Apoptosis Signaling Is Altered in CD4+CD25+FoxP3+ T Regulatory Lymphocytes in Pre-Eclampsia
Abstract
:1. Introduction
2. Results and Discussion
3. Material and Methods
3.1. Patients
3.2. Blood Sampling and Cell Preparation
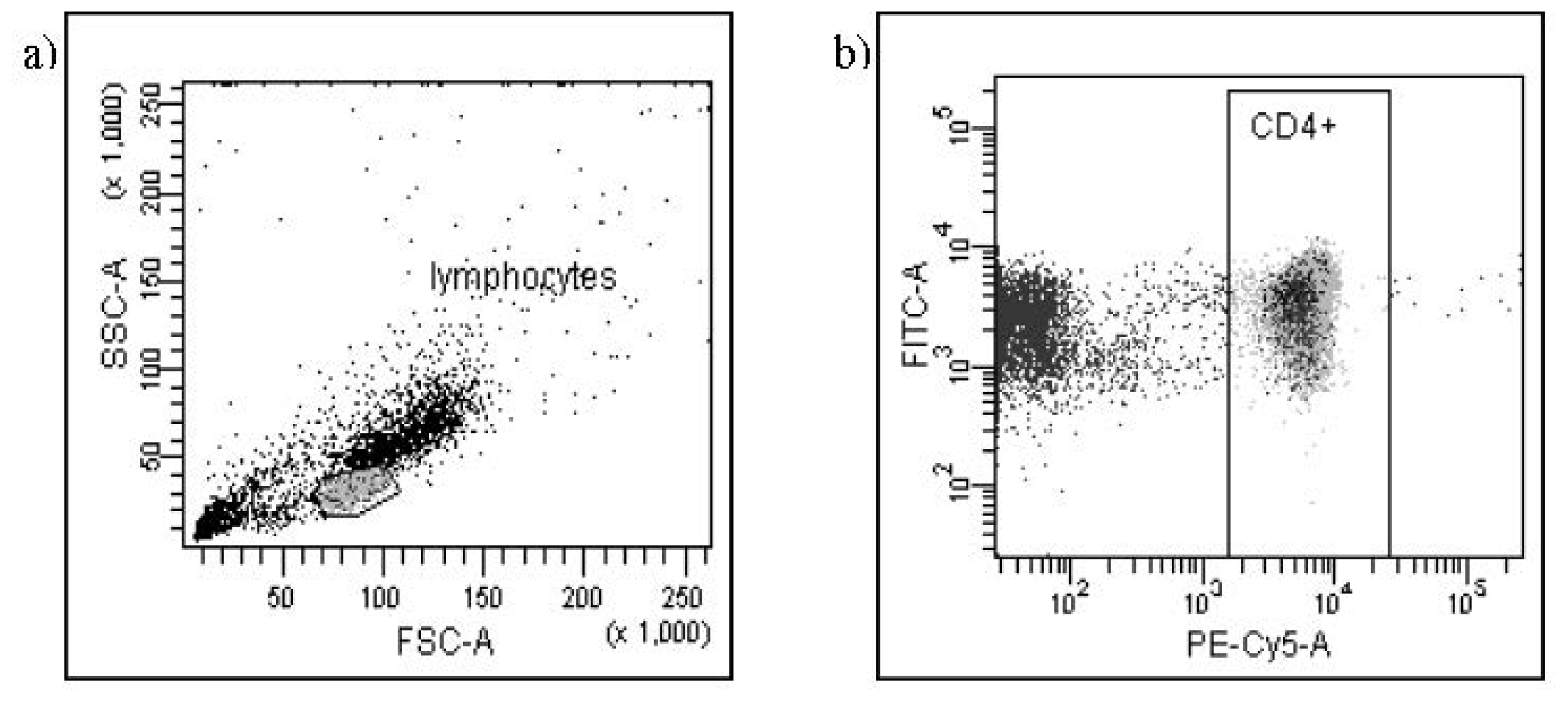
3.3. Isolation of Peripheral Blood Cells
3.4. Phenotyping of T Cells
- anti-Human CD8 (FITC) and anti-Human CD28 (PE) monoclonal antibodies;
- anti-Human CD95 (FITC), anti-Human CD25 (PE), anti-Human CD4 (PE-Cy5) and anti-Human FoxP3 (Pacific Blue) monoclonal antibodies;
- anti-Human Bax (FITC), anti-Human CD25 (PE), anti-Human CD4 (PE-Cy5) and anti-Human FoxP3 (Pacific Blue) monoclonal antibodies;
- anti-Human Bcl-2 Oncoprotein (FITC), anti-Human CD25 (PE), anti-Human CD4 (PE-Cy5) and anti-Human FoxP3 (Pacific Blue) monoclonal antibodies.
