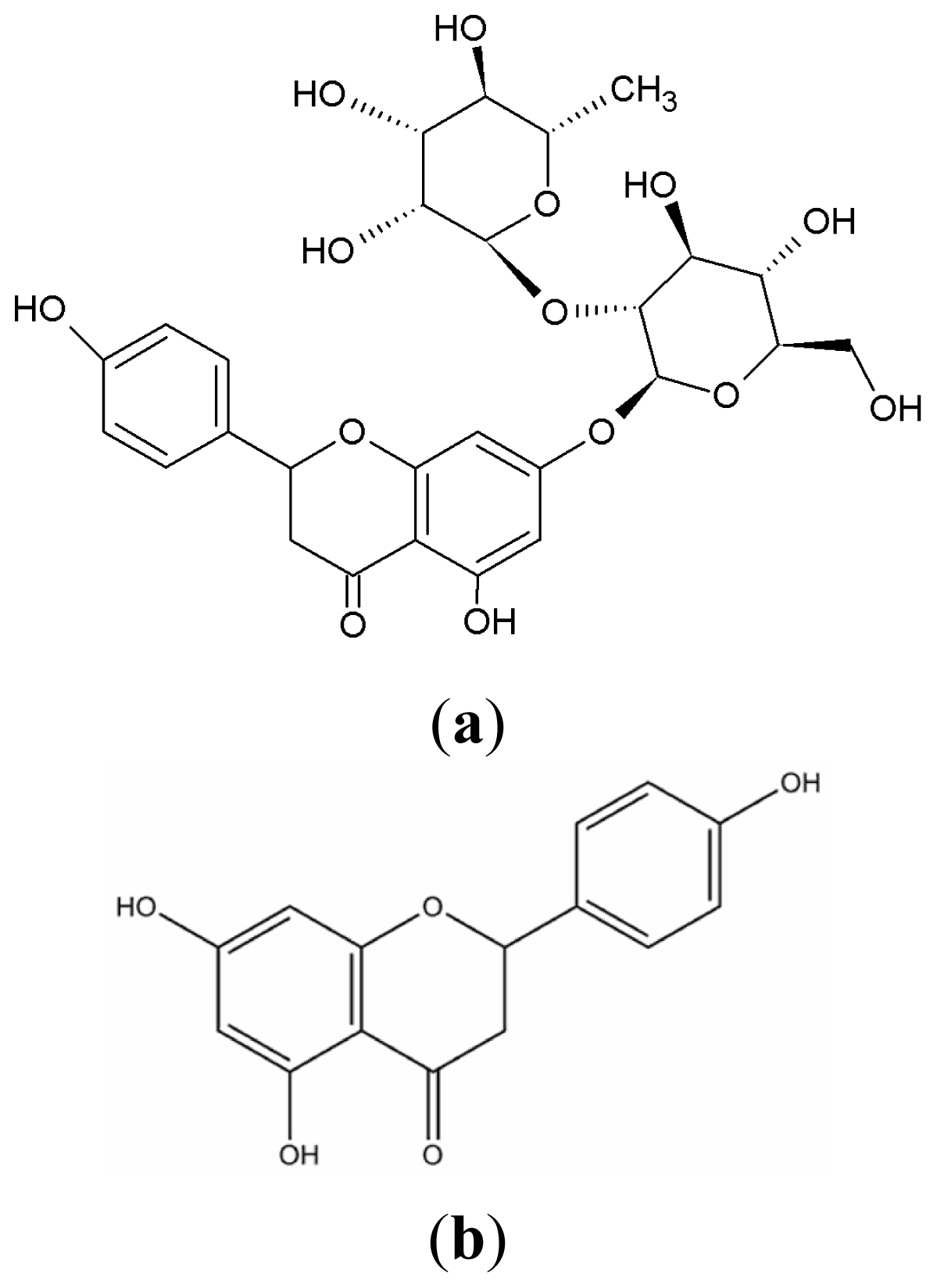
:1. Introduction
2. Results and Discussion
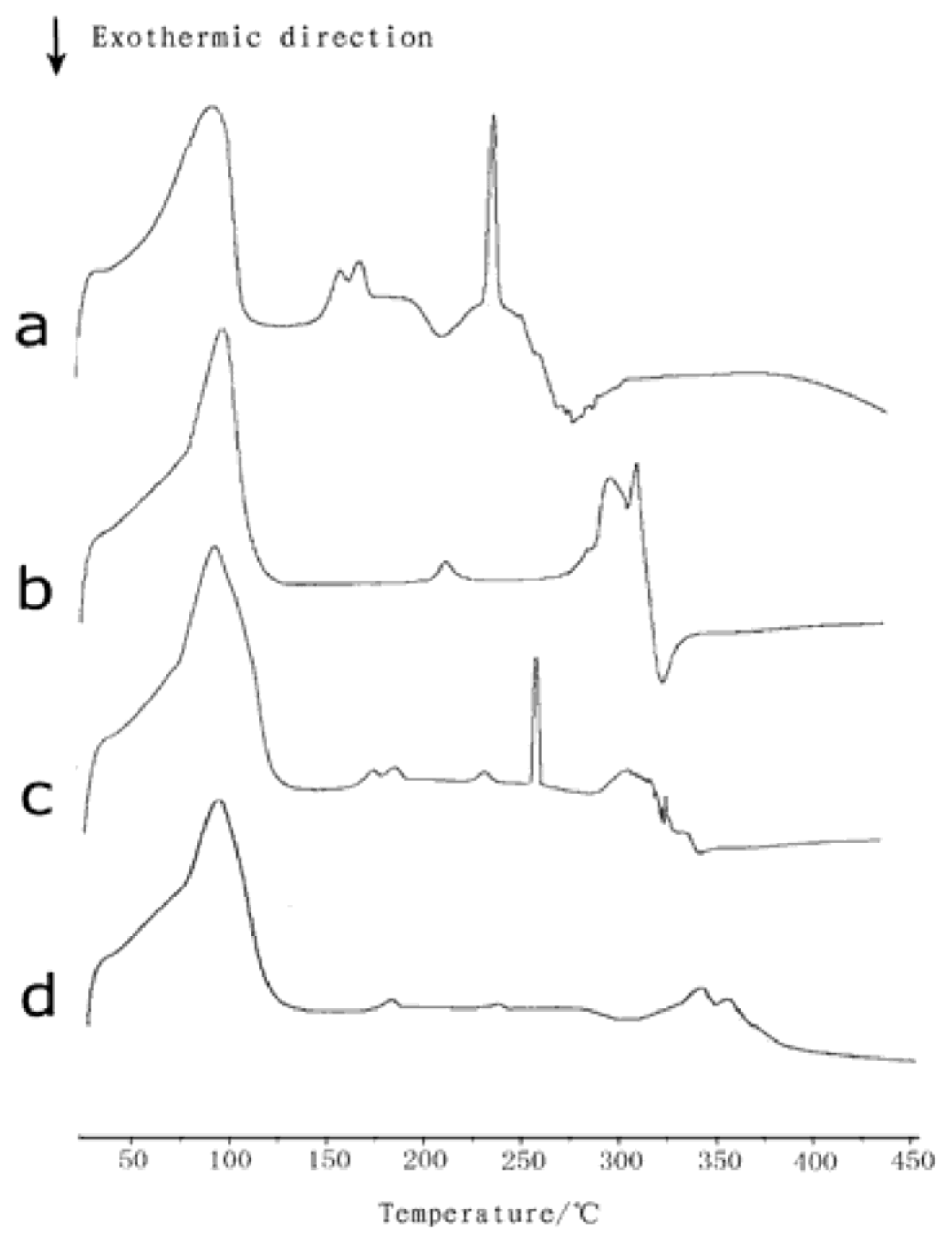
2.1. Differential Scanning Calorimetry
2.2. Analytical Method Validation
2.3. Solubility of Inclusion Complex
2.4. Univariate Experiments
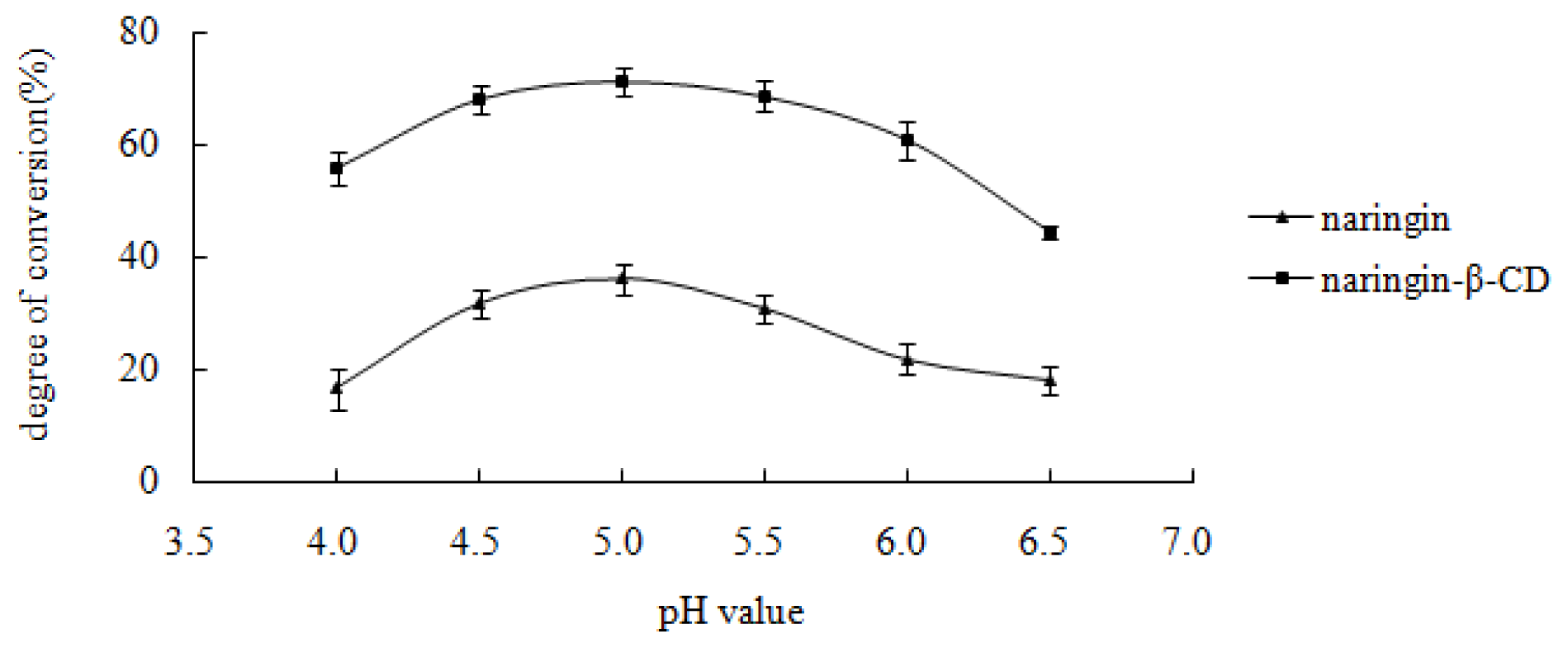
2.4.1. Effect of pH Value on Enzymatic Hydrolysis of the Inclusion Complex and Free Naringin
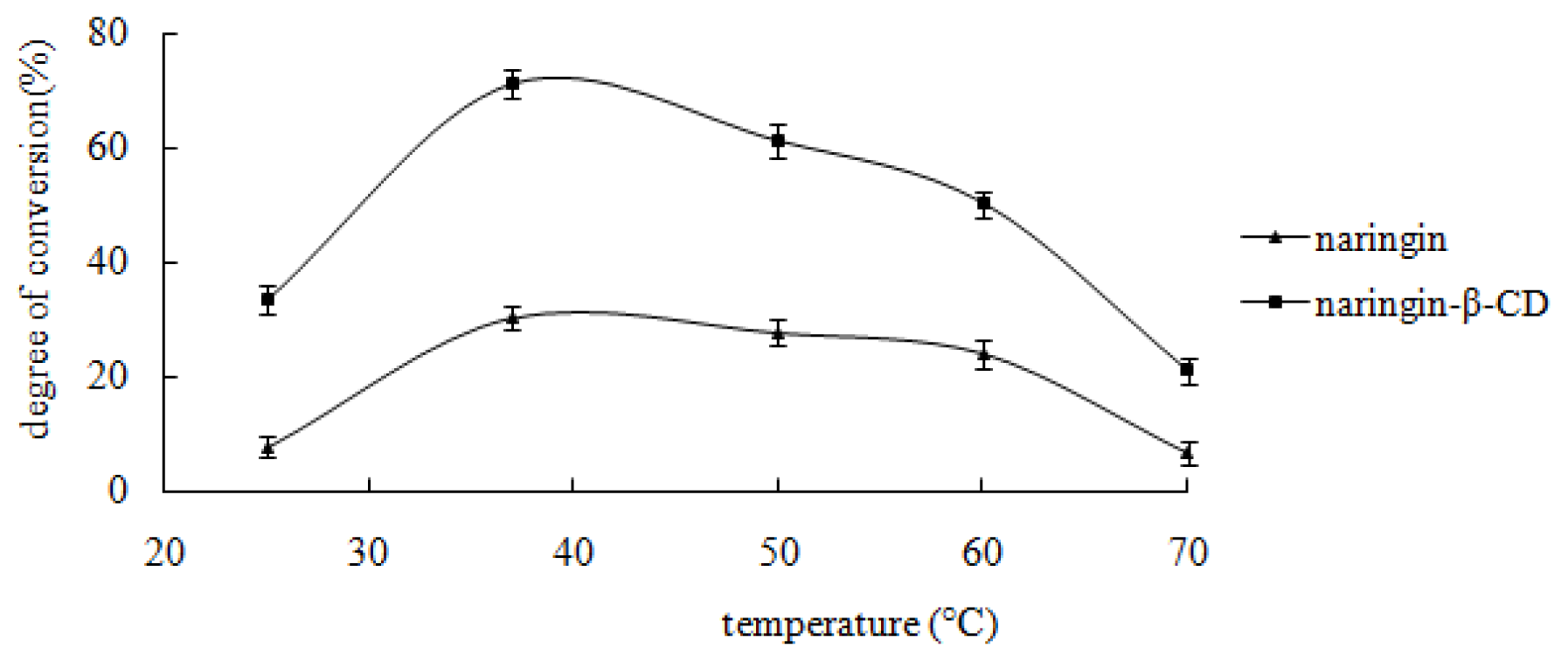
2.4.2. Effect of Temperature on Enzymatic Hydrolysis of the Inclusion Complex and Free Naringin
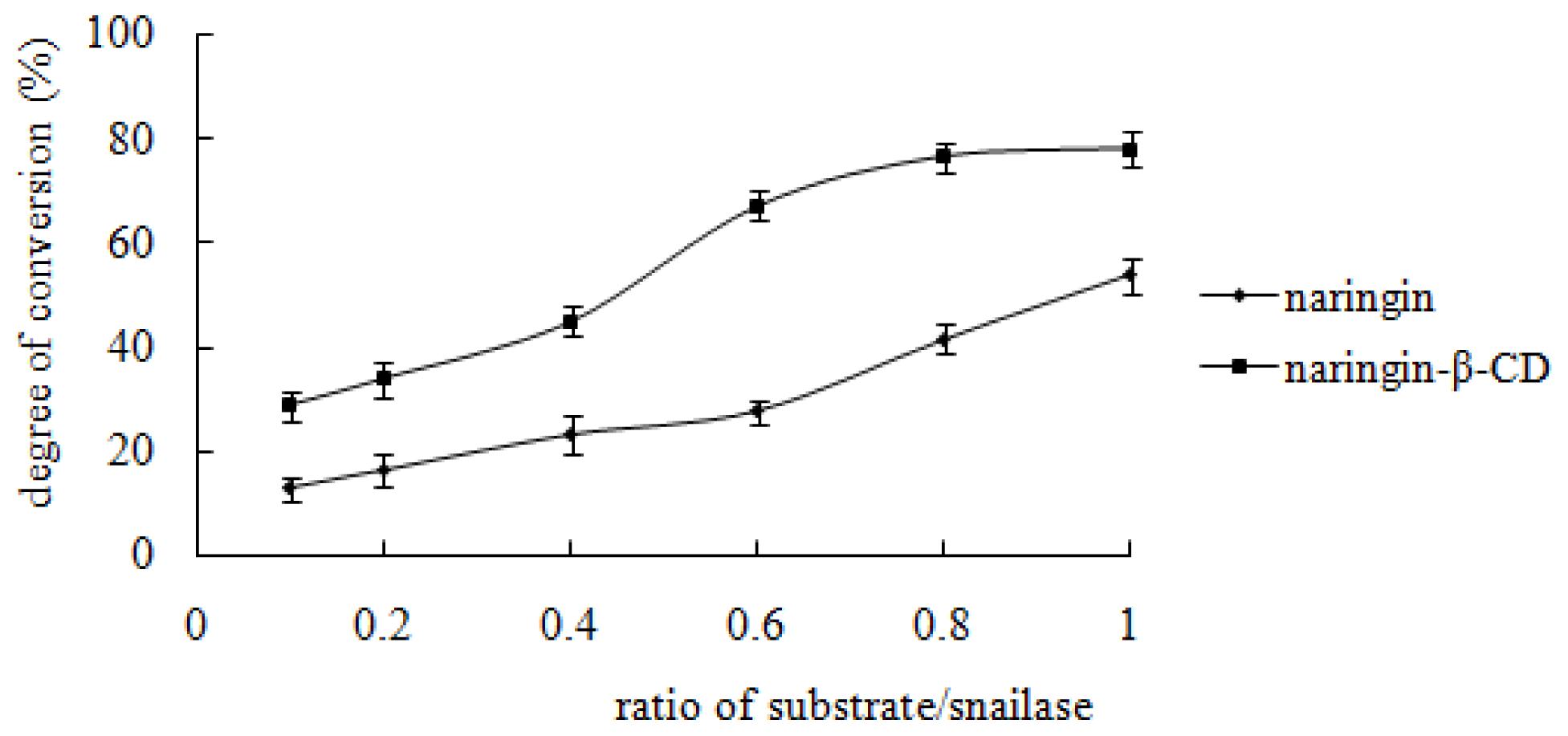
2.4.3. Effect of Snailase/Substrate Ratio on Enzymatic Hydrolysis of the Inclusion Complex and Free Naringin
2.4.4. Effect of the Substrate Concentration on Enzymatic Hydrolysis of the Inclusion Complex and Free Naringin
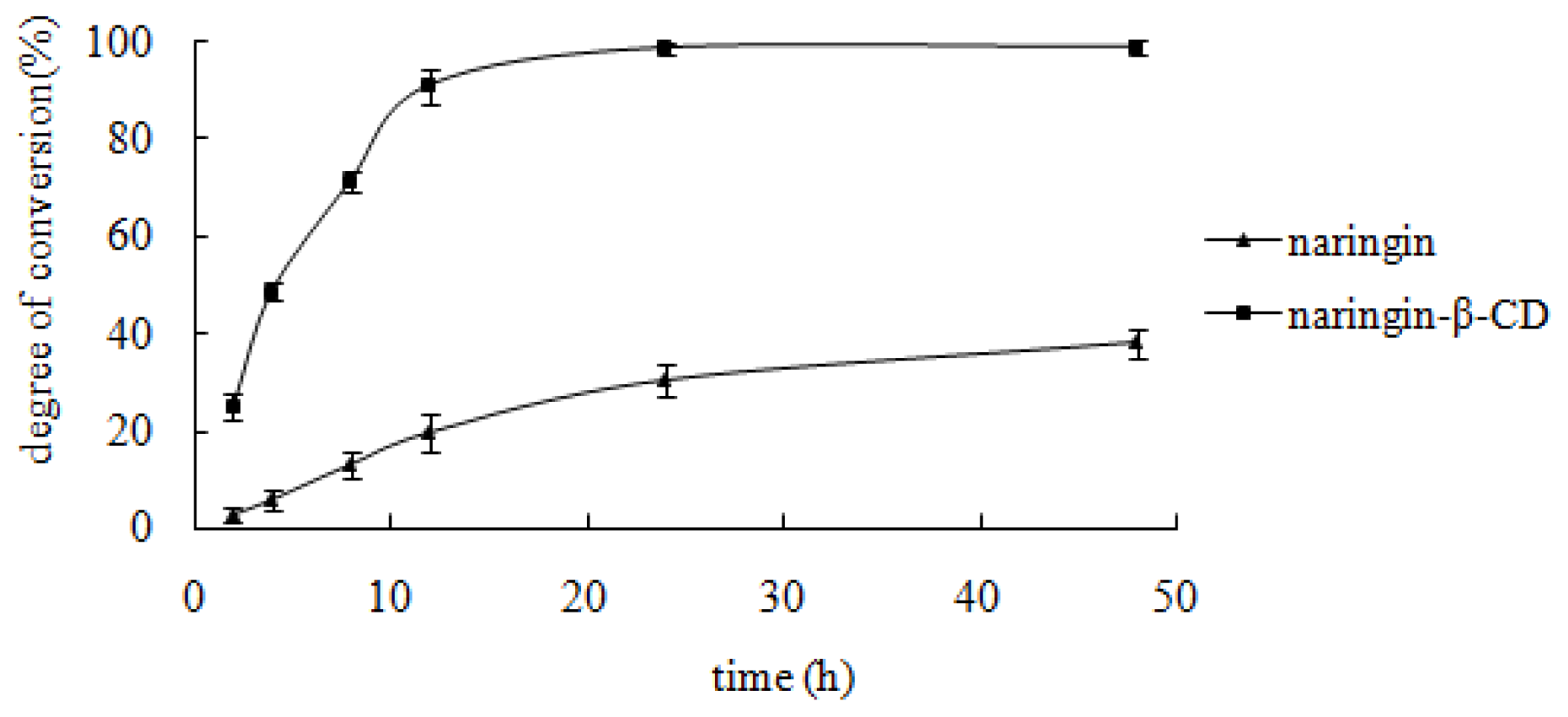
2.4.5. Effect of the Reaction Time on Enzymatic Hydrolysis of the Inclusion Complex and Free Naringin
3. Experimental Section
