Wogonin Induces Reactive Oxygen Species Production and Cell Apoptosis in Human Glioma Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. MTT Assay
2.4. Quantification of Apoptosis by Flow Cytometry
2.5. Western Blot Analysis
2.6. Reactive Oxygen Species (ROS) Assay
2.7. Statistics
3. Results and Discussion
3.1. Wogonin Induces Cell Apoptosis in Human Glioma Cells
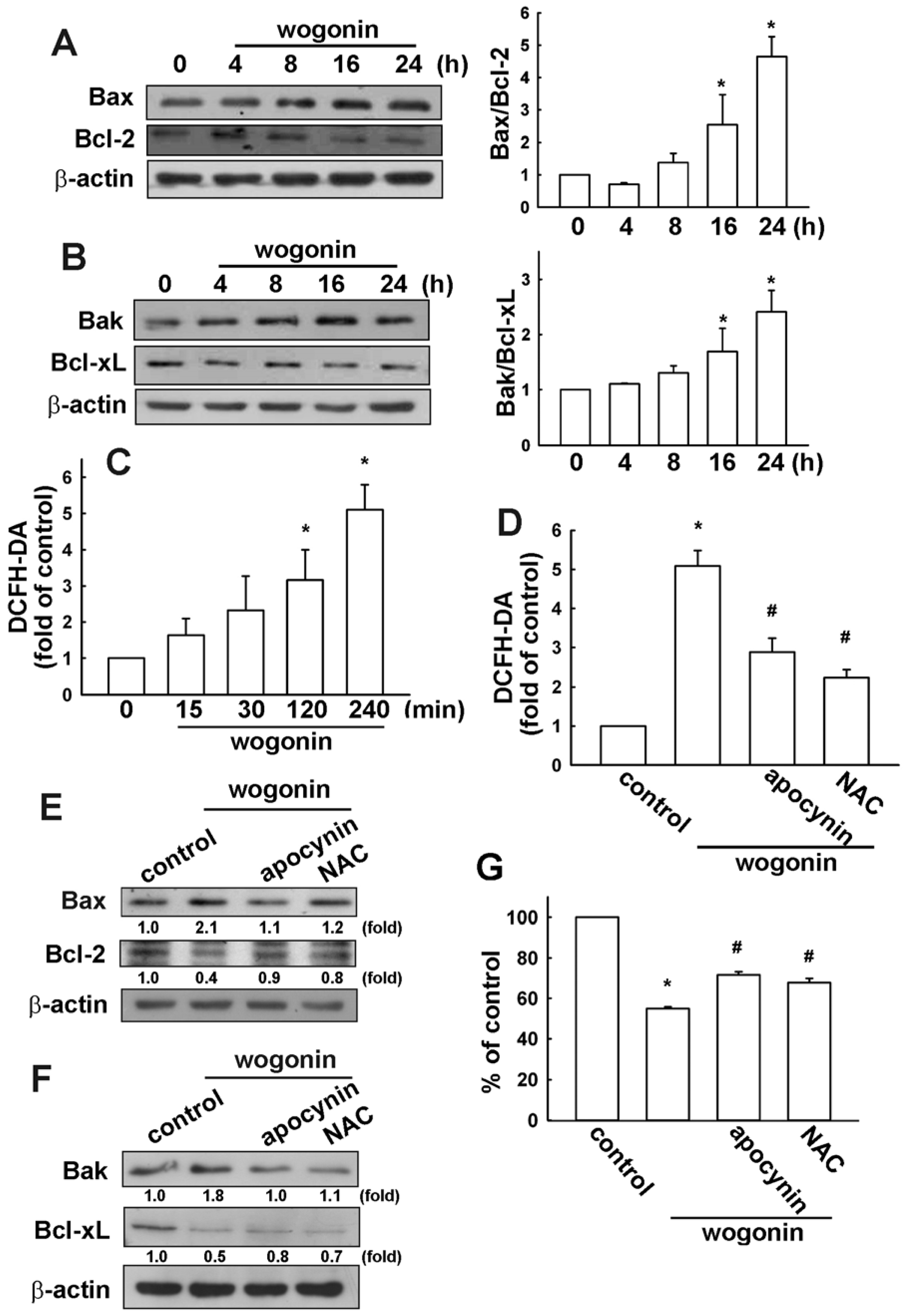
3.2. Wogonin Induces Cell Apoptosis through Reactive Oxygen Species Production in U251 Human Glioma Cells
3.3. Wogonin Induces ER Stress-Related Protein Expression in U251 Human Glioma Cells
3.4. Involvement of Caspase-9, Caspase-3 and PARP Cleavage in Wogonin Treatment in U251 Human Glioma Cells
4. Discussion and Conclusions
Supplementary Materials
ijms-13-09877-s001.pdfAcknowledgments
References
- Kleihues, P.; Soylemezoglu, F.; Schauble, B.; Scheithauer, B.W.; Burger, P.C. Histopathology, classification, and grading of gliomas. Glia 1995, 15, 211–221. [Google Scholar]
- Griscelli, F.; Li, H.; Cheong, C.; Opolon, P.; Bennaceur-Griscelli, A.; Vassal, G.; Soria, J.; Soria, C.; Lu, H.; Perricaudet, M.; et al. Combined effects of radiotherapy and angiostatin gene therapy in glioma tumor model. Proc. Natl. Acad. Sci. USA 2000, 97, 6698–6703. [Google Scholar]
- Amberger, V.R.; Hensel, T.; Ogata, N.; Schwab, M.E. Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res 1998, 58, 149–158. [Google Scholar]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med 2005, 352, 987–996. [Google Scholar]
- Koul, D.; Shen, R.; Bergh, S.; Sheng, X.; Shishodia, S.; Lafortune, T.A.; Lu, Y.; de Groot, J.F.; Mills, G.B.; Yung, W.K. Inhibition of Akt survival pathway by a small-molecule inhibitor in human glioblastoma. Mol. Cancer Ther 2006, 5, 637–644. [Google Scholar]
- Soboloff, J.; Berger, S.A. Sustained ER Ca2+ depletion suppresses protein synthesis and induces activation-enhanced cell death in mast cells. J. Biol. Chem 2002, 277, 13812–13820. [Google Scholar]
- Abcouwer, S.F.; Marjon, P.L.; Loper, R.K.; Vander Jagt, D.L. Response of VEGF expression to amino acid deprivation and inducers of endoplasmic reticulum stress. Invest. Ophthalmol. Vis. Sci 2002, 43, 2791–2798. [Google Scholar]
- Feldman, D.E.; Chauhan, V.; Koong, A.C. The unfolded protein response: A novel component of the hypoxic stress response in tumors. Mol. Cancer Res 2005, 3, 597–605. [Google Scholar]
- Moenner, M.; Pluquet, O.; Bouchecareilh, M.; Chevet, E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res 2007, 67, 10631–10634. [Google Scholar]
- Lee, A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci 2001, 26, 504–510. [Google Scholar]
- Kubota, H.; Suzuki, T.; Lu, J.; Takahashi, S.; Sugita, K.; Sekiya, S.; Suzuki, N. Increased expression of GRP94 protein is associated with decreased sensitivity to X-rays in cervical cancer cell lines. Int. J. Radiat. Biol 2005, 81, 701–709. [Google Scholar]
