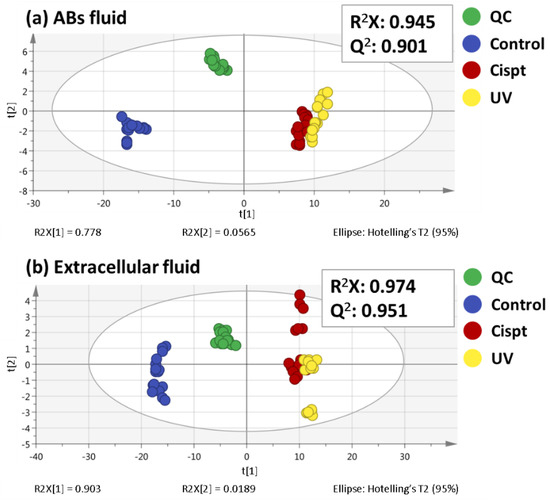
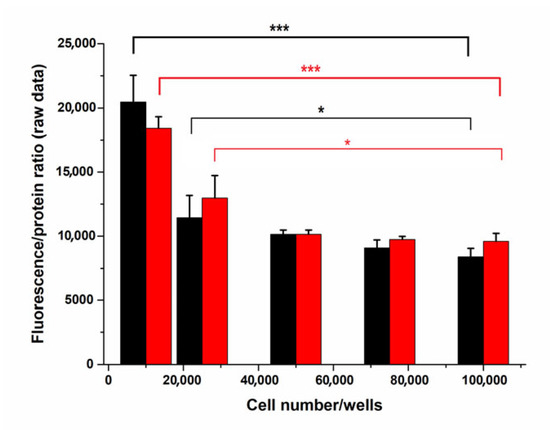
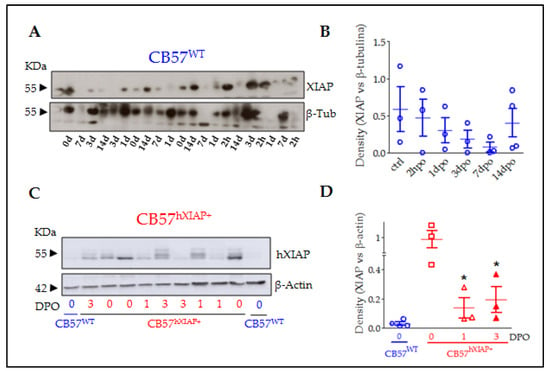
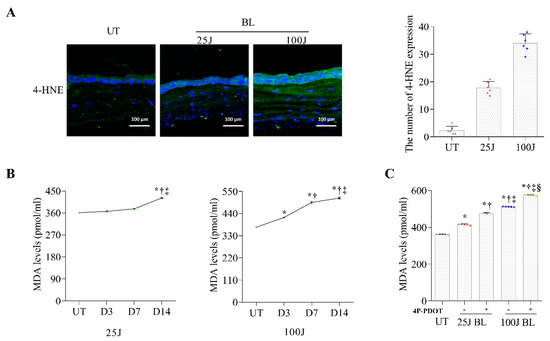
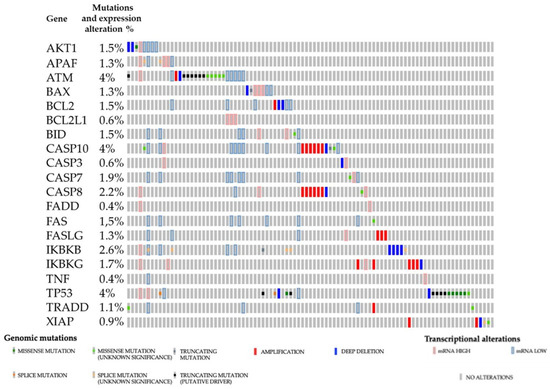
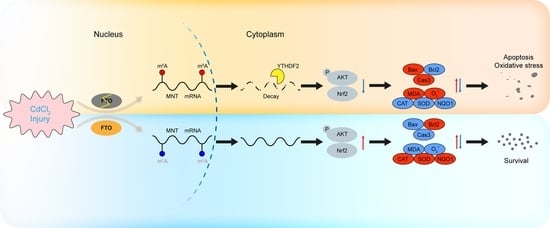
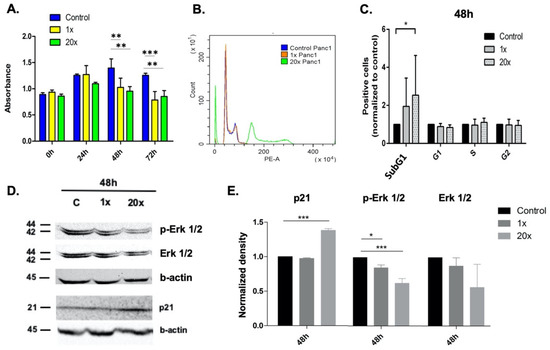
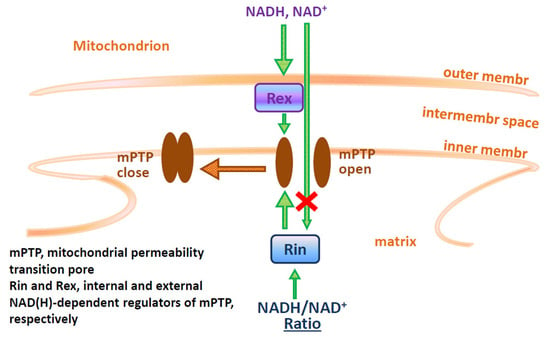
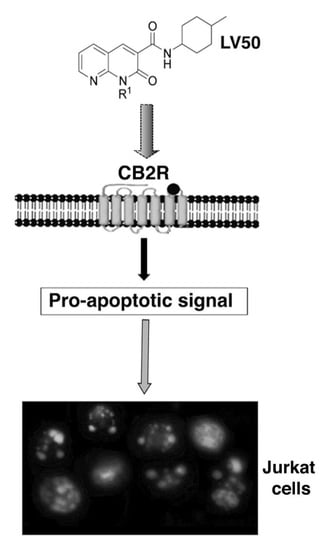
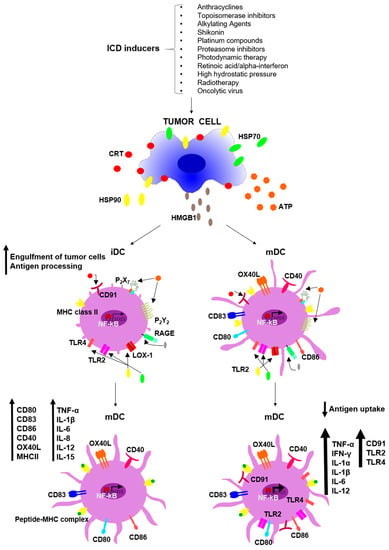
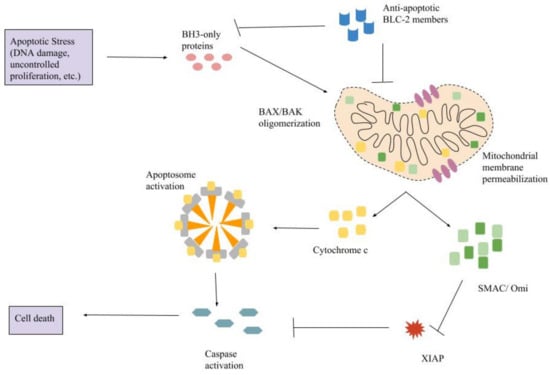
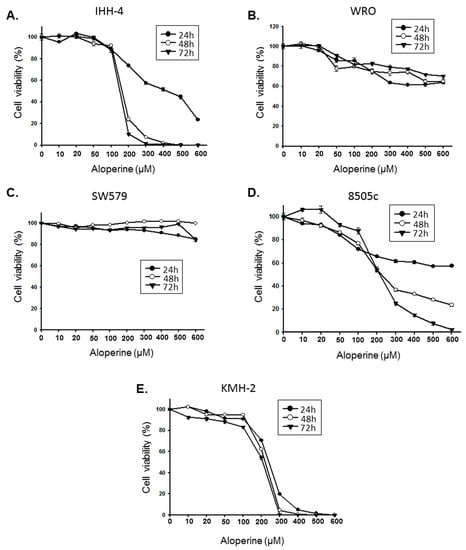