3.1. Materials and Equipment
3.2. Preparation and Characterization of Inclusion Complex of Naringin
3.2.1. Preparation of Inclusion Complex
3.2.2. Preparation of the Physical Mixture of Naringin and β-CD
3.2.3. Differential Scanning Calorimetry (DSC)
3.3. Analytical Method Validation
3.3.1. The Calibration Curve of Naringin
3.3.2. The Calibration Curve of Naringenin
3.4. Calculation of Conversion
3.5. Solubility of Inclusion Complex
3.6. Univariate Experiment for Naringin-β-CD and Naringin
4. Conclusions
Acknowledgments
References
- Ali, G.; Hawa, Z.E.J.; Asmah, R. Synthesis of Phenolics and Flavonoids in Ginger (Zingiber officinale Roscoe) and Their Effects on Photosynthesis Rate. Int. J. Mol. Sci 2010, 11, 4539–4555. [Google Scholar]
- Raja, N.Z.R.A.R.; Iffah, I.Z.; Abu, B.S.; Mahiran, B. Enzymatic Properties and Mutational Studies of Chalcone Synthase from Physcomitrella patens. Int. J. Mol. Sci. 2012, 13, 9673–9691. [Google Scholar]
- Chen, H.J.; Baskaran, S.I.; Chen, B.H. Determination of Phenolic Acids and Flavonoids in Taraxacum formosanum Kitam by Liquid Chromatography-Tandem Mass Spectrometry Coupled with a Post-Column Derivatization Technique. Int. J. Mol. Sci 2012, 13, 260–285. [Google Scholar]
- Badary, O.A.; Maksoud, S.A.; Ahmed, W.A.; Owieda, G.H. Naringenin attenuates cisplatin nephrotoxicity in rats. Life Sci 2005, 76, 2125–2135. [Google Scholar]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res 2004, 2, 851–874. [Google Scholar]
- Isabel, A.C.; Maria, H.L.R. Kinetic modelling of naringin hydrolysis using a bitter sweet alfa-rhamnopyranosidase immobilized in k-carrageenan. J. Mol. Catal. B 2008, 51, 10–18. [Google Scholar]
- Vila Real, H.J.; Alfaia, A.J.; Calado, A.R.T.; Ribeiro, M.H.L. High pressure-temperature effects on enzymatic activity:Naringin bioconversion. Food Chem 2007, 102, 565–570. [Google Scholar]