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Ahn, H.; Park, J.; Gilman-Sachs, A.; Kwak-Kim, J. Immunologic characteristics of preeclampsia, a comprehensive review. Am. J. Reprod. Immunol 2010. [Google Scholar] [CrossRef]
- Redman, C.W.G.; Sacks, G.P.; Sargent, I.L. Pre-eclampsia: An excessive maternal inflammatory response to pregnancy. Am. J. Obstet. Gynekol 1999, 180, 499–506. [Google Scholar]
- Saito, S.; Umekage, H.; Sakamoto, Y. Increased Th1-type immunity and decreased Th2-type immunity in patients with preeclampsia. Am. J. Reprod. Immunol 1999, 41, 297–306. [Google Scholar]
- Darmochwal-Kolarz, D.; Leszczynska-Gorzelak, B.; Rolinski, J.; Oleszczuk, J. T helper 1 type and T helper 2 type cytokines imbalance in pregnant women with pre-eclampsia. Eur. J. Obstet. Gynecol 1999, 86, 165–170. [Google Scholar]
- Baecher-Allan, C.; Bron, J.A.; Freeman, G.J.; Hafler, D.A. CD4+CD25 high regulatory cells in human peripheral blood. J. Immunol 2001, 167, 1245–1253. [Google Scholar]
- Kuniyasu, Y.; Takahashi, T.; Itoh, M.; Shimizu, J.; Toda, G.; Sakaguchi, S. Naturally anergic and suppressive CD25+CD4+ T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. Int. Immunol 2000, 12, 1145–1155. [Google Scholar]
- Sakaguchi, S. Immunological tolerance maintained by CD4+CD25+ regulatory T cells: Their common role in controlling autoimmunity, tumor immunity and transplantation tolerance. Immunol. Rev 2001, 182, 18–32. [Google Scholar]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol 2003, 4, 337–342. [Google Scholar]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cells development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar]
- Waldmann, H.; Graca, I.; Cobbold, S.; Adams, E.; Tone, M.; Tone, Y. Regulatory T cells and organ transplantation. Semin. Immunol 2004, 16, 119–126. [Google Scholar]
- Tafuri, A.; Alferink, J.; Möller, P.; Hämmerling, G.J.; Arnold, B. T cell awareness of paternal alloantigens during pregnancy. Science 1995, 270, 630–633. [Google Scholar]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol 2004, 5, 266–271. [Google Scholar]
- Sasaki, Y.; Sakai, M.; Miyazaki, S.; Higuma, S.; Shiozaki, A.; Saito, S. Decidual and peripheral blond regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod 2004, 10, 347–353. [Google Scholar]
- Zenclussen, A.C.; Gerlof, K.; Zenclussen, M.L.; Sollwedel, A.; Bertoja, A.Z.; Ritter, T.; Kotsch, K.; Leber, J.; Volk, H.D. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: Adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am. J. Pathol 2005, 166, 811–822. [Google Scholar]
- Darrasse-Jèze, G.; Klatzmann, D.; Charlotte, F.; Salomon, B.L.; Cohen, J.L. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol. Lett 2006, 102, 106–109. [Google Scholar]
- Sasaki, Y.; Darmochwal-Kolarz, D.; Suzuki, D.; Sakai, M.; Ito, M.; Shima, T.; Shiozaki, A.; Rolinski, J.; Saito, S. Proportion of peripheral blood and decidual CD4+ CD25bright regulatory T cells in pre-eclampsia. Clin. Exp. Immunol 2007, 149, 139–145. [Google Scholar]
- Darmochwal-Kolarz, D.; Saito, S.; Rolinski, J.; Tabarkiewicz, J.; Kolarz, B.; Leszczynska-Gorzelak, B.; Oleszczuk, J. Activated T lymphocytes in pre-eclampsia. Am. J. Reprod. Immunol 2007, 58, 39–45. [Google Scholar]
- Santner-Nanan, B.; Peek, M.J.; Khanam, R.; Richarts, L.; Zhu, E.; Fazekas de St Groth, B.; Nanan, R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J. Immunol 2009, 183, 7023–7030. [Google Scholar]
- Darmochwal-Kolarz, D.; Kludka-Sternik, M.; Tabarkiewicz, J.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J. Reprod. Immunol 2012, 93, 75–81. [Google Scholar]