- Wang, X.Z.; Lawson, B.; Brewer, J.W.; Zinszner, H.; Sanjay, A.; Mi, L.J.; Boorstein, R.; Kreibich, G.; Hendershot, L.M.; Ron, D. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Mol. Cell. Biol 1996, 16, 4273–4280. [Google Scholar]
- Rao, R.V.; Ellerby, H.M.; Bredesen, D.E. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 2004, 11, 372–380. [Google Scholar]
- Harding, H.P.; Calfon, M.; Urano, F.; Novoa, I.; Ron, D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu. Rev. Cell Dev. Biol 2002, 18, 575–599. [Google Scholar]
- McCullough, K.D.; Martindale, J.L.; Klotz, L.O.; Aw, T.Y.; Holbrook, N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol 2001, 21, 1249–1259. [Google Scholar]
- Wek, R.C.; Jiang, H.Y.; Anthony, T.G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans 2006, 34, 7–11. [Google Scholar]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death by apoptosis. Trends Mol. Med 2006, 12, 440–450. [Google Scholar]
- Gupta, S.C.; Hevia, D.; Patchva, S.; Park, B.; Koh, W.; Aggarwal, B.B. Upsides and downsides of reactive oxygen species for cancer: The roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid. Redox Sign 2012, 16, 1295–1322. [Google Scholar]
- Tai, M.C.; Tsang, S.Y.; Chang, L.Y.; Xue, H. Therapeutic potential of wogonin: A naturally occurring flavonoid. CNS Drug Rev 2005, 11, 141–150. [Google Scholar]
- Wakabayashi, I.; Yasui, K. Wogonin inhibits inducible prostaglandin E(2) production in macrophages. Eur. J. Pharmacol 2000, 406, 477–481. [Google Scholar]
- Lee, H.; Kim, Y.O.; Kim, H.; Kim, S.Y.; Noh, H.S.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Suk, K. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J 2003, 17, 1943–1944. [Google Scholar]
- Huang, G.C.; Chow, J.M.; Shen, S.C.; Yang, L.Y.; Lin, C.W.; Chen, Y.C. Wogonin but not Nor-wogonin inhibits lipopolysaccharide and lipoteichoic acid-induced iNOS gene expression and NO production in macrophages. Int. Immunopharmacol 2007, 7, 1054–1063. [Google Scholar]
- Piao, H.Z.; Choi, I.Y.; Park, J.S.; Kim, H.S.; Cheong, J.H.; Son, K.H.; Jeon, S.J.; Ko, K.H.; Kim, W.K. Wogonin inhibits microglial cell migration via suppression of nuclear factor-kappa B activity. Int. Immunopharmacol 2008, 8, 1658–1662. [Google Scholar]
- Chi, Y.S.; Lim, H.; Park, H.; Kim, H.P. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: In vivo regulation of inflammation-associated gene expression. Biochem. Pharmacol 2003, 66, 1271–1278. [Google Scholar]
- Huang, W.H.; Lee, A.R.; Yang, C.H. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis GEORGI. Biosci. Biotechnol. Biochem 2006, 70, 2371–2380. [Google Scholar]
- Cho, J.; Lee, H.K. Wogonin inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Biol. Pharm. Bull 2004, 27, 1561–1564. [Google Scholar]
- Piao, H.Z.; Jin, S.A.; Chun, H.S.; Lee, J.C.; Kim, W.K. Neuroprotective effect of wogonin: Potential roles of inflammatory cytokines. Arch. Pharm. Res 2004, 27, 930–936. [Google Scholar]
- Son, D.; Lee, P.; Lee, J.; Kim, H.; Kim, S.Y. Neuroprotective effect of wogonin in hippocampal slice culture exposed to oxygen and glucose deprivation. Eur. J. Pharmacol 2004, 493, 99–102. [Google Scholar]