- Pedro, H.A.L.; Alfaia, A.J.; Marques, J.; Vila-Real, H.J.; Calado, A.; Ribeiro, M.H.L. Design of an immobilized enzyme system for naringin hydrolysis at high-pressure. Enzyme Microb. Technol 2007, 40, 442–446. [Google Scholar]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. Drug solubilization and stabilization. J. Pharm. Sci 1996, 85, 1017–1025. [Google Scholar]
- Anca, H.; Cristina, P.; Aurel, A.; Miruna, S.; Nicoleta, H.; Mihali, C.V.; Marieta, C.; Anca, D. Hepatoprotective Effects of Berberis vulgaris L. Extract/β Cyclodextrin on Carbon Tetrachloride–Induced Acute Toxicity in Mice. Int. J. Mol. Sci 2012, 13, 9014–9034. [Google Scholar]
- Sharifah, M.; Hemavathy, S.; Muggundha, R.; Tilagam, M.; Kumuthini, C.; Puvaneswary, S. Conventional Study on Novel Dicationic Ionic Liquid Inclusion with β-Cyclodextrin. Int. J. Mol. Sci 2011, 12, 6329–6345. [Google Scholar]
- Sun, T.; Jiang, B.; Pan, B.L. Microwave Accelerated Transglycosylation of Rutin by Cyclodextrin Glucanotransferase from Bacillus sp. SK13.002. Int. J. Mol. Sci 2011, 12, 3786–3796. [Google Scholar]
