Preparation and Characterization of Micronized Artemisinin via a Rapid Expansion of Supercritical Solutions (RESS) Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of RESS-SC Micronization
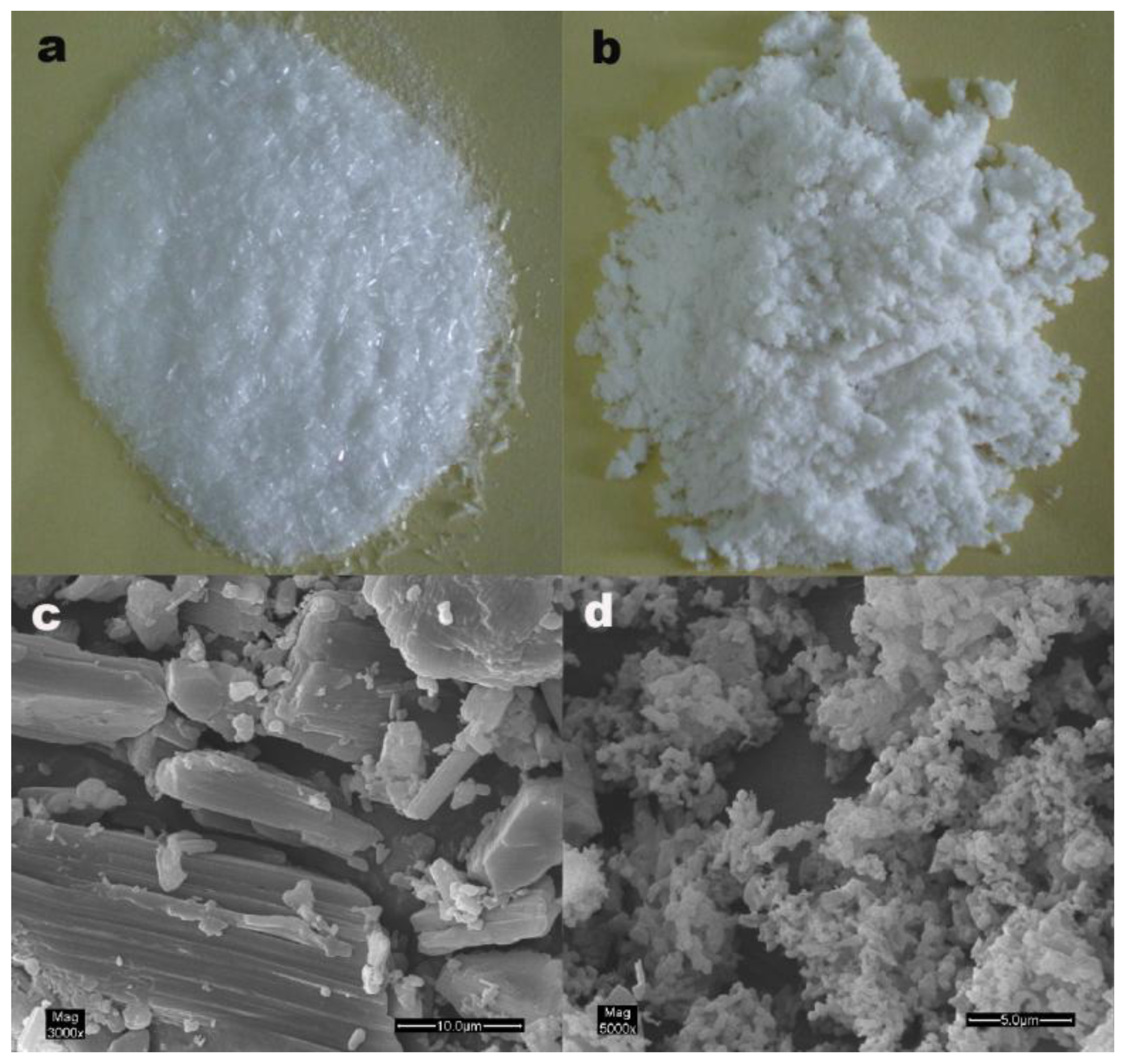
2.2. Particle Morphology
2.3. FTIR Analysis
2.4. LC-MS Analysis
2.5. DSC Analysis
2.6. X-ray Analysis
2.7. Bulk Density
3. Experimental Section
3.1. Materials
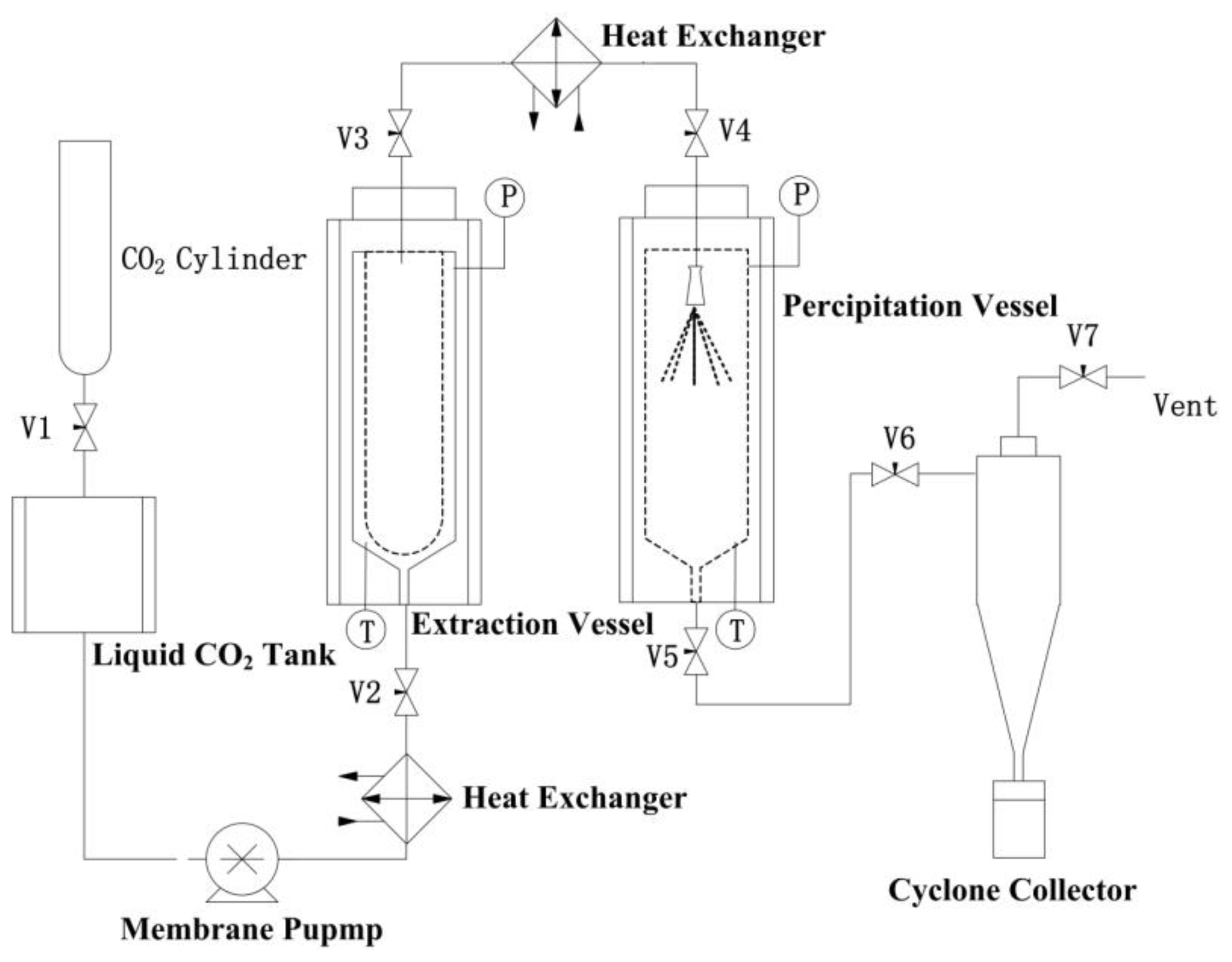
3.2. RESS Apparatus
3.3. Optimization of RESS-SC Micronization
3.4. Analytical Methods
3.4.1. Observation of Particle Morphology
3.4.2. FTIR Analysis
3.4.3. LC-MS Analysis
3.4.4. DSC Analysis
3.4.5. X-ray Diffraction
3.4.6. Bulk Density
4. Conclusions
Acknowledgments
References
- Klayman, D.L. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049–1055. [Google Scholar]
- Dong, J.X.; Dan, Y.M.; Tan, Z.C.; Zhao, J.N.; Liu, Y. Low temperature molar heat capacities and thermal stability of crystalline artemisinin. Thermochim. Acta 2007, 463, 2–5. [Google Scholar]
- O’Neill, P.M.; Posner, G.H. A medicinal chemistry perspective on artemisinin and related endoperoxides. J. Med. Chem 2004, 47, 2945–2964. [Google Scholar]
- Haynes, R.K.; Fugmann, B.; Stetter, J.; Rieckmann, K.; Heilmann, H.D.; Chan, H.W.; Cheung, M.K.; Lam, W.L.; Wong, H.N.; Croft, S.L.; et al. Artemisone—A highly active antimalarial drug of the artemisinin class. Angew. Chem. Int. Ed. Engl 2006, 45, 2082–2088. [Google Scholar]