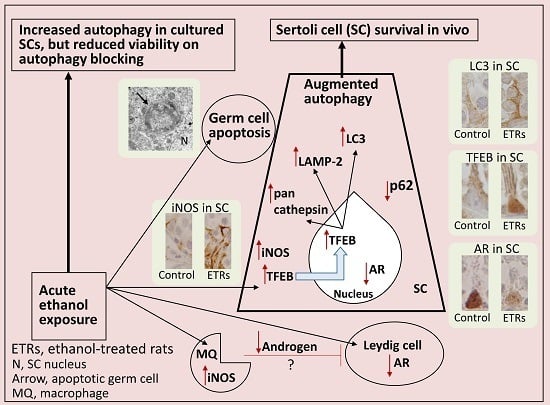
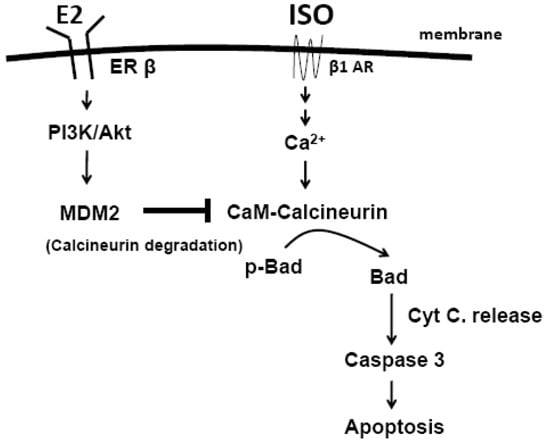
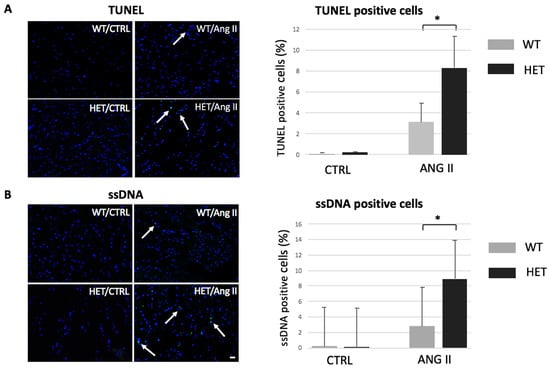
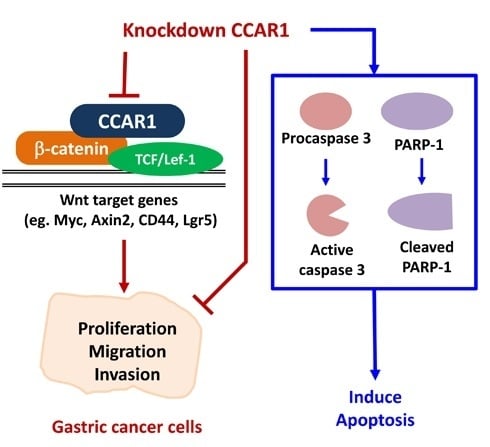
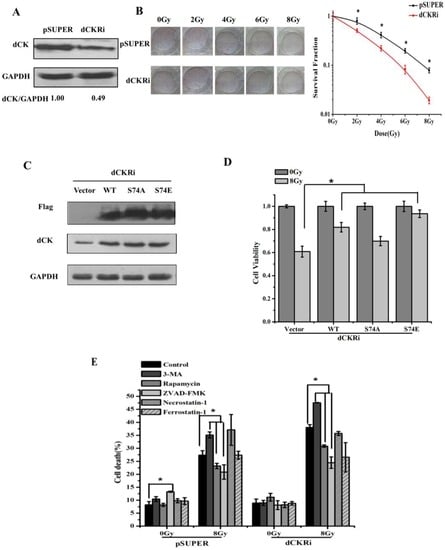
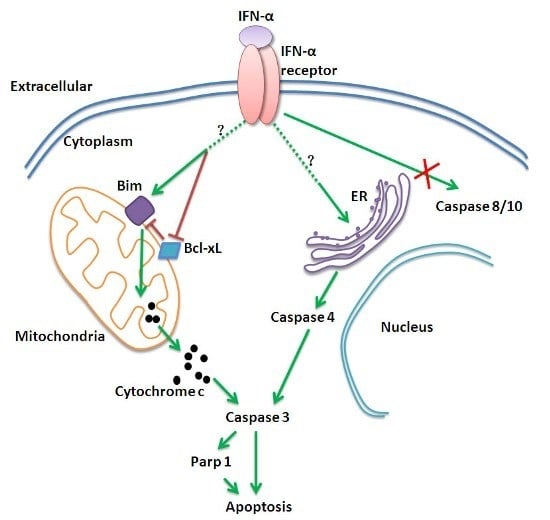
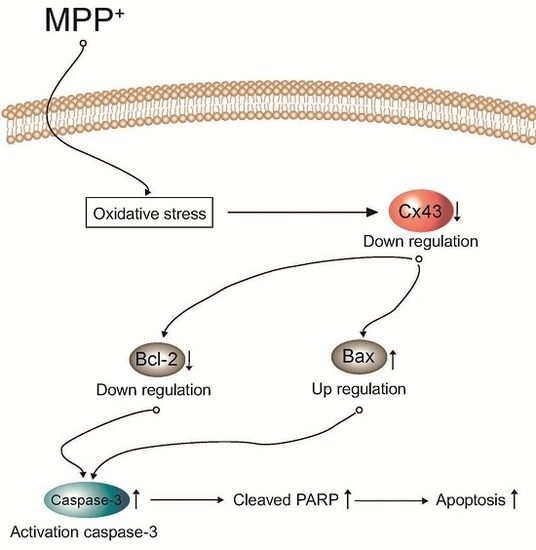
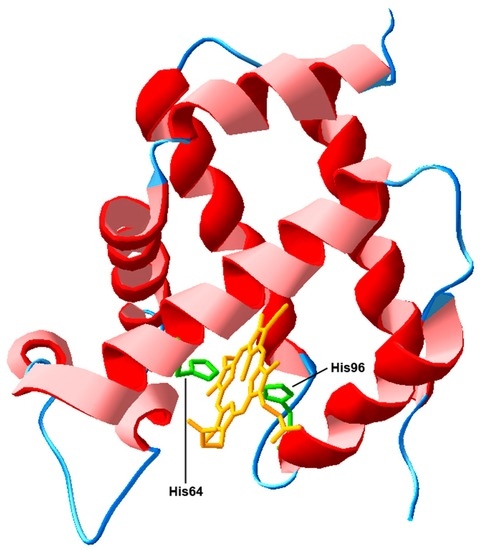
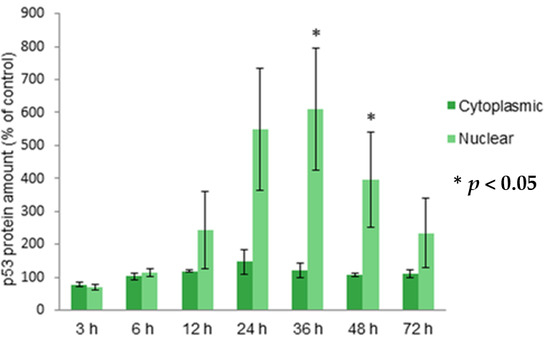
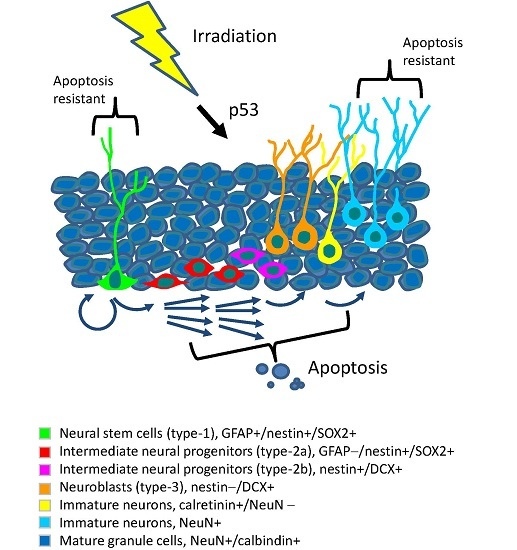
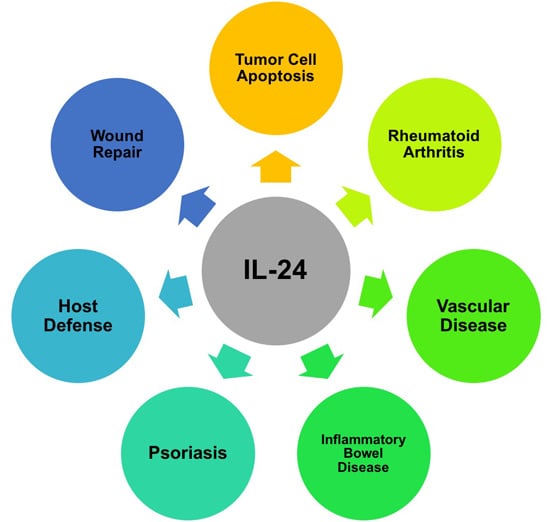
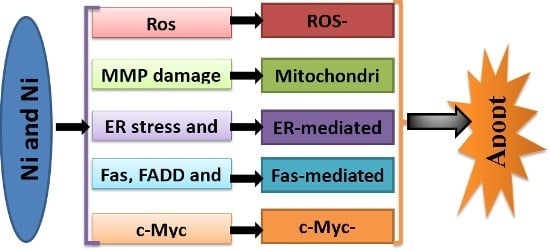
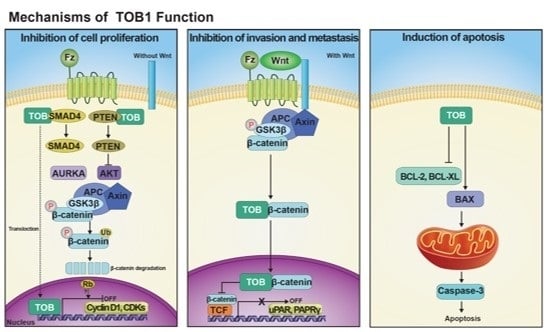