- Ratnasooriya, C.C.; Rupasinghe, H.P.V. Extraction of phenolic compounds from grapes and their pomace using beta-cyclodextrin. Food Chem 2012, 134, 625–631. [Google Scholar]
- Casella, R.; Williams, D.A.; Jambhekar, S.S. Solid-state β-cyclodextrin complexes containing indomethacin, ammonia and water. II. Solubility studies. Int. J. Pharm 1998, 165, 15–22. [Google Scholar]
- Divakar, S. A structural study of the naringin-β-cyclodextrin complex. J. Incl. Phenom. Macrocycl. Chem 1993, 15, 305–316. [Google Scholar]
- Swati, R.; Sanjay, K.J. Solubility enhancement of celecoxib using β-cyclodextrin inclusion complexes. Eur. J. Pharm. Biopharm 2004, 57, 263–267. [Google Scholar]
- Germain, P.; Bilal, M.; de Brauer, C. Beta-cyclodextrin/citric acid complexation equilibrium thermodynamic study. Apparent solubility of β-CD in aqueous solutions of citric acid. Thermochim. Acta 1995, 259, 187–198. [Google Scholar]
- Flari, V.; Matoub, M.; Rouland, C. Purification and characterization of a b-mannanase from the digestive tract of the edible snail Helix lucorum L. Carbohydr. Res. 1995, 275, 207–213. [Google Scholar]
- Got, R.; Marnay, A.; Jarrige, P.; Font, J. Beta-galactosidase of Helix pomatia. Nature 1964, 204, 686–687. [Google Scholar]
- Bilensoy, E.; Cırpanlı, Y.; Sen, M.; Doğan, A.L.; Calıs, S. Thermosensitive mucoadhesive gel formulation loaded with 5-Fu: Cyclodextrin complex for HPV-induced cervical cancer. J. Incl. Phenom. Macrocycl. Chem 2007, 57, 363–370. [Google Scholar]
- Calderini, A.; Pessine, F.B.T. Synthesis and characterization of inclusion complex of the vasodilator drug minoxidil with beta-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem 2008, 60, 369–377. [Google Scholar]
- Cevher, E.; Sensoy, D.; Zloh, M.; Mulazimoglu, L. Preparation and characterisation of natamycin: γ-Cyclodextrin inclusion complex and its evaluation in vaginal mucoadhesive formulations. J. Pharm. Sci 2008, 97, 4319–4335. [Google Scholar]