- Cho, J.; Lee, H.K. Wogonin inhibits excitotoxic and oxidative neuronal damage in primary cultured rat cortical cells. Eur. J. Pharmacol 2004, 485, 105–110. [Google Scholar]
- Chen, C.C.; Hung, T.H.; Wang, Y.H.; Lin, C.W.; Wang, P.Y.; Lee, C.Y.; Chen, S.F. Wogonin improves histological and functional outcomes, and reduces activation of TLR4/NF-kappaB signaling after experimental traumatic brain injury. PLoS One 2012, 7, e30294. [Google Scholar]
- Himeji, M.; Ohtsuki, T.; Fukazawa, H.; Tanaka, M.; Yazaki, S.; Ui, S.; Nishio, K.; Yamamoto, H.; Tasaka, K.; Mimura, A. Difference of growth-inhibitory effect of Scutellaria baicalensis-producing flavonoid wogonin among human cancer cells and normal diploid cell. Cancer Lett 2007, 245, 269–274. [Google Scholar]
- Lin, C.C.; Kuo, C.L.; Lee, M.H.; Lai, K.C.; Lin, J.P.; Yang, J.S.; Yu, C.S.; Lu, C.C.; Chiang, J.H.; Chueh, F.S.; et al. Wogonin triggers apoptosis in human osteosarcoma U-2 OS cells through the endoplasmic reticulum stress, mitochondrial dysfunction and caspase-3-dependent signaling pathways. Int. J. Oncol 2011, 39, 217–224. [Google Scholar]
- Yu, C.S.; Yu, F.S.; Chuang, Y.C.; Lu, H.F.; Lin, S.Y.; Chiu, T.H.; Chung, J.G. Wogonin inhibits N-acetyltransferase activity and gene expression in human leukemia HL-60 cells. Anticancer Res 2005, 25, 127–132. [Google Scholar]
- Chung, H.; Jung, Y.M.; Shin, D.H.; Lee, J.Y.; Oh, M.Y.; Kim, H.J.; Jang, K.S.; Jeon, S.J.; Son, K.H.; Kong, G. Anticancer effects of wogonin in both estrogen receptor-positive and -negative human breast cancer cell lines in vitro and in nude mice xenografts. Int. J. Cancer 2008, 122, 816–822. [Google Scholar]
- Parajuli, P.; Joshee, N.; Rimando, A.M.; Mittal, S.; Yadav, A.K. In vitro antitumor mechanisms of various Scutellaria extracts and constituent flavonoids. Planta Med 2009, 75, 41–48. [Google Scholar]
- Perez, A.T.; Arun, B.; Tripathy, D.; Tagliaferri, M.A.; Shaw, H.S.; Kimmick, G.G.; Cohen, I.; Shtivelman, E.; Caygill, K.A.; Grady, D.; et al. A phase 1B dose escalation trial of Scutellaria barbata (BZL101) for patients with metastatic breast cancer. Breast Cancer Res. Treat 2010, 120, 111–118. [Google Scholar]
- Rugo, H.; Shtivelman, E.; Perez, A.; Vogel, C.; Franco, S.; Tan Chiu, E.; Melisko, M.; Tagliaferri, M.; Cohen, I.; Shoemaker, M.; et al. Phase I trial and antitumor effects of BZL101 for patients with advanced breast cancer. Breast Cancer Res. Treat 2007, 105, 17–28. [Google Scholar]
- Wang, W.; Guo, Q.L.; You, Q.D.; Zhang, K.; Yang, Y.; Yu, J.; Liu, W.; Zhao, L.; Gu, H.Y.; Hu, Y.; et al. The anticancer activities of wogonin in murine sarcoma S180 both in vitro and in vivo. Biol. Pharm. Bull 2006, 29, 1132–1137. [Google Scholar]
- Baumann, S.; Fas, S.C.; Giaisi, M.; Muller, W.W.; Merling, A.; Gulow, K.; Edler, L.; Krammer, P.H.; Li-Weber, M. Wogonin preferentially kills malignant lymphocytes and suppresses T-cell tumor growth by inducing PLCgamma1- and Ca2+-dependent apoptosis. Blood 2008, 111, 2354–2363. [Google Scholar]