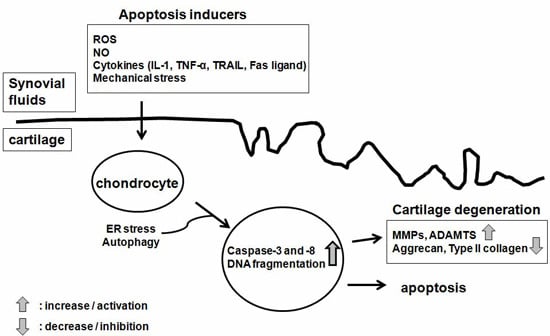
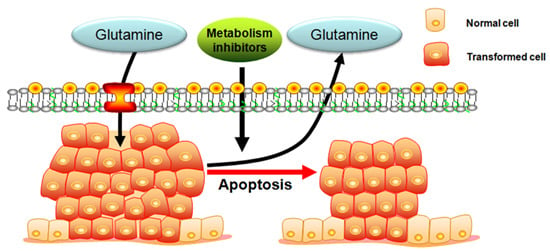
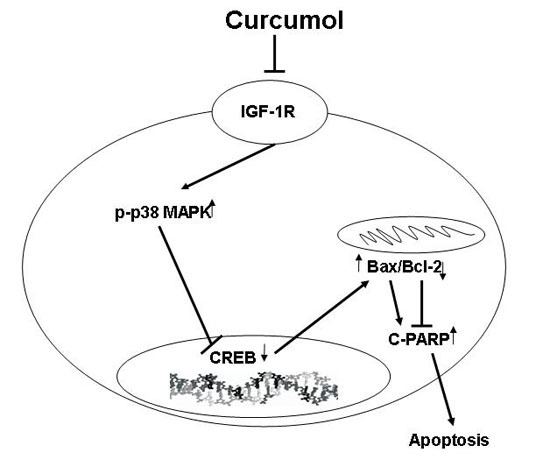
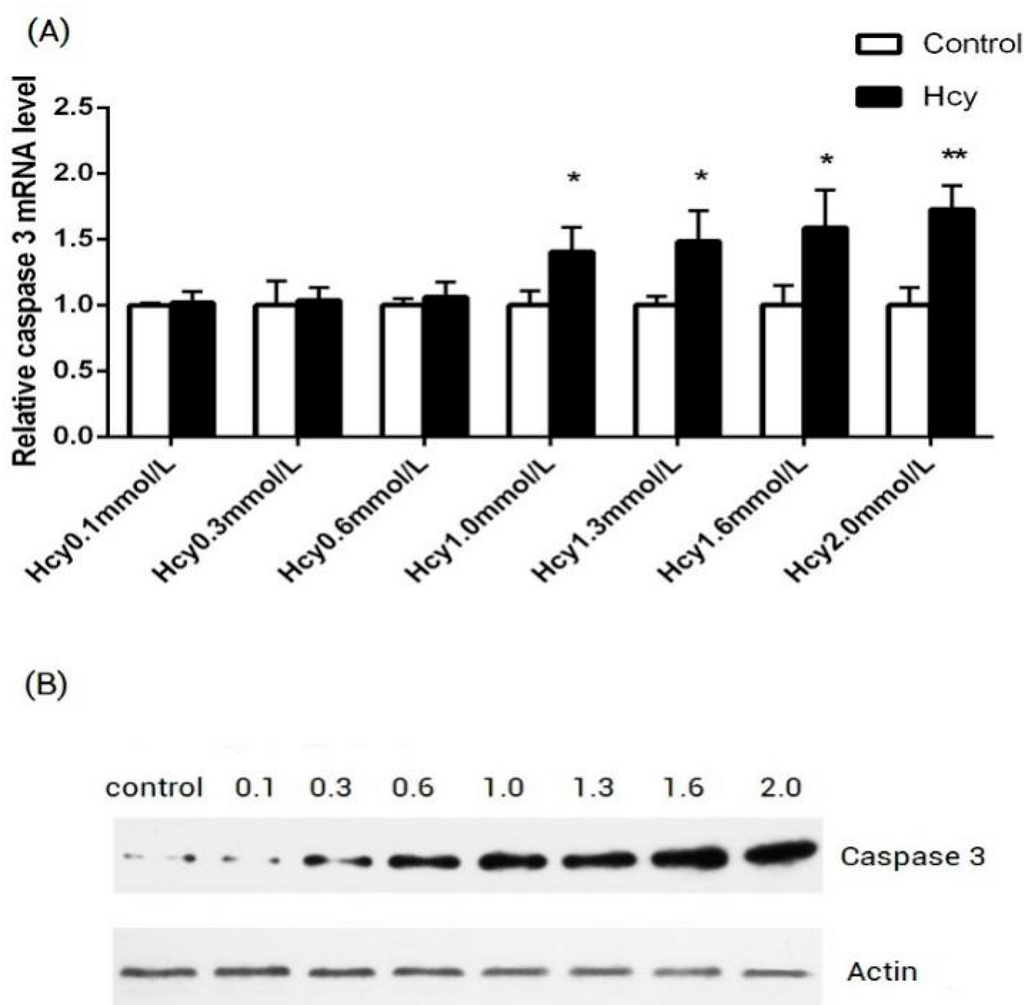
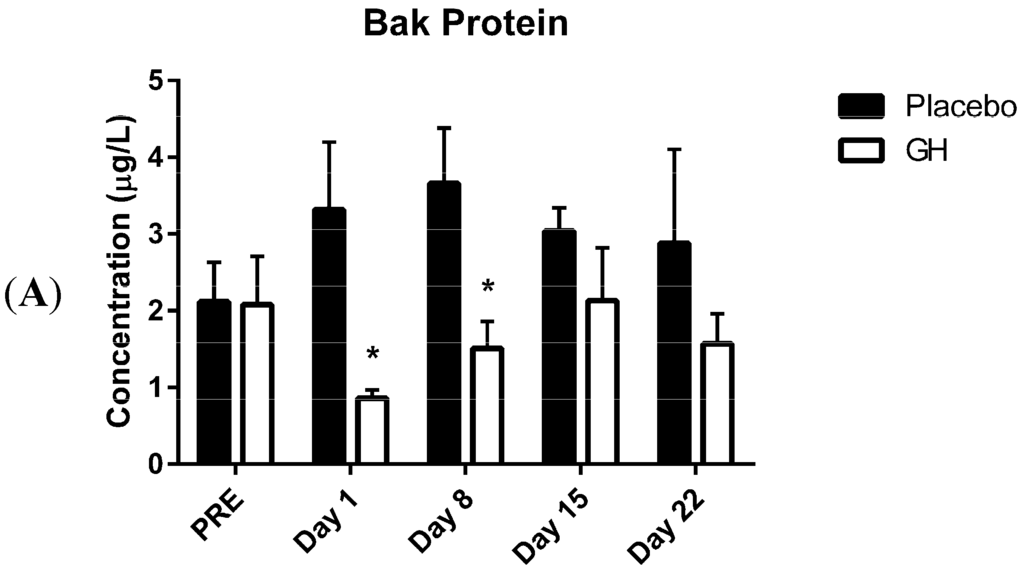
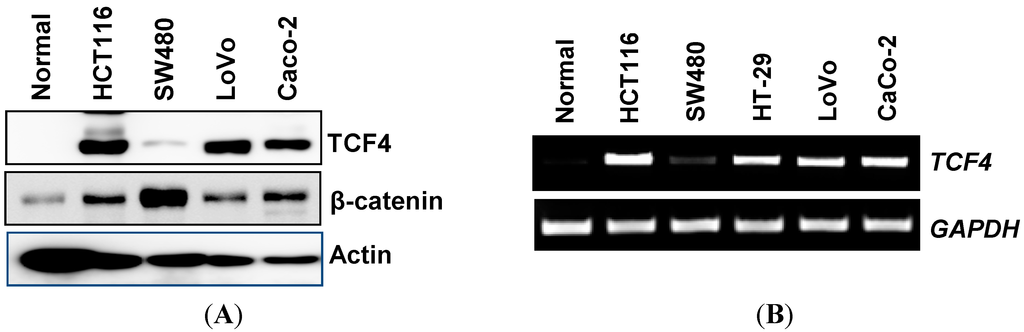
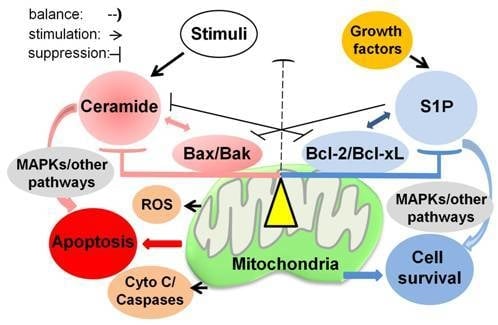
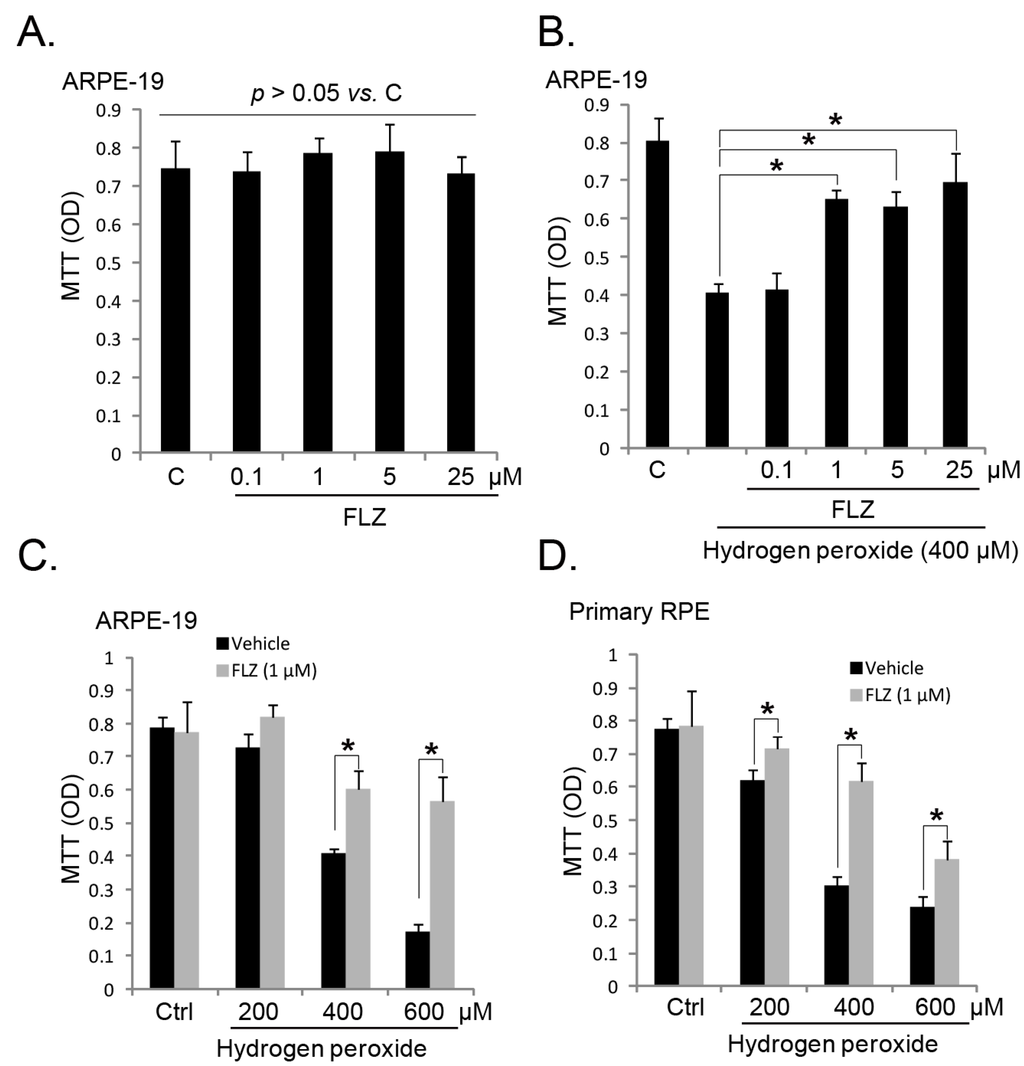
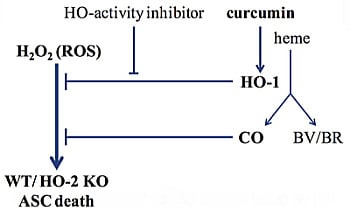
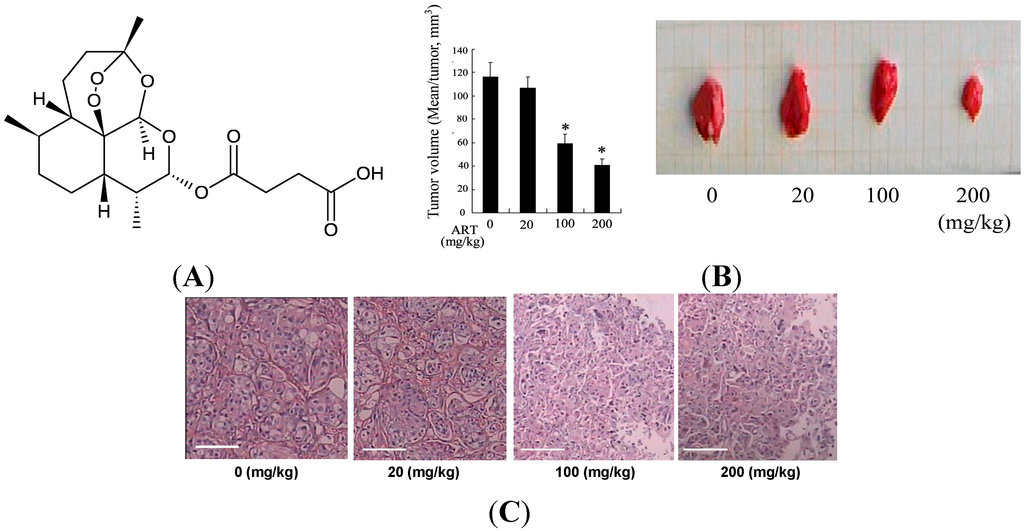
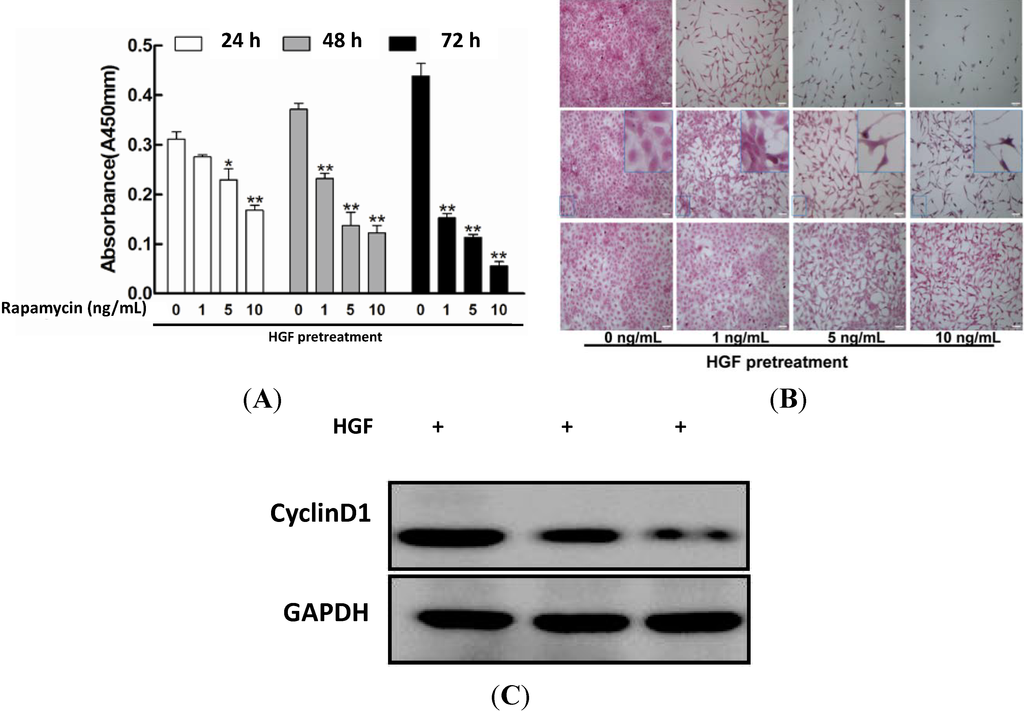
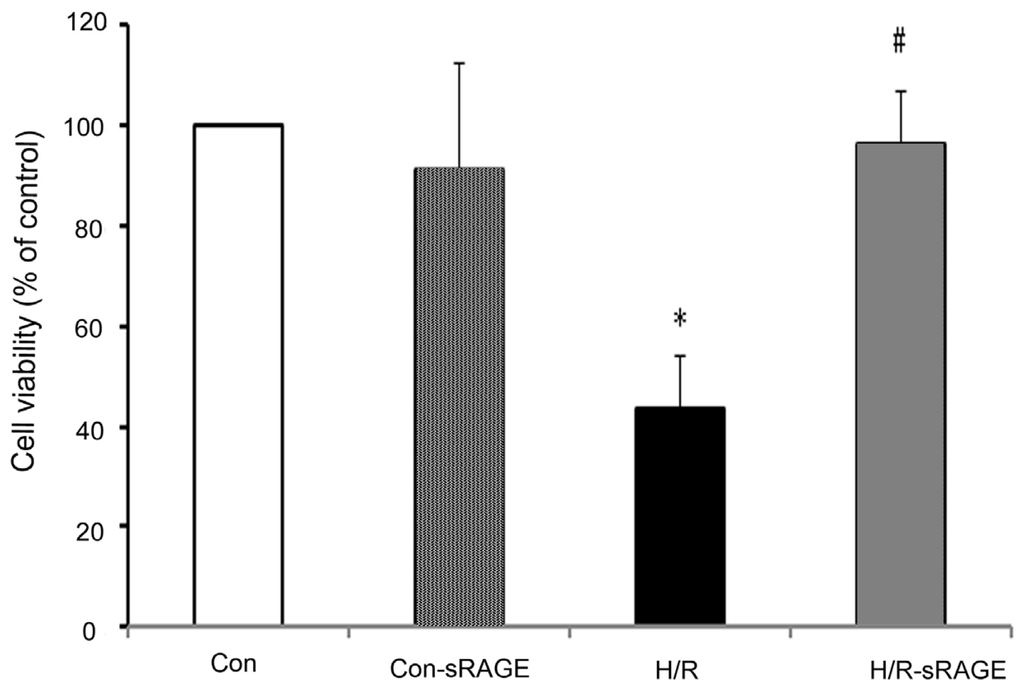