- World Health Organization (WHO), Assessment of the Safety of Artemisinin Compounds in Pregnancy; WHO: Geneva, Switzerland, 2003; Volume 14.
- Dhingra, V.; Rao, K.V.; Narasu, M.L. Current status of artemisinin and its derivatives as antimalarial drugs. Life Sci 2000, 66, 279–300. [Google Scholar]
- Meshnick, S.R. Artemisin: Mechanisms of action, resistance and toxicity. Int. J. Parasitol 2002, 32, 1655–1660. [Google Scholar]
- Hien, T.T.; White, N.J. Qinghaosu. Lancet 1993, 341, 603–608. [Google Scholar]
- Wiesner, J.; Ortmann, R.; Jomaa, H.; Schlitzer, M. New antimalarial drugs. Angew. Chem. Int. Ed. Engl 2003, 42, 5274–5293. [Google Scholar]
- Luo, X.D.; Shen, C.C. The chemistry, pharmacology and clinical application of qinghaosu (artemisinin) and its derivatives. Med. Res. Rev 1987, 7, 29–52. [Google Scholar]
- Perrut, M.; Jung, J.; Leboeuf, F. Enhancement of dissolution rate of poorly-soluble active ingredients by supercritical fluid processes: Part I: Micronization of neat particles. Int. J. Pharm 2005, 288, 3–10. [Google Scholar]
- Kim, M.S.; Lee, S.; Park, J.S.; Woo, J.S.; Hwang, S.J. Micronization of cilostazol using supercritical antisolvent (SAS) process: Effect of process parameters. Powder Technol 2007, 177, 64–70. [Google Scholar]
- Pasquali, I.; Bettini, R.; Giordano, F. Solid-state chemistry and particle engineering with supercritical fluids in pharmaceutics. Eur. J. Pharm. Sci 2006, 27, 299–310. [Google Scholar]