- Lu, N.; Gao, Y.; Ling, Y.; Chen, Y.; Yang, Y.; Gu, H.Y.; Qi, Q.; Liu, W.; Wang, X.T.; You, Q.D.; Guo, Q.L. Wogonin suppresses tumor growth in vivo and VEGF-induced angiogenesis through inhibiting tyrosine phosphorylation of VEGFR2. Life Sci 2008, 82, 956–963. [Google Scholar]
- Fas, S.C.; Baumann, S.; Zhu, J.Y.; Giaisi, M.; Treiber, M.K.; Mahlknecht, U.; Krammer, P.H.; Li-Weber, M. Wogonin sensitizes resistant malignant cells to TNFα- and TRAIL-induced apoptosis. Blood 2006, 108, 3700–3706. [Google Scholar]
- Huang, S.M.; Cheung, C.W.; Chang, C.S.; Tang, C.H.; Liu, J.F.; Lin, Y.H.; Chen, J.H.; Ko, S.H.; Wong, K.L.; Lu, D.Y. Phloroglucinol derivative MCPP induces cell apoptosis in human colon cancer. J. Cell. Biochem 2011, 112, 643–652. [Google Scholar]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 9, 501–507. [Google Scholar]
- Leber, B.; Lin, J.; Andrews, D.W. Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene 2010, 29, 5221–5230. [Google Scholar]
- Lau, A.T.; Wang, Y.; Chiu, J.F. Reactive oxygen species: Current knowledge and applications in cancer research and therapeutic. J. Cell. Biochem 2008, 104, 657–667. [Google Scholar]
- Mates, J.M.; Sanchez-Jimenez, F.M. Role of reactive oxygen species in apoptosis: Implications for cancer therapy. Int. J. Biochem. Cell Biol 2000, 32, 157–170. [Google Scholar]
- Zu, K.; Hawthorn, L.; Ip, C. Up-regulation of c-Jun-NH2-kinase pathway contributes to the induction of mitochondria-mediated apoptosis by alpha-tocopheryl succinate in human prostate cancer cells. Mol. Cancer Ther 2005, 4, 43–50. [Google Scholar]
- Iwamaru, A.; Iwado, E.; Kondo, S.; Newman, R.A.; Vera, B.; Rodriguez, A.D.; Kondo, Y. Eupalmerin acetate, a novel anticancer agent from Caribbean gorgonian octocorals, induces apoptosis in malignant glioma cells via the c-Jun NH2-terminal kinase pathway. Mol. Cancer Ther 2007, 6, 184–192. [Google Scholar]
- Wenzel, U.; Nickel, A.; Kuntz, S.; Daniel, H. Ascorbic acid suppresses drug-induced apoptosis in human colon cancer cells by scavenging mitochondrial superoxide anions. Carcinogenesis 2004, 25, 703–712. [Google Scholar]
- Reddy, R.K.; Mao, C.; Baumeister, P.; Austin, R.C.; Kaufman, R.J.; Lee, A.S. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: Role of ATP binding site in suppression of caspase-7 activation. J. Biol. Chem 2003, 278, 20915–20924. [Google Scholar]
- Masud, A.; Mohapatra, A.; Lakhani, S.A.; Ferrandino, A.; Hakem, R.; Flavell, R.A. Endoplasmic reticulum stress-induced death of mouse embryonic fibroblasts requires the intrinsic pathway of apoptosis. J. Biol. Chem 2007, 282, 14132–14139. [Google Scholar]
- Kamata, H.; Hirata, H. Redox regulation of cellular signalling. Cell. Signal 1999, 11, 1–14. [Google Scholar]
- Annunziato, L.; Amoroso, S.; Pannaccione, A.; Cataldi, M.; Pignataro, G.; D’Alessio, A.; Sirabella, R.; Secondo, A.; Sibaud, L.; Di Renzo, G.F. Apoptosis induced in neuronal cells by oxidative stress: Role played by caspases and intracellular calcium ions. Toxicol. Lett 2003, 139, 125–133. [Google Scholar]