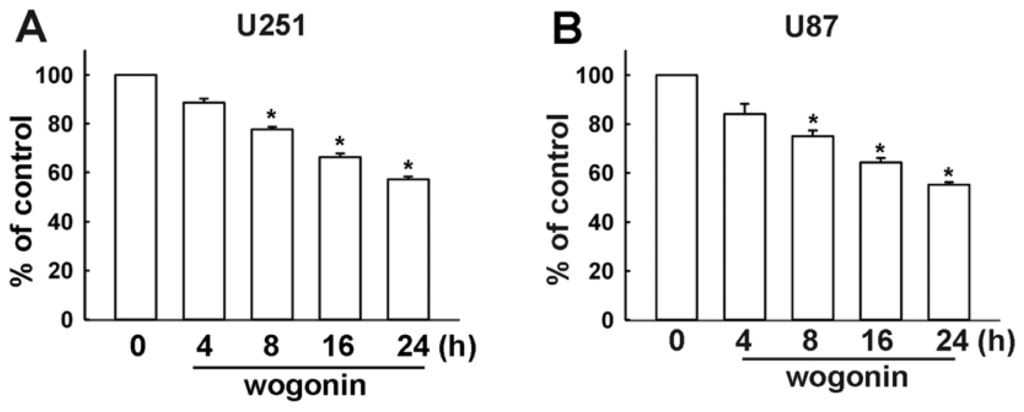
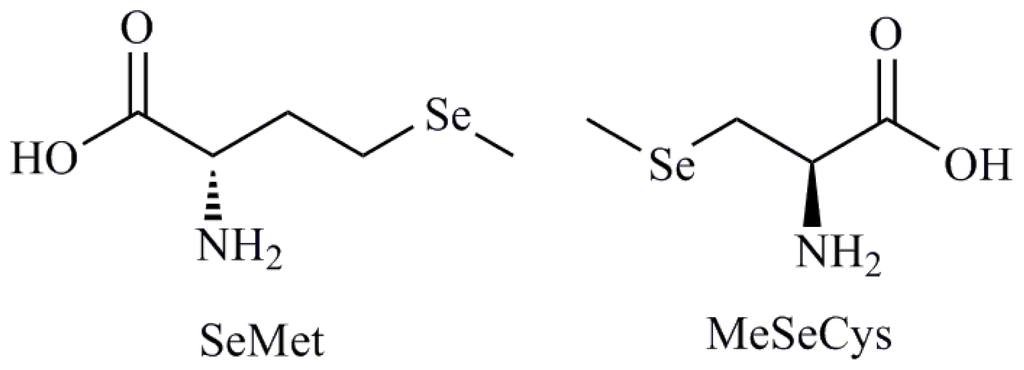
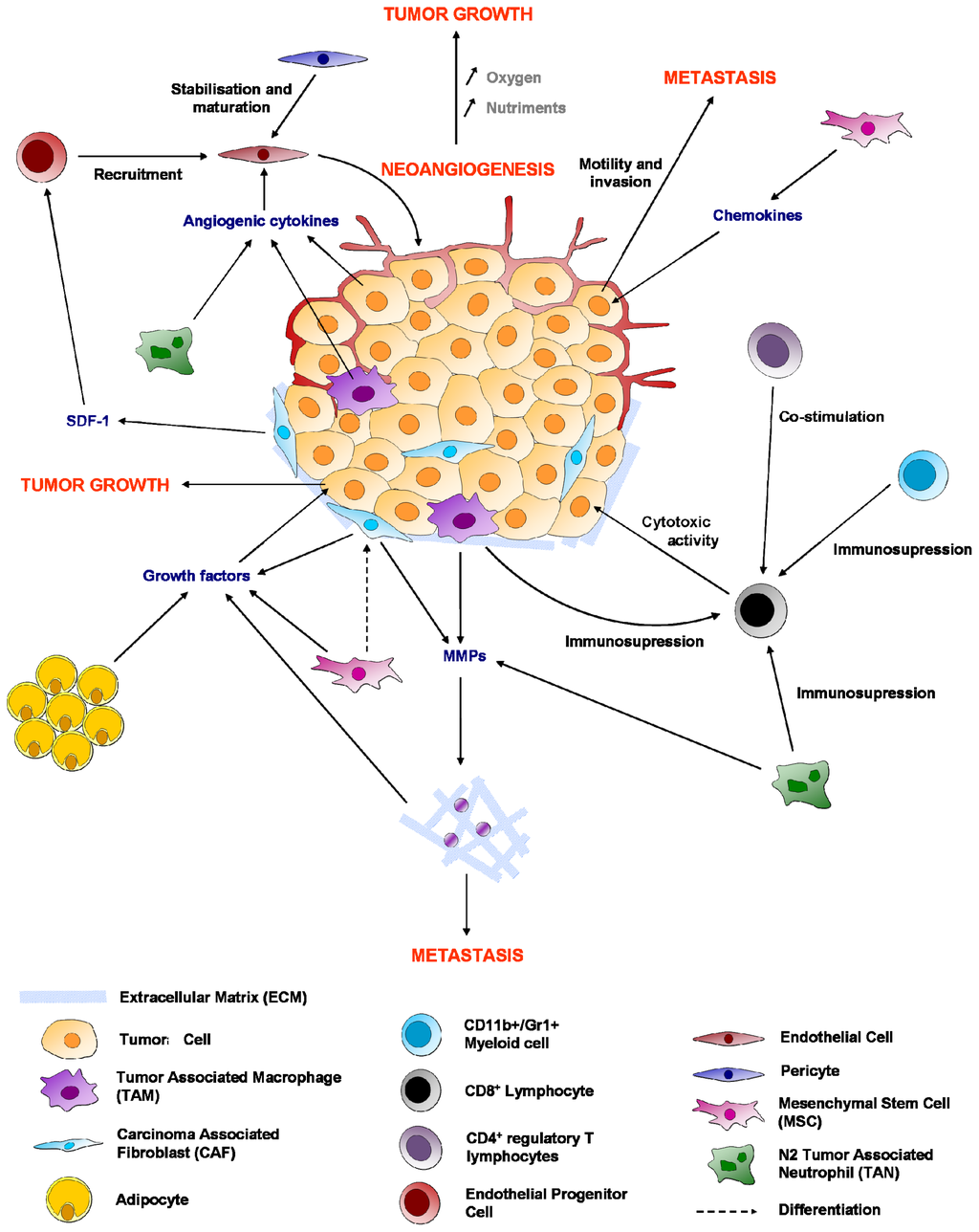
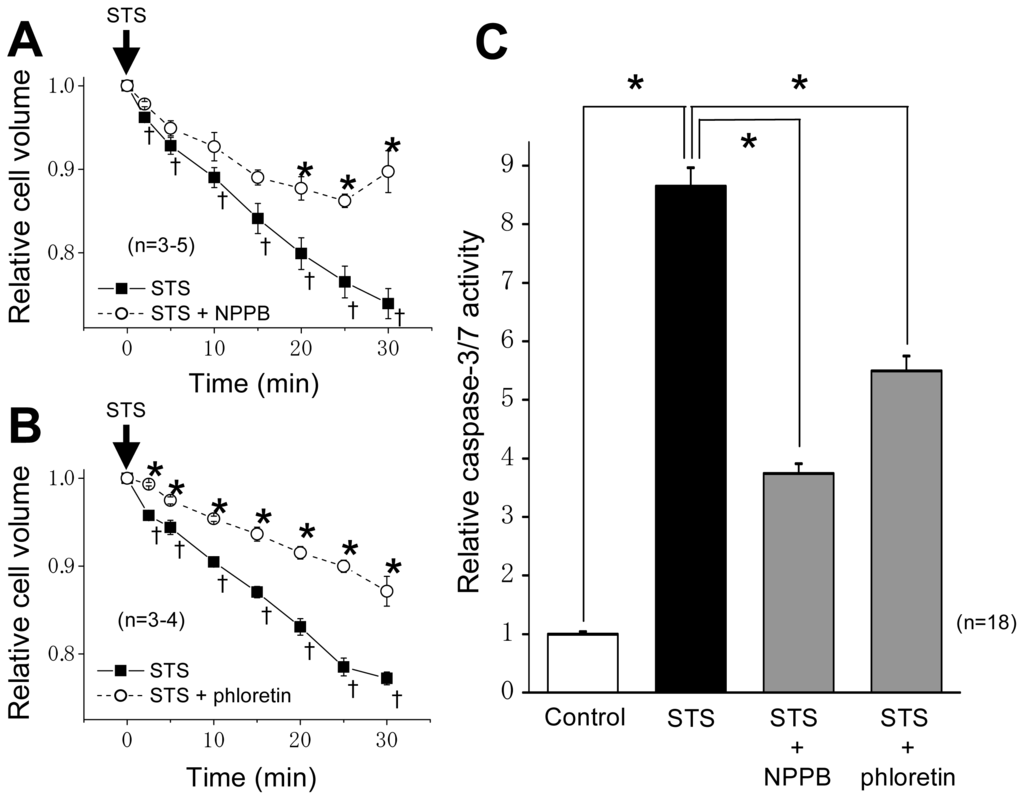
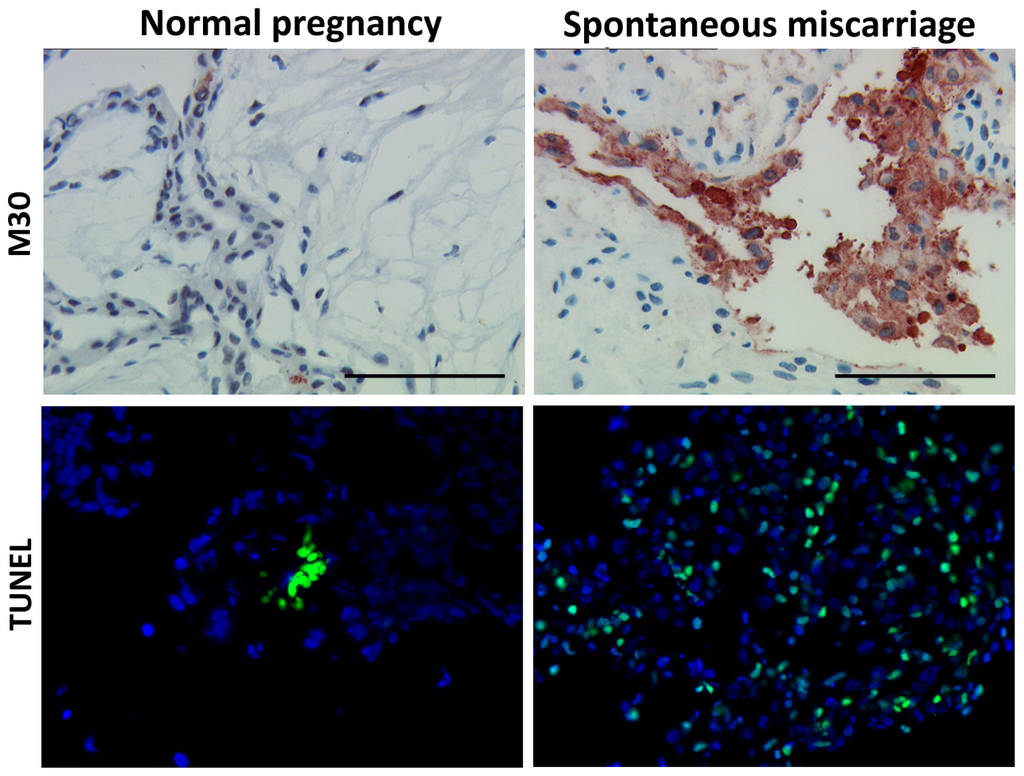
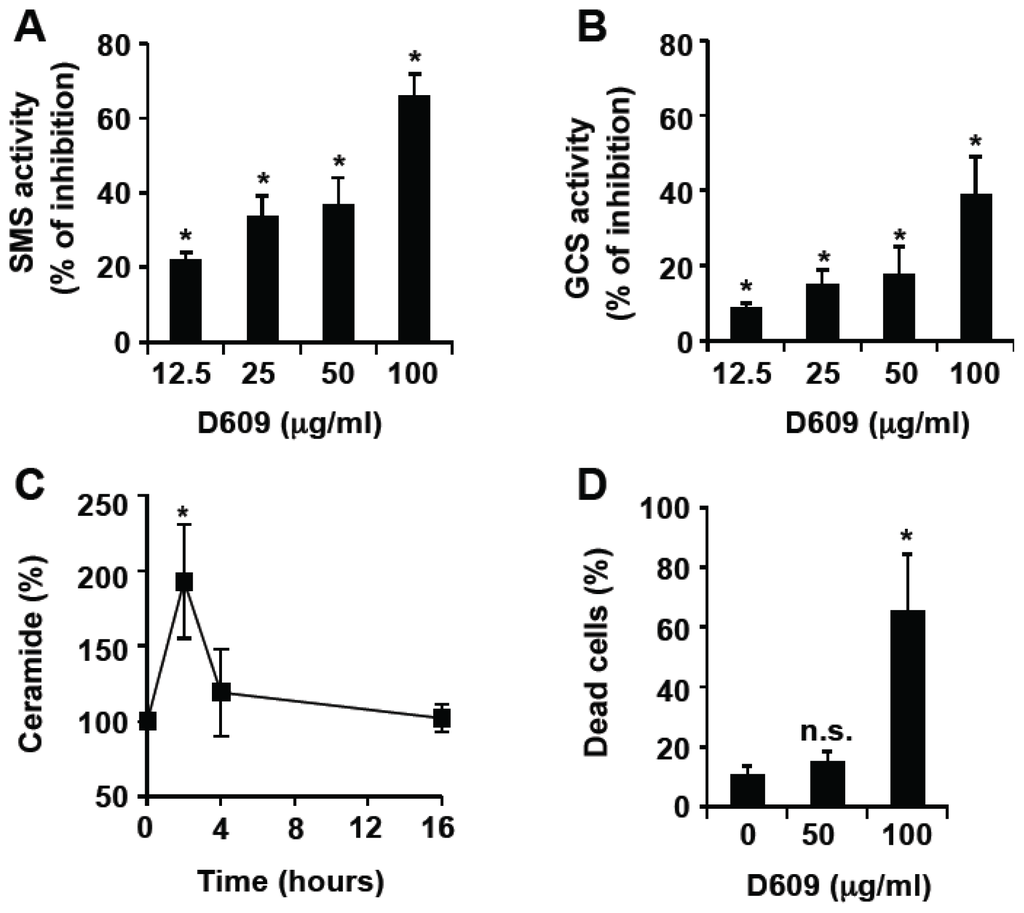
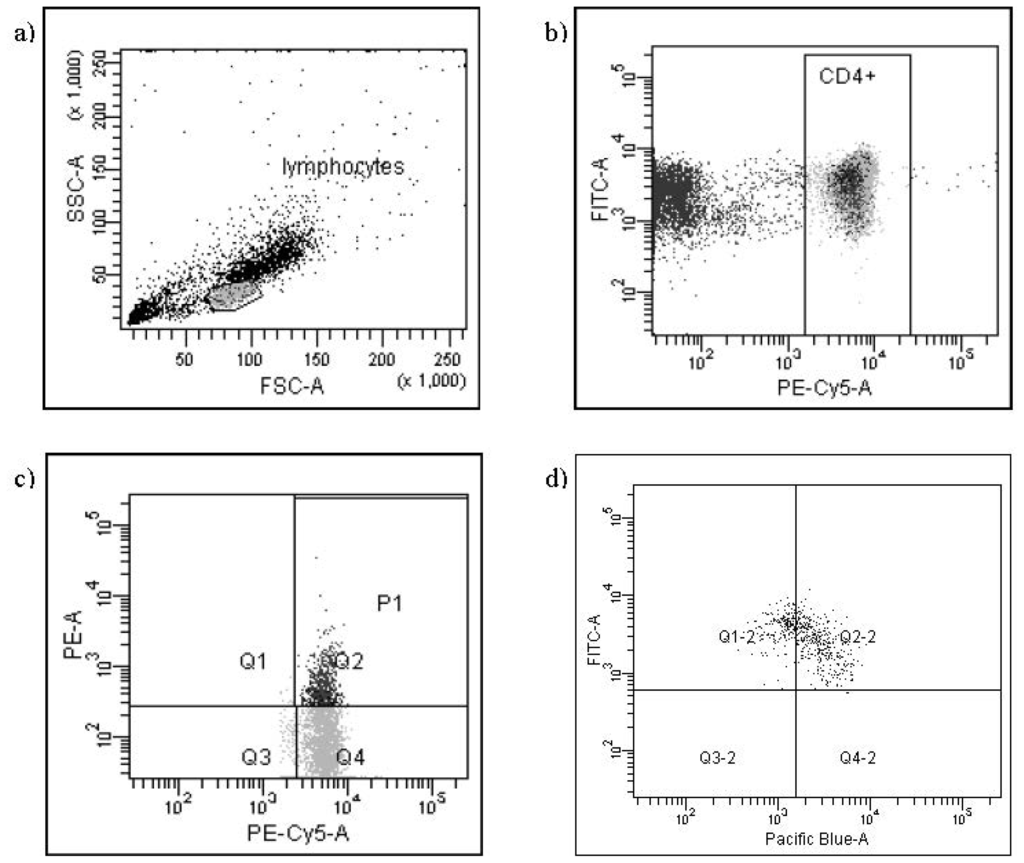
Programmed Cell Death and Apoptosis (Closed)
A topical collection in International Journal of Molecular Sciences (ISSN 1422-0067). This collection belongs to the section "Molecular Pathology, Diagnostics, and Therapeutics".