- Paeschke, S.; Chen, F.; Horn, N.; Fotopoulou, C.; Zambon-Bertoja, A.; Sollwedel, A.; Zenclussen, M.L.; Casalis, P.A.; Dudenhausen, J.W.; Volk, H.D.; et al. Pre-eclampsia is not associated with changes in the levels of regulatory T cells in peripheral blood. Am. J. Reprod. Immunol 2005, 54, 384–389. [Google Scholar]
- Hu, D.; Chen, Y.; Zhang, W.; Wang, H.; Wang, Z.; Dong, M. Alteration of peripheral CD4+CD25+ regulatory T lymphocytes in pregnancy and pre-eclampsia. Acta Obstet. Gynecol. Scand 2008, 87, 190–194. [Google Scholar]
- Civil, A.; Geerts, M.; Aarden, L.A.; Verweij, C.L. Evidence for a role of CD28RE as a response element for distinct mitogenic T cell activation signals. Eur. J. Immunol 1992, 22, 3041–3043. [Google Scholar]
- Tilburgs, T.; Roelen, D.L.; van der Mast, B.J.; van Schip, J.J.; Kleijburg, C.; de Groot-Swings, G.M.; Kanhai, H.H.; Claas, F.H.; Scherjon, S.A. Differential distribution of CD4+CD25bright and CD8+CD28− T-cells in decidua and maternal blood during human pregnancy. Placenta 2006, 27, S47–S53. [Google Scholar]
- Reed, J.C. Bcl-2 family proteins. Oncogene 1998, 17, 3225–3236. [Google Scholar]
- Oltavi, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619. [Google Scholar]
- Nagata, S.; Golstein, P. The fast death factor. Science 1995, 267, 1449–1456. [Google Scholar]
- Lynch, D.H.; Ramsdell, F.; Alderson, M.R. Fas and FasL in the homeostatic regulation of immune responses. Immunol. Today 1995, 16, 569–574. [Google Scholar]
- Wegmann, T.G. Bi-directional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a Th2 phenomenon? Immunol. Today 1993, 14, 353–356. [Google Scholar]
- Chaouat, G.; Tranchot Diallo, J.; Volumenie, J.L.; Menu, E.; Gras, G.; Delage, G.; Mognetti, B. Immune suppression and Th1/Th2 balance in pregnancy revisited: A (very) personal tribute to Tom Wegmann. Am. J. Reprod. Immunol 1997, 37, 427–434. [Google Scholar]
- Polanczyk, M.J.; Carson, B.D.; Subramanian, S.; Afentoulls, M.; Vandenbark, A.A.; Ziegler, F.S.; Offner, H. Estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J. Immunol 2004, 173, 2227–2230. [Google Scholar]
- Zeisler, H.; Jirecek, S.; Hohlagschwandtner, M.; Knöfler, M.; Tempfer, C.; Livingston, J.C. Concentrations of estrogens in patients with preeclampsia. Wien. Klin. Wochenschr 2002, 114, 458–461. [Google Scholar]
- Casart, Y.C.; Tarrazzi, K.; Camejo, M.I. Serum levels of interleukin-6, interleukin-1beta and human chorionic gonadotropin in pre-eclamptic and normal pregnancy. Gynecol. Endocrinol 2007, 23, 300–303. [Google Scholar]
- Pasare, C.; Medzhitov, R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 2003, 299, 1033–1036. [Google Scholar]
- Weaver, C.T.; Hatton, R.D. Interplay between the TH17 and TReg cell lineages: A (co-)evolutionary perspective. Nat. Rev. Immunol 2009, 9, 883–889. [Google Scholar]
- Afzali, B.; Mitchell, P.; Lechler, R.I.; John, S.; Lombardi, G. Translational mini-review series on Th17 cells: Induction of interleukin-17 production by regulatory T cells. Clin. Exp. Immunol 2009, 159, 120–130. [Google Scholar]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol 2007, 8, 942–949. [Google Scholar]
- Reinhard, G.; Noll, A.; Schlebusch, H.; Mallmann, P.; Ruecker, A.V. Shifts in the TH1/TH2 balance during human pregnancy correlate with apoptotic changes. Biochem. Biophys. Res. Commun 1998, 245, 933–938. [Google Scholar]
- Fritzschining, B.; Oberle, N.; Eberhardt, N.; Quick, S.; Haas, J.; Wildemann, B.; Krammer, P.H.; Suri-Payer, E. In contrast to effector cells, CD4+CD25+Foxp3+ regulatory T cells are highly susceptible to CD95 ligand—But not to TCR-mediated cell death. J. Immunol 2005, 175, 32–36. [Google Scholar]