- Berg, H.; Turner, C.; Dahlberg, L.; Mathiasson, L. Determination of food constituents based on SFE: Applications to vitamins A and E in meatand milk. J. Biochem. Biophys. Methods 2000, 43, 391–401. [Google Scholar]
- Jarzebski, A.B.; Janusz, J.M. Potentials and prospects for application of supercritical fluid technology in bioprocessing. Process Biochem 1995, 30, 343–352. [Google Scholar]
- Hauthal, W.H. Advances with supercritical fluids. Chemosphere 2001, 43, 123–135. [Google Scholar]
- Mandel, F.S.; Wang, J.D. Manufacturing of specialty materials in supercritical fluid carbon dioxide. Inorg. Chim. Acta 1999, 294, 214–223. [Google Scholar]
- Turk, M. Formation of small organic particles by RESS: Experimental and theoretical investigations. J. Supercrit. Fluids 1999, 15, 79–89. [Google Scholar]
- Tom, J.W.; Debenedetti, P.G. Formation of bioerodable polymeric microspheres and microparticles by rapid expansion of supercritical solutions. Biotechnol. Prog 1991, 7, 403–411. [Google Scholar]
- Hirunsit, P.; Huang, Z.; Srinophakun, T.; Charoenchaitrakool, A.; Kawi, S. Particle formation of ibuprofen-supercritical CO2 system from rapid expansion of supercritical solutions (RESS): A mathematical model. Powder Technol 2005, 154, 83–94. [Google Scholar]