Viewed by 1103102Editor
Interests: cell death; apoptosis; cellular differentiation; cellular and mitochondrial metabolism; cellular and mitochondrial homoeostasis; oxidative stress; cancer; cancer stem cells; glioma; radiotherapy & radioresistance
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
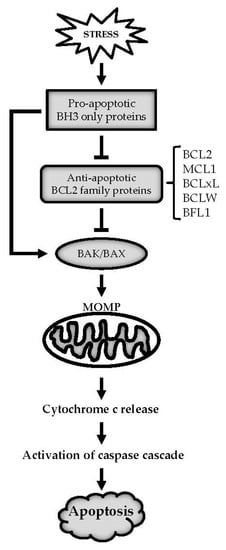
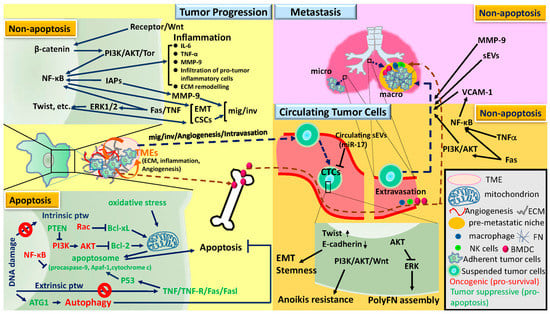
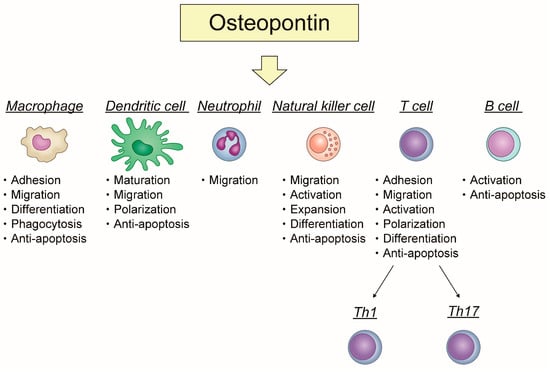
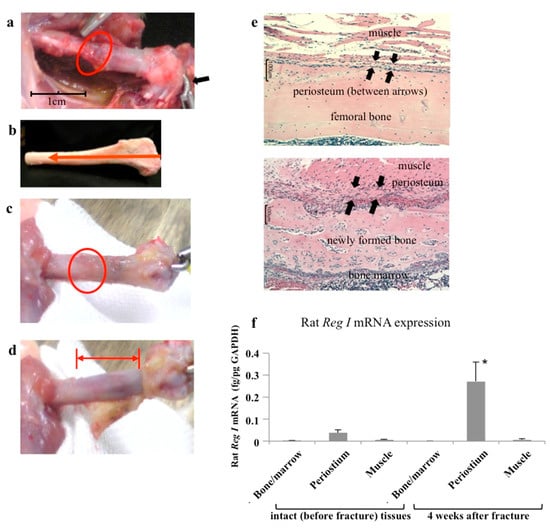
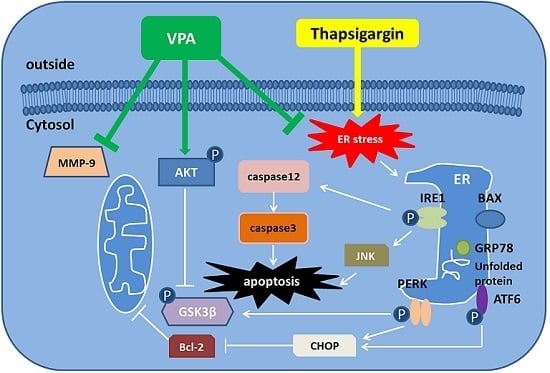
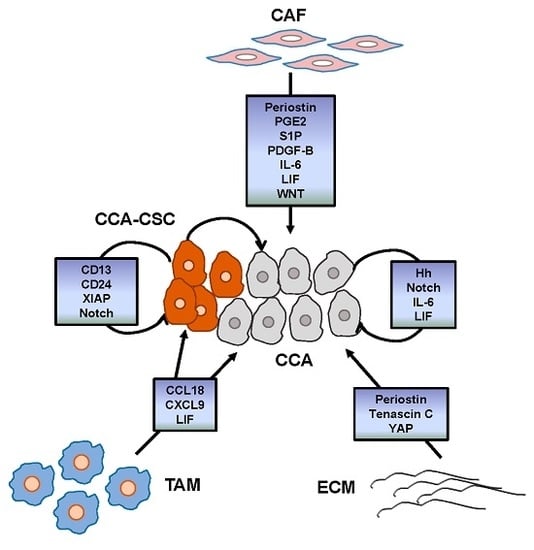
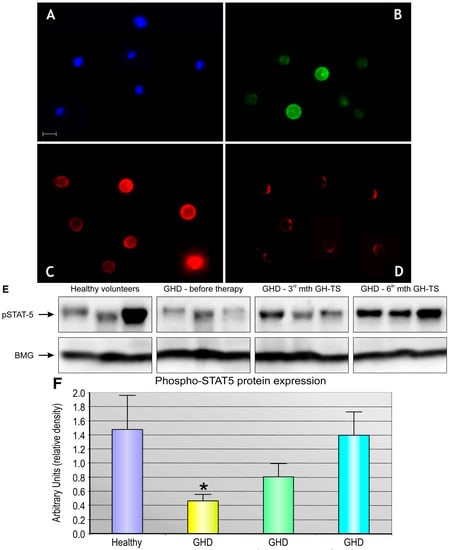
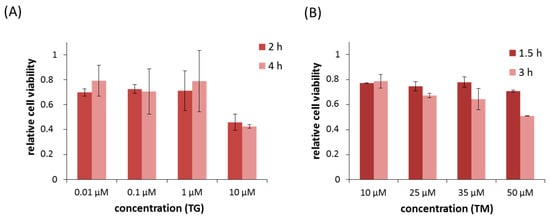
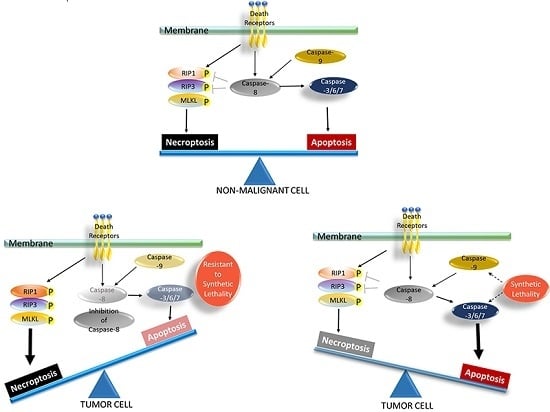
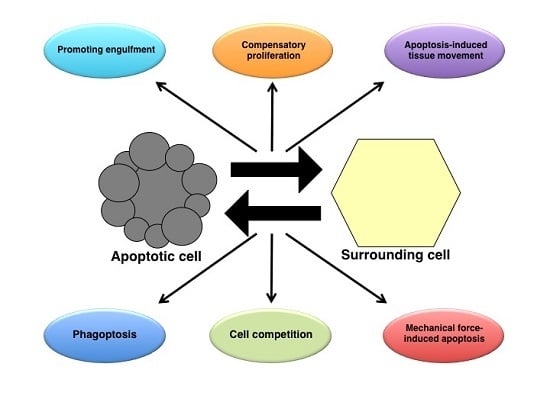
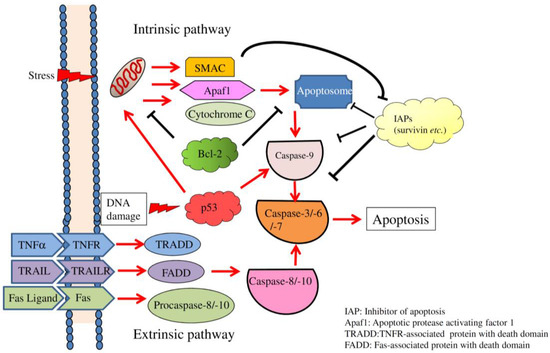
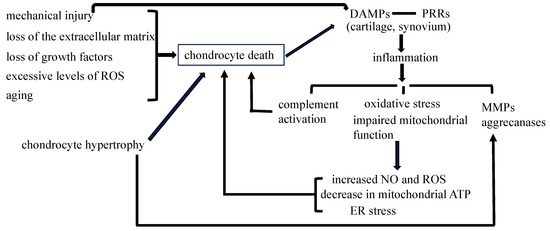
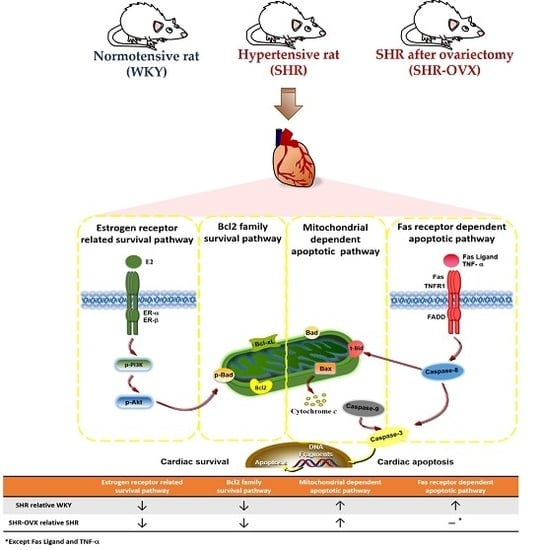
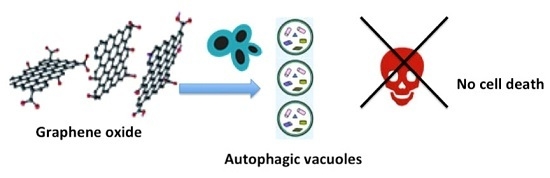
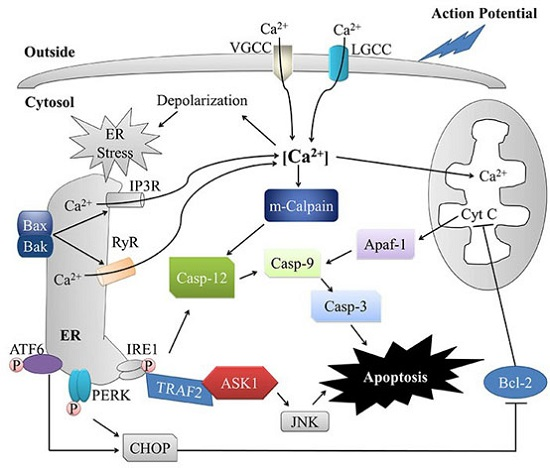
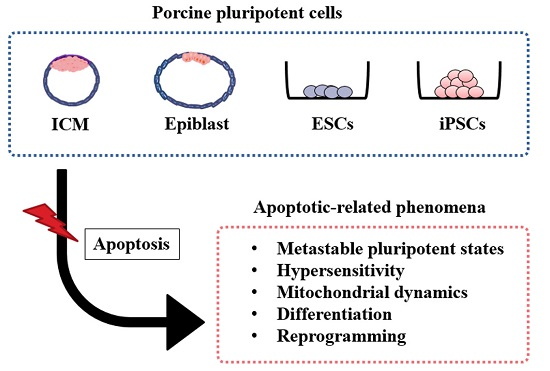
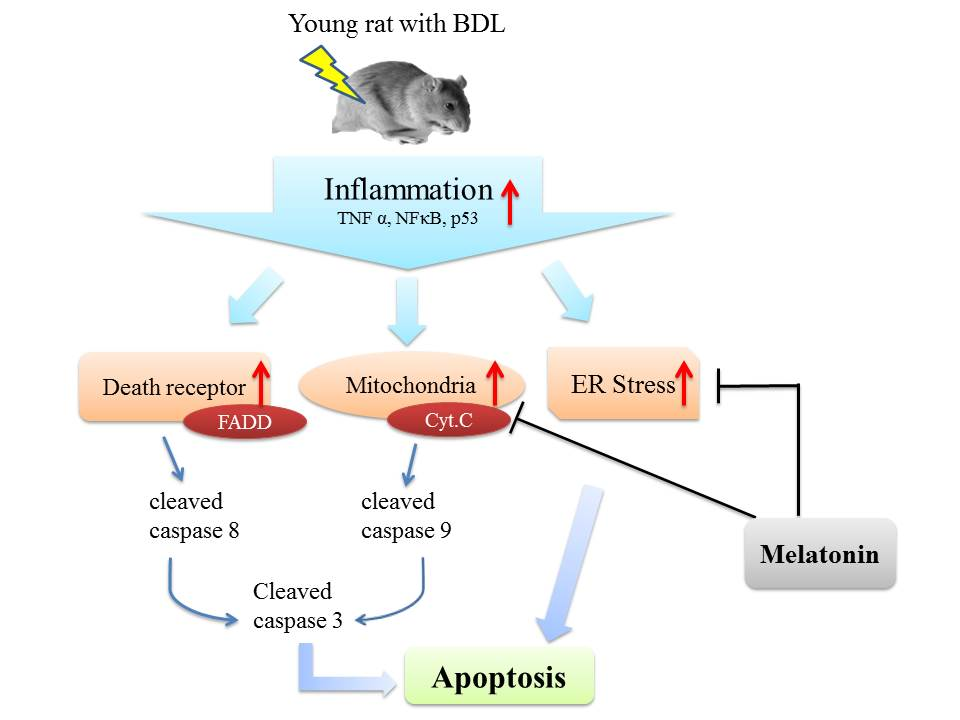
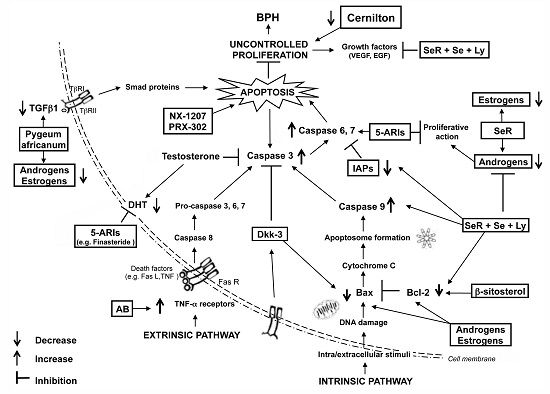
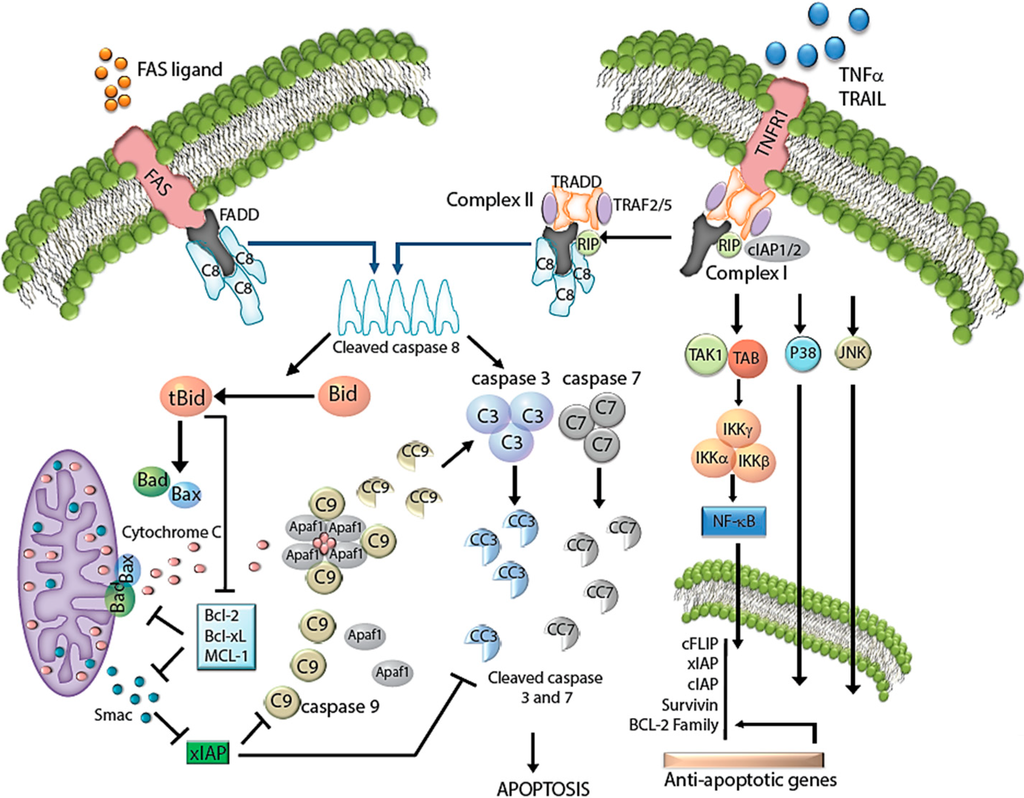