- Rudd, C.E.; Taylor, A.; Schneider, H. CD28 and CTLA4 coreceptor expression and Signal transduction. Immunol. Rev 2009, 229, 12–26. [Google Scholar]


| Patients with pre-eclampsia (n = 24) median and interquartile ranges | Normal third trimester pregnancy (n = 20) median and interquartile ranges | p | |
|---|---|---|---|
| CD4+CD25+FoxP3+ Treg cells | 3.60% (2.30%–6.01%) | 6.20% (5.15%–7.60%) | p < 0.05 |
| CD95 antigen on CD4+CD25+FoxP3+ Treg cells | 63.34% (49.38%–68.60%) | 64.32% (51.90%–72.89%) | NS |
| Bcl-2 protein on CD4+CD25+FoxP3+ Treg cells | 69.10% (52.00%–88.00%) | 97.45% (95.70%–99.30%) | p < 0.05 |
| Bax protein on CD4+CD25+FoxP3+ Treg cells | 21.15% (19.59%–28.95%) | 23.97% (19.77%–30.66%) | NS |
| CD8+CD28+ T cells | 6.50% (3.59%–12.85%) | 6.75% (4.07%–9.41%) | NS |
| Patients with pre-eclampsia (n = 24) median and interquartile ranges (arbitral units) | Healthy third trimester pregnant women (n = 20) median and interquartile ranges (arbitral units) | p | |
|---|---|---|---|
| MFI CD95 antigen on CD4+CD25+FoxP3+ cells | 52.05 a.u. (46.93 a.u.–56.62 a.u.) | 49.41 a.u. (42.79 a.u.–55.60 a.u.) | NS |
| MFI Bax antigen on CD4+CD25+FoxP3+ cells | 185.34 a.u. (150.51 a.u.–206.13 a.u.) | 102.76 a.u. (86.13 a.u.–124.15 a.u.) | p < 0.01 |
| MFI Bcl-2 antigen on CD4+CD25+FoxP3+ cells | 2343 a.u. (2077.00 a.u.–2592 a.u.) | 2800.00 a.u. (2315.00 a.u.–3858.00 a.u.) | p < 0.05 |
| MFI CD28 antigen on T CD8+ cells | 131.26 a.u. (98.47 a.u.–146.14 a.u.) | 63.34 a.u. (68.56 a.u.–88.95 a.u.) | p < 0.001 |
| Study group mean ± SD n = 24 | Control group mean ± SD n = 20 | Significance (p) | |
|---|---|---|---|
| Maternal age | 28.68 ± 4.76 | 27.68 ± 5.31 | NS |
| Gravidity | 1.84 ± 1.12 | 1.93 ± 0.85 | NS |
| Parity | 1.63 ± 0.95 | 1.81 ± 0.81 | NS |
| Time of blood collection (weeks of gestation) | 34.05 ± 2.14 | 34.62 ± 1.63 | NS |
| Systolic pressure (mmHg) | 155.35 ± 15.85 | 110.25 ± 20.15 | <0.01 |
| Diastolic pressure (mmHg) | 96.47 ± 5.66 | 73.56 ± 7.18 | <0.05 |
| Proteinuria (g/24 h) | 1.45 ± 0.65 | absent | - |
| Uric acid (mg/dL) | 5.47 ± 1.38 | 3.15 ± 1.43 | <0.01 |
| Fetal weight (g) | 2560 ± 615 | 3280 ± 365 | <0.05 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Darmochwal-Kolarz, D.; Saito, S.; Tabarkiewicz, J.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. Apoptosis Signaling Is Altered in CD4+CD25+FoxP3+ T Regulatory Lymphocytes in Pre-Eclampsia. Int. J. Mol. Sci. 2012, 13, 6548-6560. https://doi.org/10.3390/ijms13066548
Darmochwal-Kolarz D, Saito S, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, Oleszczuk J. Apoptosis Signaling Is Altered in CD4+CD25+FoxP3+ T Regulatory Lymphocytes in Pre-Eclampsia. International Journal of Molecular Sciences. 2012; 13(6):6548-6560. https://doi.org/10.3390/ijms13066548
Chicago/Turabian StyleDarmochwal-Kolarz, Dorota, Shigeru Saito, Jacek Tabarkiewicz, Bogdan Kolarz, Jacek Rolinski, Bozena Leszczynska-Gorzelak, and Jan Oleszczuk. 2012. "Apoptosis Signaling Is Altered in CD4+CD25+FoxP3+ T Regulatory Lymphocytes in Pre-Eclampsia" International Journal of Molecular Sciences 13, no. 6: 6548-6560. https://doi.org/10.3390/ijms13066548
APA StyleDarmochwal-Kolarz, D., Saito, S., Tabarkiewicz, J., Kolarz, B., Rolinski, J., Leszczynska-Gorzelak, B., & Oleszczuk, J. (2012). Apoptosis Signaling Is Altered in CD4+CD25+FoxP3+ T Regulatory Lymphocytes in Pre-Eclampsia. International Journal of Molecular Sciences, 13(6), 6548-6560. https://doi.org/10.3390/ijms13066548