- Dennis, B.; Michael, T. Micronisation of carbamazepine through rapid expansion of supercritical solution (RESS). J. Supercrit. Fluids 2012, 62, 32–40. [Google Scholar]
- Ali, K.; Javad, K.S.; Alborz, F.; Aboo, A.G.; Morteza, R.T.; Farid, A.D. Preparation and characterization of raloxifene nanoparticles using rapid expansion of supercritical solution (RESS). J. Supercrit. Fluids 2012, 63, 169–179. [Google Scholar]
- Helfgen, B.; Turk, M.; Schaber, K. Hydrodynamic and aerosol modelling of the rapid expansion of supercritical solutions (RESS-process). J. Supercrit. Fluids 2003, 26, 225–242. [Google Scholar]
- Weber, M.; Thies, M.C. A simplified and generalized model for the rapid expansion of supercritical solutions. J. Supercrit. Fluids 2007, 40, 402–419. [Google Scholar]
- Defne, K.; Ugur, A.; Oner, H. Micronization of ibuprofen by RESS. J. Supercrit. Fluids 2003, 26, 17–31. [Google Scholar]
- Xing, H.B.; Yang, Y.W.; Su, B.G.; Huang, M.; Ren, Q.L. Solubility of artemisinin in supercritical carbon dioxide. J. Chem. Eng. Data 2003, 48, 330–332. [Google Scholar]
- Viktor, M.; Gérard, C.; Elisabeth, B.; Geza, H.; Lazlo, S.; Nathalie, B.; Eric, T. Bioavailability enhancement of an active substance by supercritical antisolvent precipitation. J. Supercrit. Fluids 2007, 40, 101–110. [Google Scholar]
- United States Pharmacopoeia; United States Pharmacopeial Convention Inc: Rockville, MD, USA, 2005; Volume XXVIII, p. 2374.