- Sansone, F.; Picerno, P.; Mencherini, T. Flavonoid microparticles by spray-drying: Influence of enhancers of the dissolution rate on properties and stability. J. Food Eng 2011, 13, 188–196. [Google Scholar]
- Liaset, B.; Julshamn, K.; Espe, M. Chemical composition and theoretical nutritional evaluation of the produced fractions from enzymic hydrolysis of salmon frames with Protamex™. Process Biochem 2003, 38, 1747–1759. [Google Scholar]
- Rosenthal, A.; Pyle, D.; Niranjan, K.; Gilmour, S.; Trinca, L. Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil and protein from soybean. Enzyme Microb. Technol 2001, 28, 499–509. [Google Scholar]
- You, J.Y.; Peng, C.; Liu, X. Enzymatic hydrolysis and extraction of arachidonic acid rich lipids from Mortierella alpine. Bioresour. Technol 2011, 102, 6088–6094. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cui, L.; Zhang, Z.-H.; Sun, E.; Jia, X.-B. Effect of ?-Cyclodextrin Complexation on Solubility and Enzymatic Conversion of Naringin. Int. J. Mol. Sci. 2012, 13, 14251-14261. https://doi.org/10.3390/ijms131114251
Cui L, Zhang Z-H, Sun E, Jia X-B. Effect of ?-Cyclodextrin Complexation on Solubility and Enzymatic Conversion of Naringin. International Journal of Molecular Sciences. 2012; 13(11):14251-14261. https://doi.org/10.3390/ijms131114251
Chicago/Turabian StyleCui, Li, Zhen-Hai Zhang, E Sun, and Xiao-Bin Jia. 2012. "Effect of ?-Cyclodextrin Complexation on Solubility and Enzymatic Conversion of Naringin" International Journal of Molecular Sciences 13, no. 11: 14251-14261. https://doi.org/10.3390/ijms131114251
APA StyleCui, L., Zhang, Z.-H., Sun, E., & Jia, X.-B. (2012). Effect of ?-Cyclodextrin Complexation on Solubility and Enzymatic Conversion of Naringin. International Journal of Molecular Sciences, 13(11), 14251-14261. https://doi.org/10.3390/ijms131114251