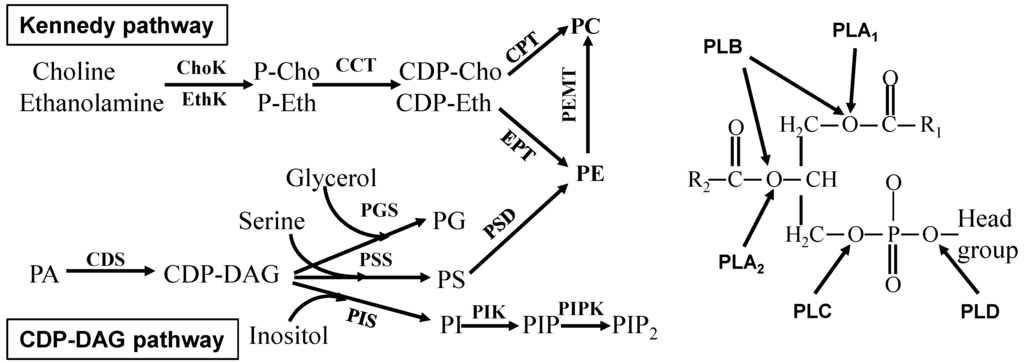
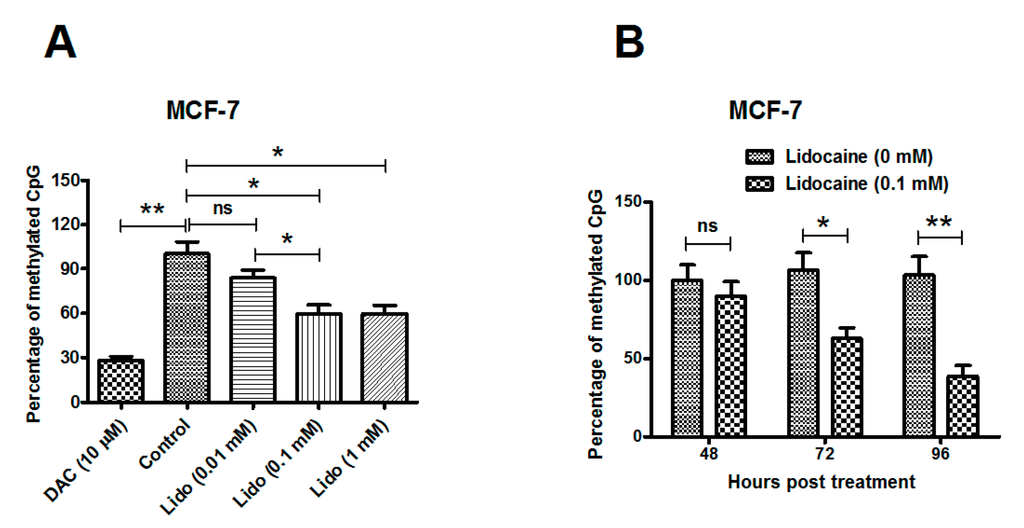
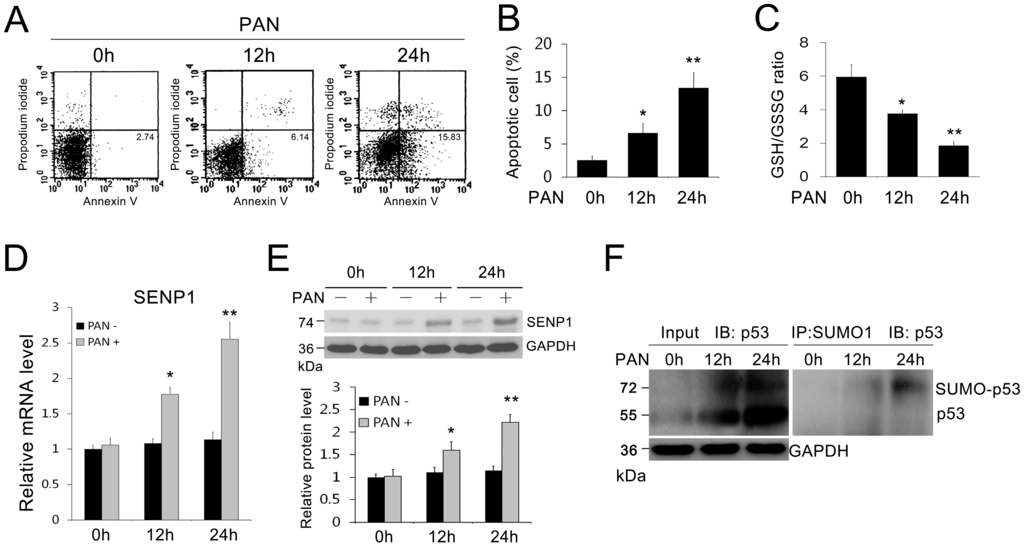
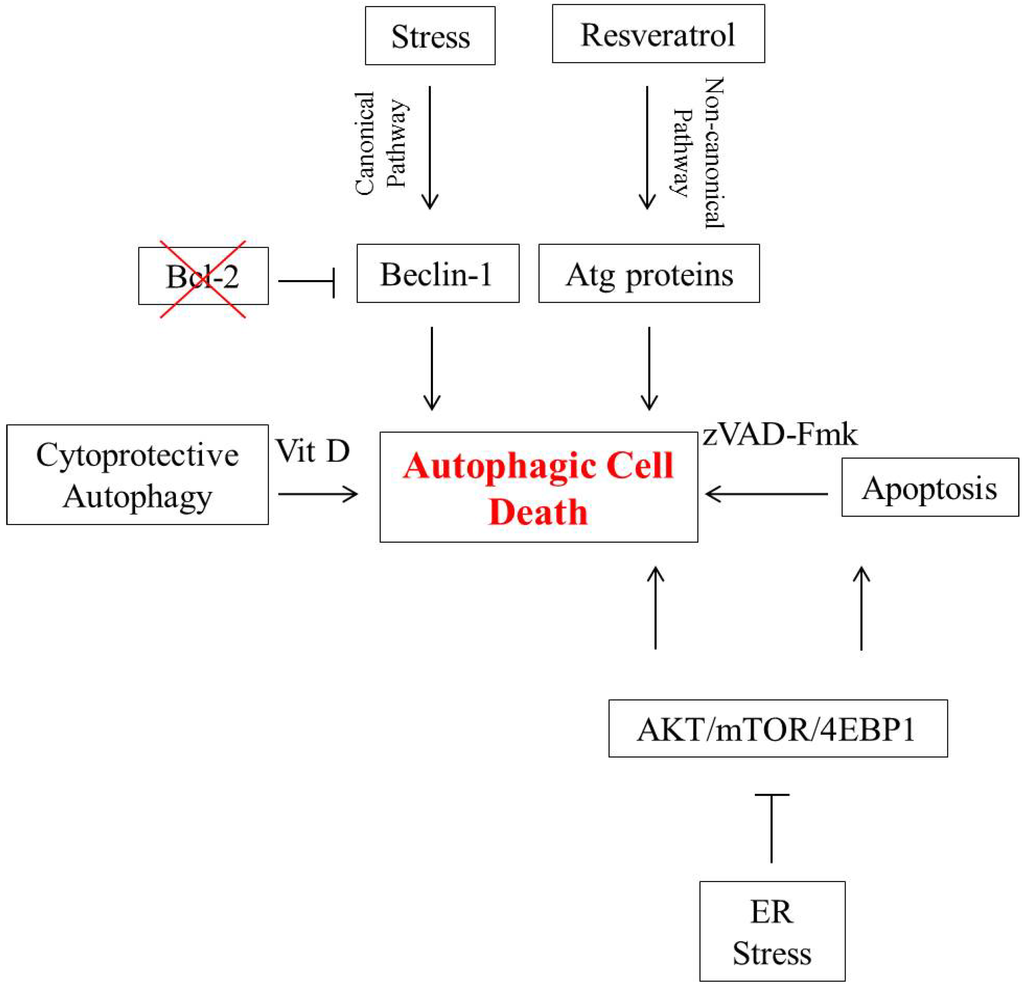
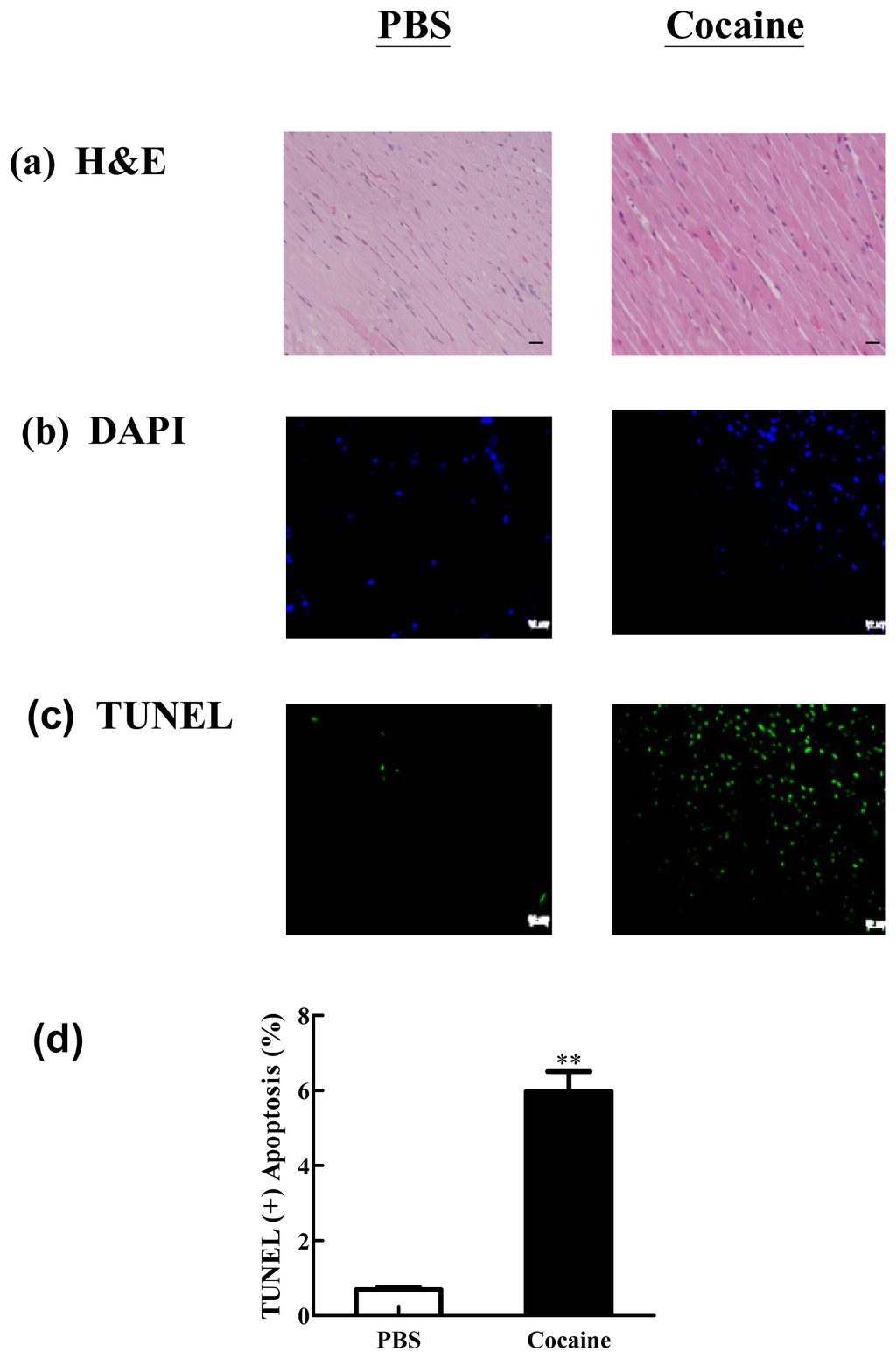
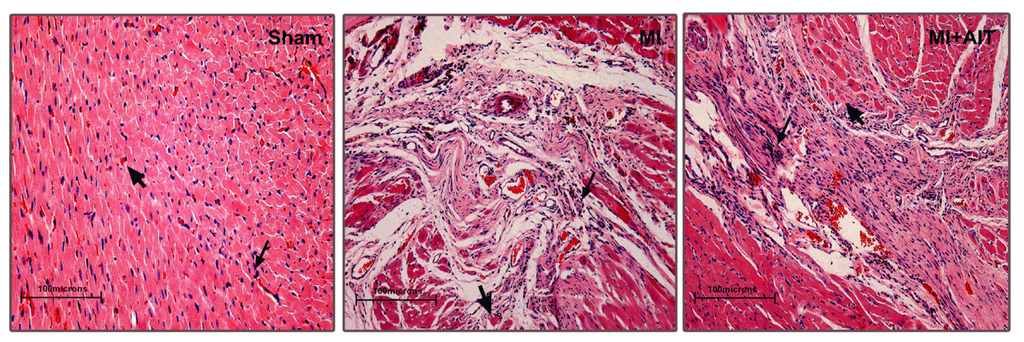
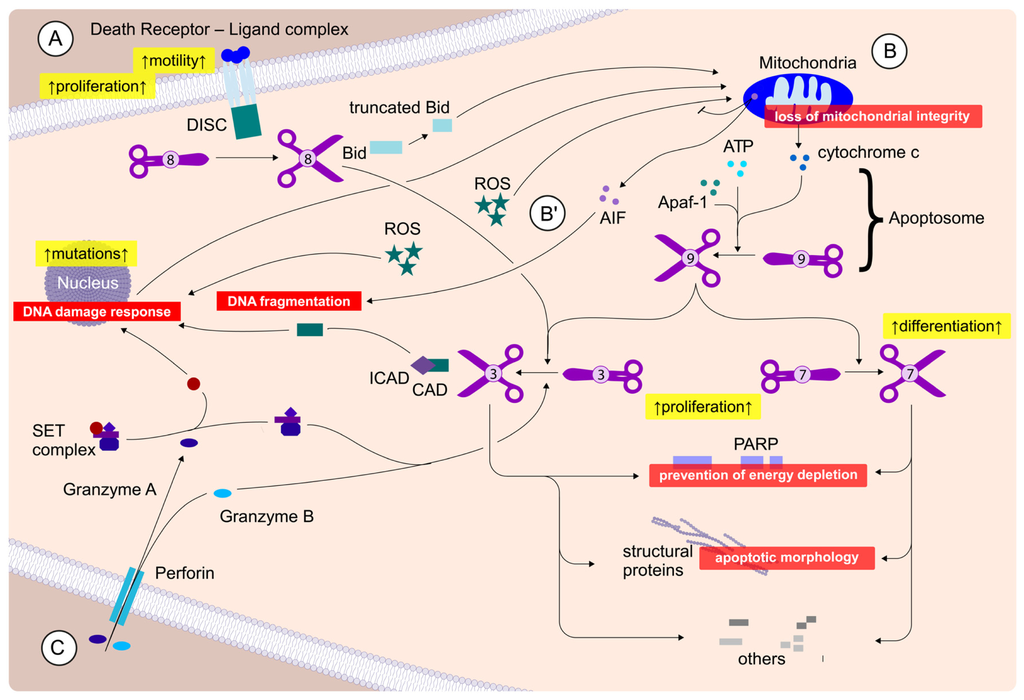
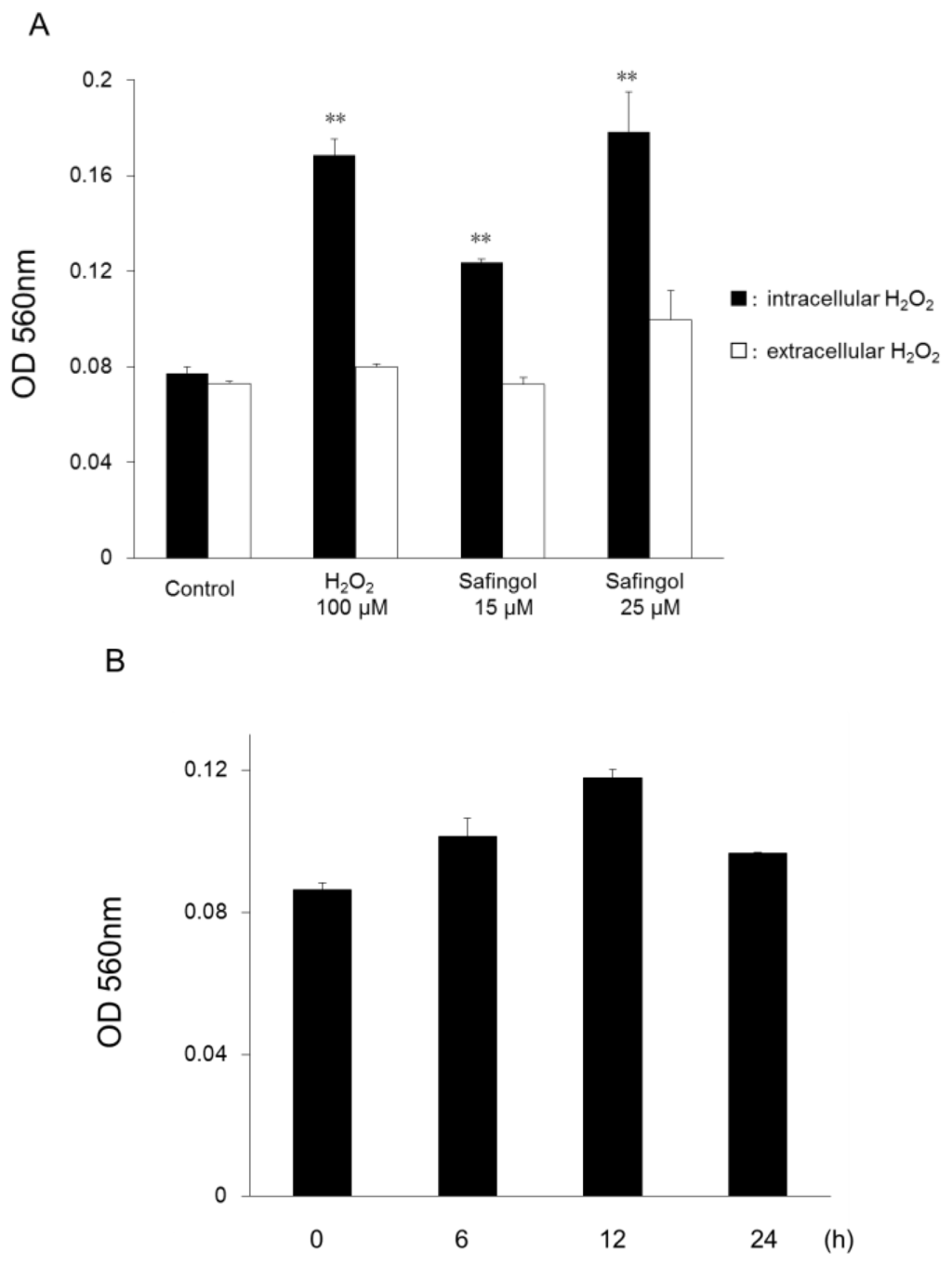
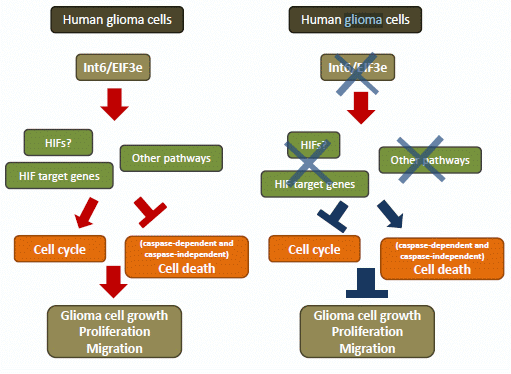
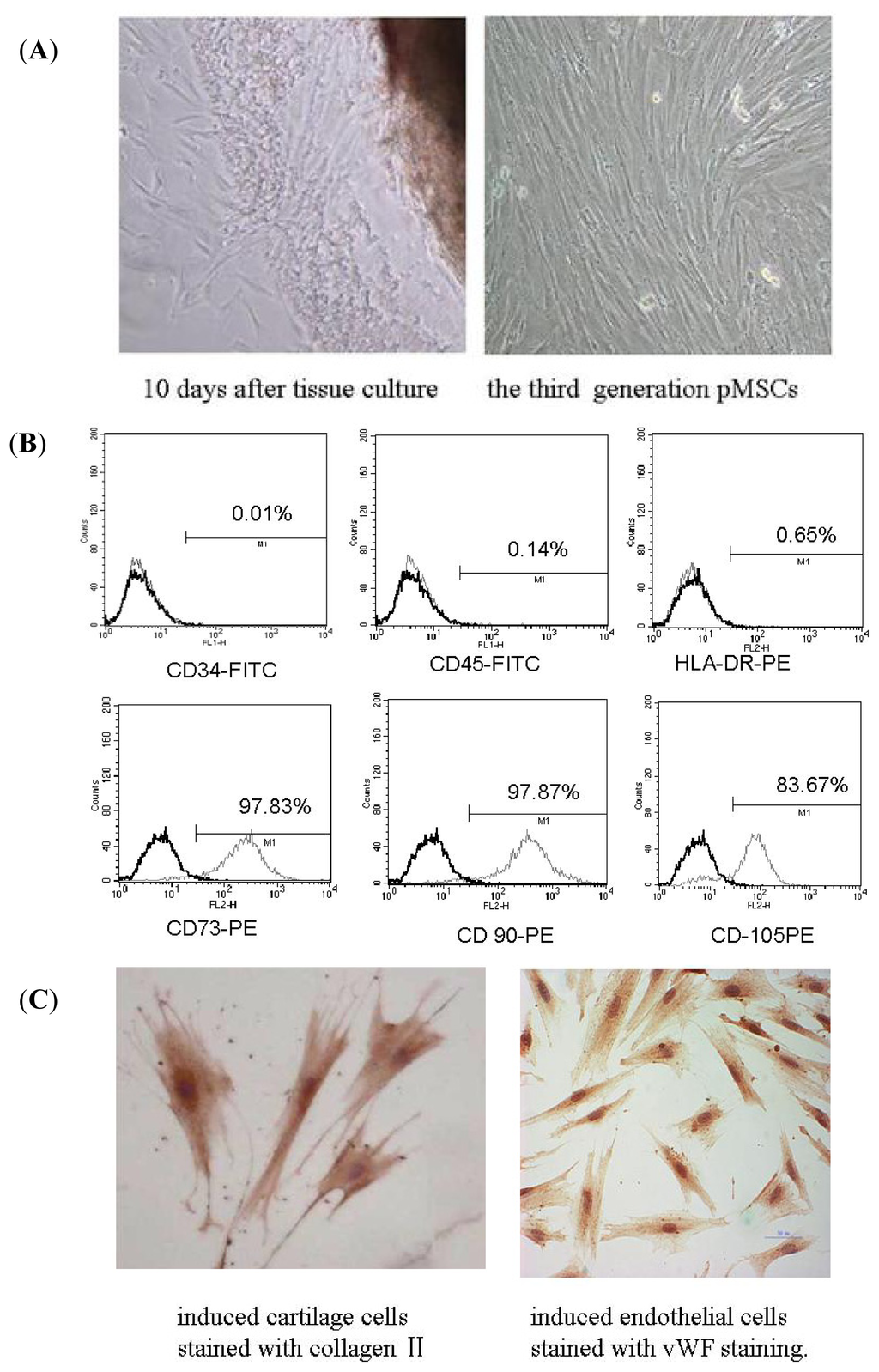
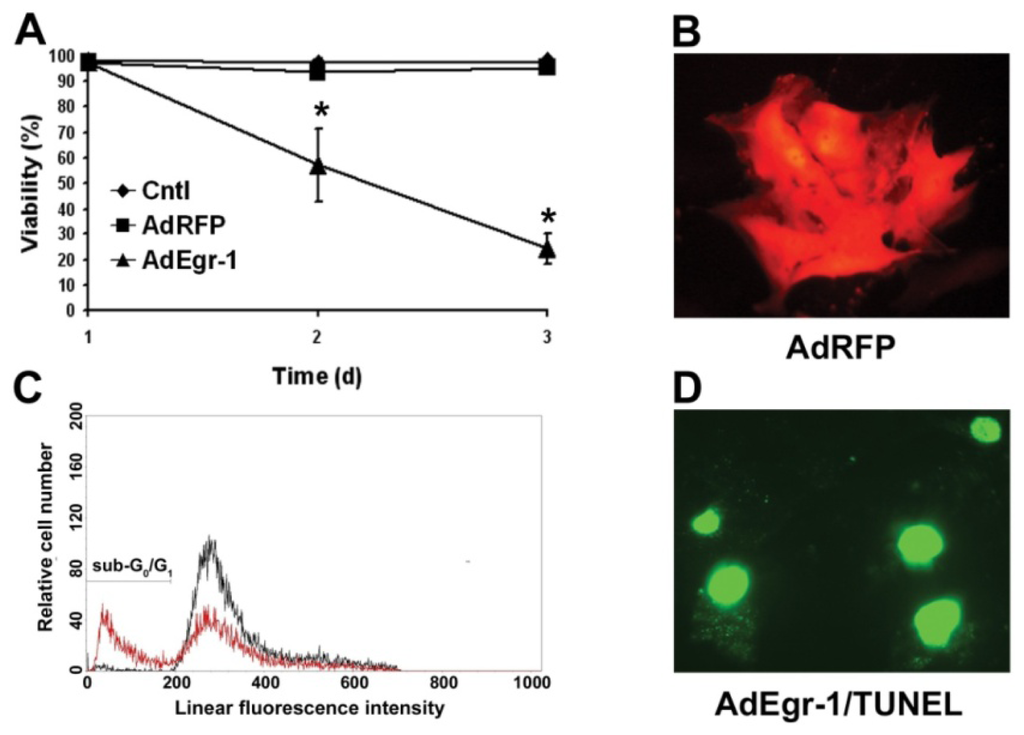
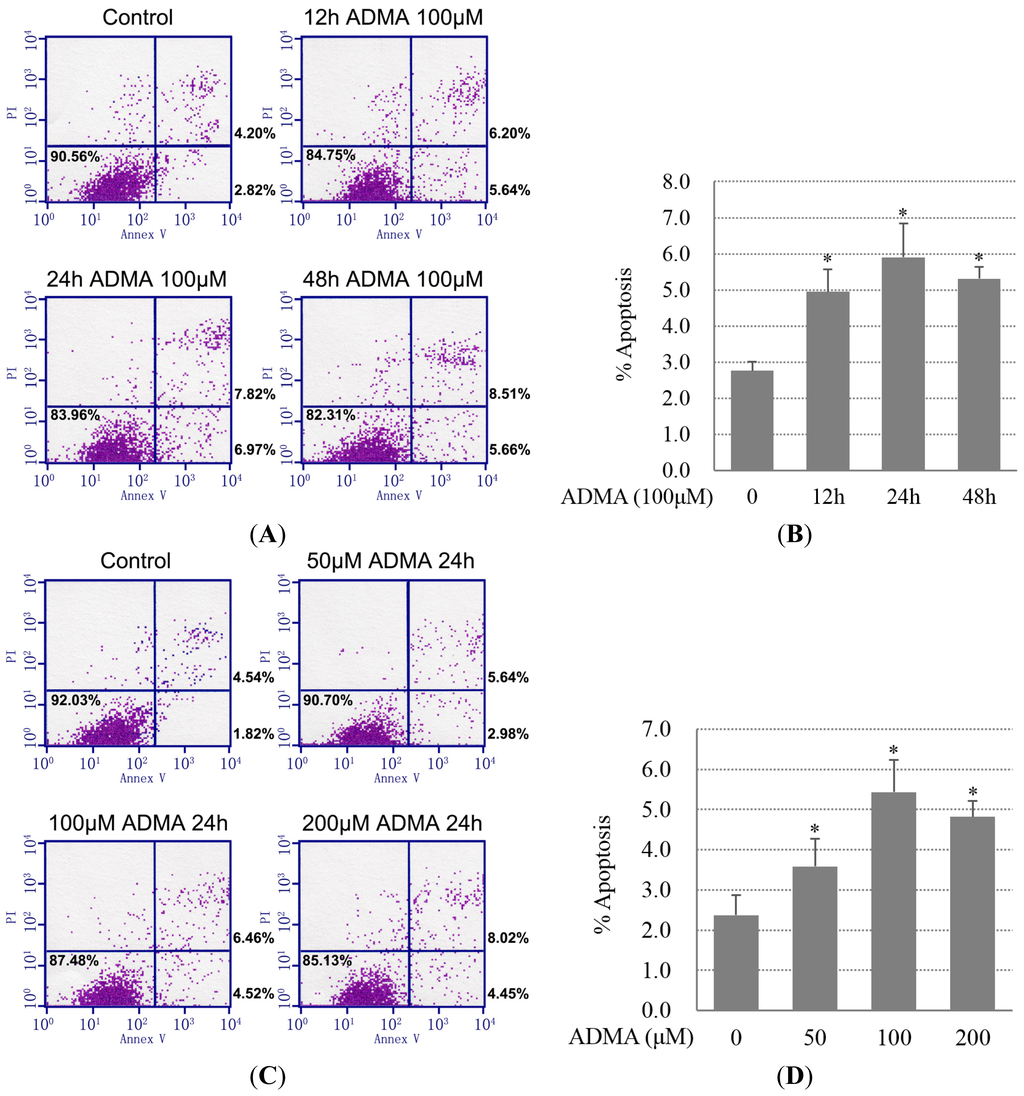
Apoptosis is considered as an essential physiological process in eukaryotes for development, tissue homeostasis, wound healing or immune response. Moreover, apoptosis appears as a key player in physiopathological deregulations, since sustained apoptotic cell death characterizes ischemic and degenerative diseases as well as toxicological responses and since impaired cell death confers to cells and tissues a hyperproliferative phenotype as observed in cancer cells or autoimmune diseases. This collection is dedicated to bring to light some recent developments in