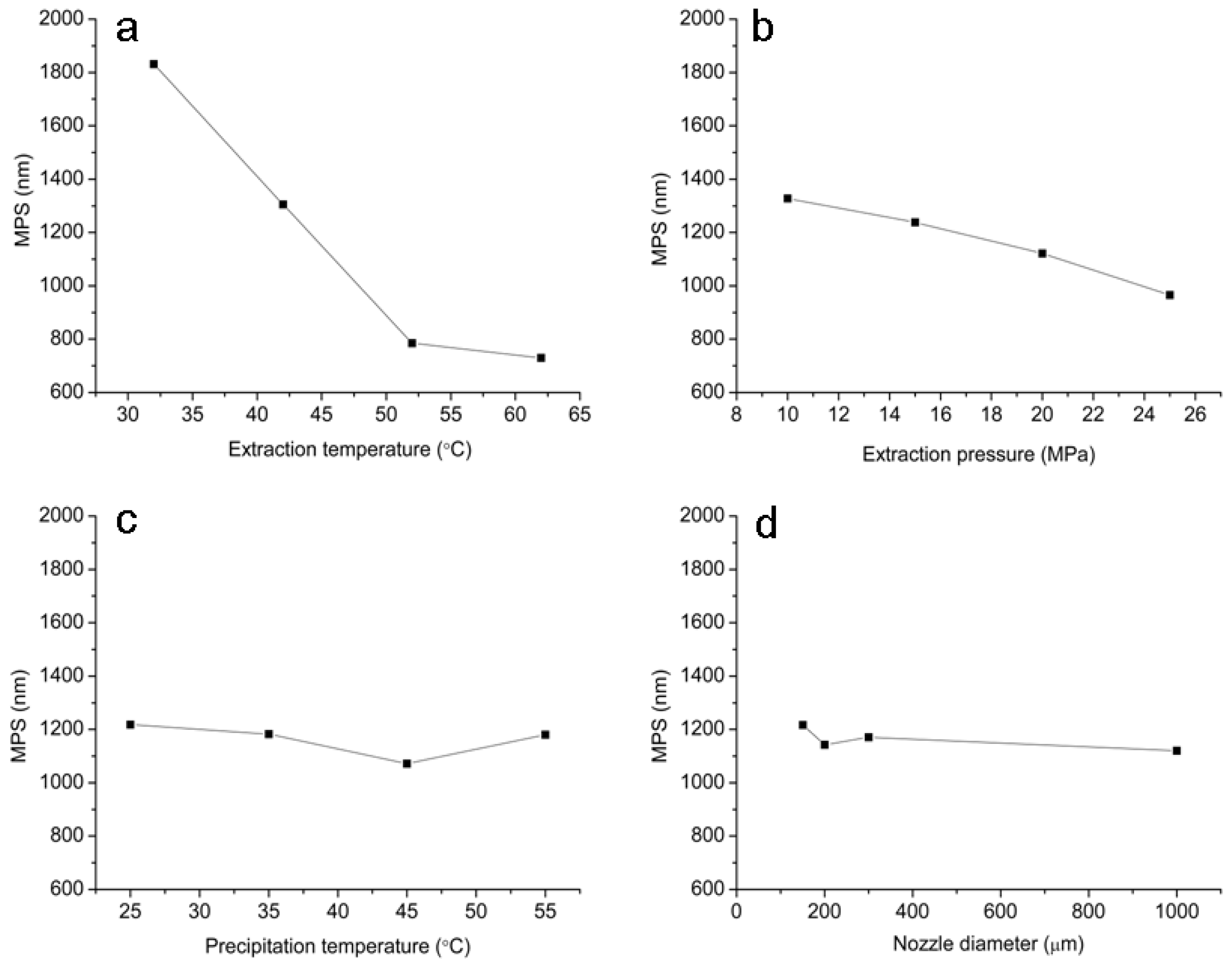




| Trial No. | Extraction Temperature (°C) | Extraction Pressure (MPa) | Precipitation Temperature (°C) | Nozzle Diameter (μm) | MPS (nm) |
|---|---|---|---|---|---|
| 1 | 1(32) | 1(10) | 1(25) | 1(150) | 2100 |
| 2 | 2(42) | 1(10) | 2(35) | 2(200) | 1930 |
| 3 | 3(52) | 1(10) | 3(45) | 3(300) | 1725 |
| 4 | 4(62) | 1(10) | 4(55) | 4(1000) | 1571 |
| 5 | 1(32) | 2(15) | 3(45) | 2(200) | 1459 |
| 6 | 2(42) | 2(15) | 4(55) | 1(150) | 1411 |
| 7 | 3(52) | 2(15) | 1(25) | 4(1000) | 1358 |
| 8 | 4(62) | 2(15) | 2(35) | 3(300) | 992 |
| 9 | 1(32) | 3(20) | 4(55) | 3(300) | 840 |
| 10 | 2(42) | 3(20) | 3(45) | 4(1000) | 880 |
| 11 | 3(52) | 3(20) | 2(35) | 1(150) | 740 |
| 12 | 4(62) | 3(20) | 1(25) | 2(200) | 680 |
| 13 | 1(32) | 4(25) | 2(35) | 4(1000) | 910 |
| 14 | 2(42) | 4(25) | 1(25) | 3(300) | 730 |
| 15 | 3(52) | 4(25) | 4(55) | 2(200) | 660 |
| 16 | 4(62) | 4(25) | 3(45) | 1(150) | 620 |
| K1 a | 1831.5 | 1327.25 | 1217.75 | 1217.0 | |
| K2 | 1305.0 | 1237.75 | 1182.25 | 1143.0 | |
| K3 | 785.0 | 1120.75 | 1071.75 | 1171.0 | |
| K4 | 730.0 | 965.75 | 1179.75 | 1120.5 | |
| R b | 1101.5 | 361.5 | 146.0 | 96.5 | |
| Optimal level | A4 | B4 | C3 | D4 |
| Source | Sum of Squares (SS) | Degrees of Freedom (df) | F-Ratio | F0.05 | Type of Effect |
|---|---|---|---|---|---|
| A | 3189716.75 | 3 | 334.045 | 9.280 | Significant |
| B | 293032.75 | 3 | 30.688 | 9.280 | Significant |
| C | 47900.75 | 3 | 5.016 | 9.280 | |
| D | 20744.75 | 3 | 2.173 | 9.280 | |
| Error | 9548.75 | 3 | 22075 |
| Artemisinin | Quality (g) | Volume (mL) | Density (g/mL) |