- Monks, T.J.; Xie, R.; Tikoo, K.; Lau, S.S. ROS-induced histone modifications and their role in cell survival and cell death. Drug Metab. Rev 2006, 38, 755–767. [Google Scholar]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 2006, 10, 175–176. [Google Scholar]
- Yu, J.S.; Kim, A.K. Wogonin induces apoptosis by activation of ERK and p38 MAPKs signaling pathways and generation of reactive oxygen species in human breast cancer cells. Mol. Cells 2011, 31, 327–335. [Google Scholar]
- Lee, D.H.; Kim, C.; Zhang, L.; Lee, Y.J. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem. Pharmacol 2008, 75, 2020–2033. [Google Scholar]
- Liu, Z.L.; Tanaka, S.; Horigome, H.; Hirano, T.; Oka, K. Induction of apoptosis in human lung fibroblasts and peripheral lymphocytes in vitro by Shosaiko-to derived phenolic metabolites. Biol. Pharm. Bull 2002, 25, 37–41. [Google Scholar]
- Lim, S.C.; Hahm, K.S.; Lee, S.H.; Oh, S.H. Autophagy involvement in cadmium resistance through induction of multidrug resistance-associated protein and counterbalance of endoplasmic reticulum stress WI38 lung epithelial fibroblast cells. Toxicology 2010, 276, 18–26. [Google Scholar]
- Park, J.W.; Woo, K.J.; Lee, J.T.; Lim, J.H.; Lee, T.J.; Kim, S.H.; Choi, Y.H.; Kwon, T.K. Resveratrol induces pro-apoptotic endoplasmic reticulum stress in human colon cancer cells. Oncol. Rep 2007, 18, 1269–1273. [Google Scholar]
- Kim, A.J.; Shi, Y.; Austin, R.C.; Werstuck, G.H. Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. J. Cell Sci 2005, 118, 89–99. [Google Scholar]
- Lu, D.Y.; Chang, C.S.; Yeh, W.L.; Tang, C.H.; Cheung, C.W.; Leung, Y.M.; Liu, J.F.; Wong, K.L. The novel phloroglucinol derivative BFP induces apoptosis of glioma cancer through reactive oxygen species and endoplasmic reticulum stress pathways. Phytomedicine 2012, in press. [Google Scholar]





© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsai, C.-F.; Yeh, W.-L.; Huang, S.M.; Tan, T.-W.; Lu, D.-Y. Wogonin Induces Reactive Oxygen Species Production and Cell Apoptosis in Human Glioma Cancer Cells. Int. J. Mol. Sci. 2012, 13, 9877-9892. https://doi.org/10.3390/ijms13089877
Tsai C-F, Yeh W-L, Huang SM, Tan T-W, Lu D-Y. Wogonin Induces Reactive Oxygen Species Production and Cell Apoptosis in Human Glioma Cancer Cells. International Journal of Molecular Sciences. 2012; 13(8):9877-9892. https://doi.org/10.3390/ijms13089877
Chicago/Turabian StyleTsai, Cheng-Fang, Wei-Lan Yeh, Ssu Ming Huang, Tzu-Wei Tan, and Dah-Yuu Lu. 2012. "Wogonin Induces Reactive Oxygen Species Production and Cell Apoptosis in Human Glioma Cancer Cells" International Journal of Molecular Sciences 13, no. 8: 9877-9892. https://doi.org/10.3390/ijms13089877
APA StyleTsai, C.-F., Yeh, W.-L., Huang, S. M., Tan, T.-W., & Lu, D.-Y. (2012). Wogonin Induces Reactive Oxygen Species Production and Cell Apoptosis in Human Glioma Cancer Cells. International Journal of Molecular Sciences, 13(8), 9877-9892. https://doi.org/10.3390/ijms13089877