the cell death area and to further present some comprehensive reviews on specific “hot” spots in the apoptotic field. In particular, it is of major interest to present the different types of programmed cell death, such as apoptosis, autophagic cell death, necroptosis and secondary necrosis, mitotic catastrophe or senescence and to depict their specific mechanisms and crossovers. The physiopathological context of each process is of particular importance. In addition, the different upstream early events leading to cell death signalling remain to be fully deciphered, notably the role of oxidative stress, ionic homeostasis, metabolic stress signals (e.g. hypoxia), DNA damages and microRNAs.
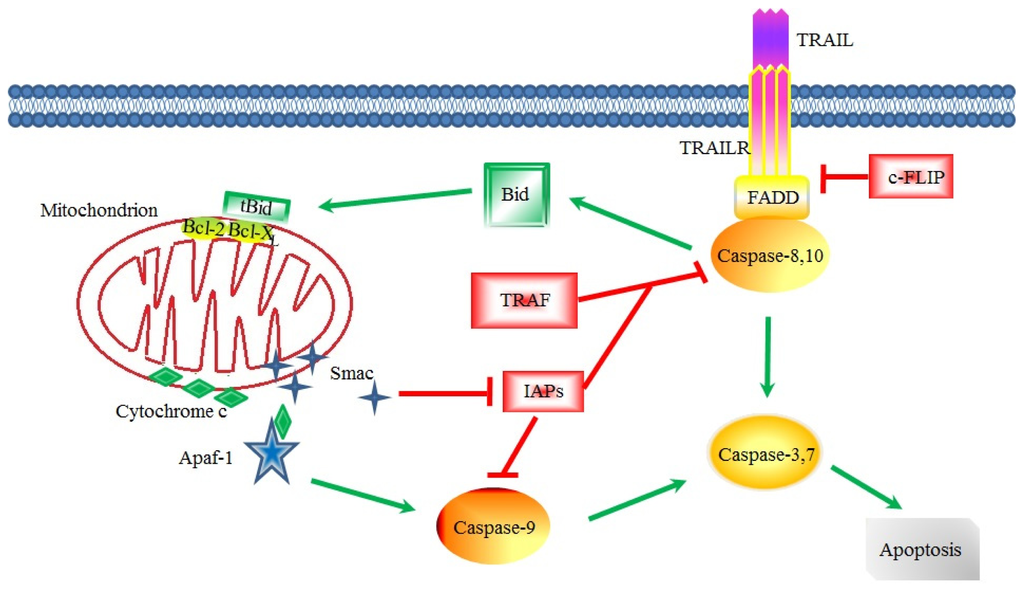
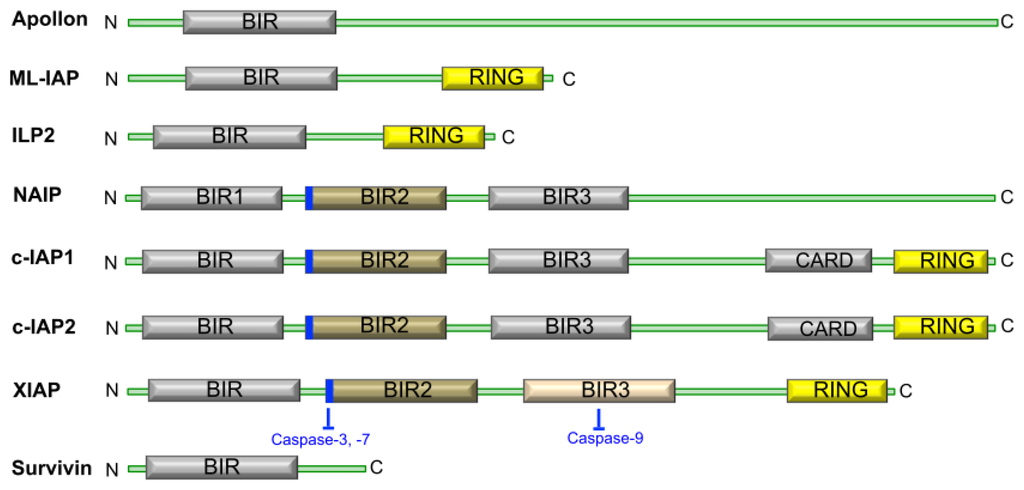
Concerning apoptosis, several aspects have to be addressed as the dependency on the caspase proteases, the involvement of extrinsic (death receptors) and intrinsic (mitochondrial) pathways and the role of the endoplasmic reticulum pathway. Finally, it would be of great interest to focus on the pro-survival vs pro-apoptotic regulation in tumour cells and during anticancer treatments (either chemo or radiotherapy), particularly towards several emerging targets such as cancer stem cells or circulating cancer cells.
Research articles, review articles as well as communications are invited.
Prof. Dr. Anthony Lemarié
Collection Editor
Manuscript Submission Information
Manuscripts for the topical collection can be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on this website. The topical collection considers regular research articles, short communications and review articles. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page.
Please visit the Instructions for Authors page before submitting a manuscript. The article processing charge (APC) for publication in this open access journal is 2900 CHF (Swiss francs).
Keywords
- cell death
- apoptosis
- cellular differentiation
- cellular and mitochondrial metabolism
- cellular and mitochondrial homoeostasis
- oxidative stress
- cancer
- cancer stem cells
- glioma
- radiotherapy & radioresistance