|---|---|---|---|
| unprocessed | 2.77 | 5 | 0.554 |
| processed | 0.64 | 5 | 0.128 |
| Factors | (A) Extraction Temperature | (B) Extraction Pressure | (C) Precipitation Temperature | (D) Nozzle Diameter |
|---|---|---|---|---|
| Levels | (°C) | (MPa) | (°C) | (μm) |
| 1 | 32 | 10 | 25 | 150 |
| 2 | 42 | 15 | 35 | 200 |
| 3 | 52 | 20 | 45 | 300 |
| 4 | 62 | 25 | 55 | 1000 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, H.; Zhao, X.; Zu, Y.; Zhang, X.; Zu, B.; Zhang, X. Preparation and Characterization of Micronized Artemisinin via a Rapid Expansion of Supercritical Solutions (RESS) Method. Int. J. Mol. Sci. 2012, 13, 5060-5073. https://doi.org/10.3390/ijms13045060
Yu H, Zhao X, Zu Y, Zhang X, Zu B, Zhang X. Preparation and Characterization of Micronized Artemisinin via a Rapid Expansion of Supercritical Solutions (RESS) Method. International Journal of Molecular Sciences. 2012; 13(4):5060-5073. https://doi.org/10.3390/ijms13045060
Chicago/Turabian StyleYu, Huimin, Xiuhua Zhao, Yuangang Zu, Xinjuan Zhang, Baishi Zu, and Xiaonan Zhang. 2012. "Preparation and Characterization of Micronized Artemisinin via a Rapid Expansion of Supercritical Solutions (RESS) Method" International Journal of Molecular Sciences 13, no. 4: 5060-5073. https://doi.org/10.3390/ijms13045060
APA StyleYu, H., Zhao, X., Zu, Y., Zhang, X., Zu, B., & Zhang, X. (2012). Preparation and Characterization of Micronized Artemisinin via a Rapid Expansion of Supercritical Solutions (RESS) Method. International Journal of Molecular Sciences, 13(4), 5060-5073. https://doi.org/10.3390/ijms13045060





