Opposite Associations of Plasma Homoarginine and Ornithine with Arginine in Healthy Children and Adolescents
Abstract
:1. Introduction
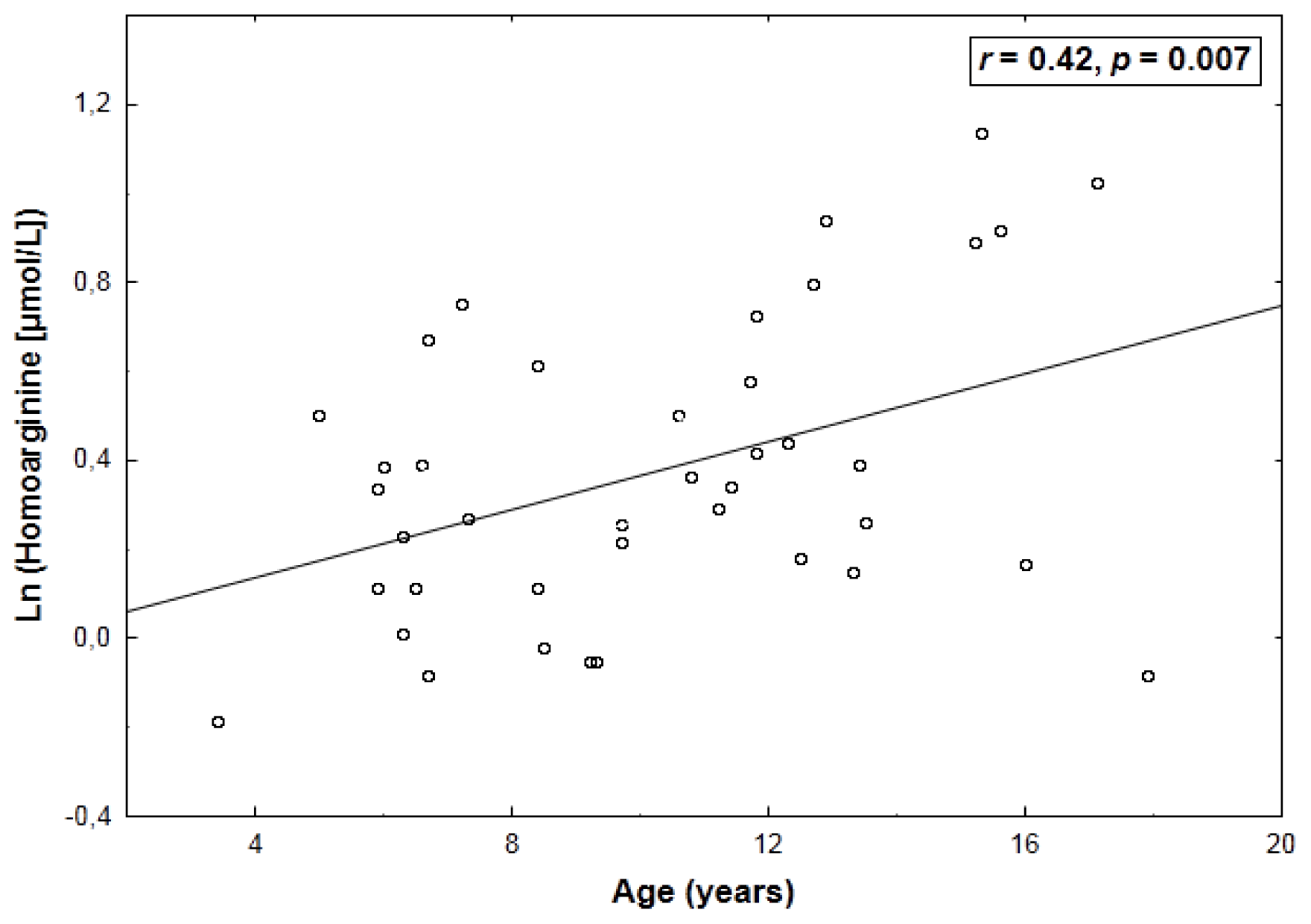
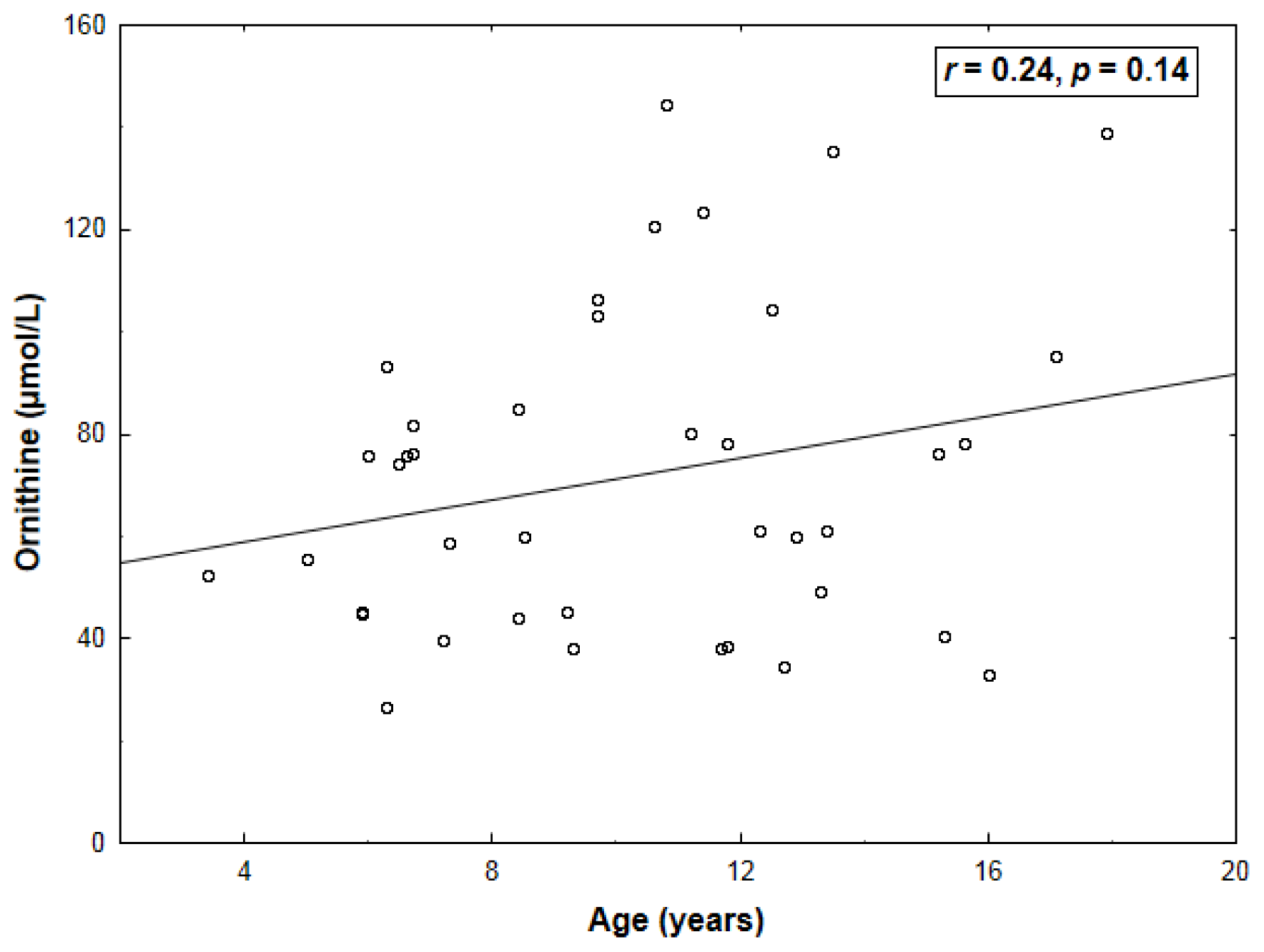
2. Results
3. Discussion
3.1. Comparison with Other Reports on Circulating Homoarginine and Ornithine
3.2. Proposed Mechanisms of the Relations between Homoarginine, Arginine and Ornithine
3.3. Homoarginine and Ornithine versus the l-Arginine—NO Pathway and Carotid Vascular Structure
3.4. Study Limitations
4. Experimental Section
4.1. Subjects
4.2. Biochemical Assays
4.3. Carotid Ultrasound
4.4. Statistical Analysis
5. Conclusions
| Variable | |
|---|---|
| Estimated GFR (mL/min per 1.73 m2) | 122 ± 22 |
| LDL cholesterol (mmol/L) | 2.3 ± 0.6 |
| HDL cholesterol (mmol/L) | 1.5 (1.3–1.8) |
| Triglycerides (mmol/L) | 0.7 (0.5–0.9) |
| Glucose (mmol/L) | 4.7 ± 0.5 |
| Homocysteine (μmol/L) | 8.9 ± 2.5 |
| Ln-homoarginine | Ornithine | |
|---|---|---|
| Estimated GFR | −0.11 (0.50) | −0.14 (0.39) |
| LDL cholesterol | −0.12 (0.45) | −0.06 (0.70) |
| HDL cholesterol | 0.11 (0.52) | 0.22 (0.18) |
| Triglycerides | −0.24 (0.14) | −0.07 (0.65) |
| Glucose | 0.25 (0.12) | −0.43 (0.006) |
| Homocysteine | 0.27 (0.10) | 0.09 (0.56) |
| Arginine | 0.43 (0.005) | −0.64 (<0.001) |
| ADMA | −0.05 (0.77) | 0.10 (0.53) |
| SDMA | 0.23 (0.15) | 0.09 (0.56) |
Acknowledgments
Conflicts of Interest
References
- Ryan, W.L.; Wells, I.C. Homocitrulline and homoarginine synthesis from lysine. Science 1964, 144, 1122–1127. [Google Scholar]
- Ryan, W.L.; Barak, A.J.; Johnson, R.J. Lysine, homocitrulline, and homoarginine metabolism by the isolated perfused rat liver. Arch. Biochem. Biophys 1968, 123, 294–297. [Google Scholar]
- Cathelineau, L.; Saudubray, J.M.; Charpentier, C.; Polonovski, C. Letter: The presence of the homoanalogues of substrates of the urea cycle in the presence of argininosuccinate synthetase deficiency. Pediatr. Res 1974, 8, 857. [Google Scholar]
- Levin, B.; Oberholzer, V.G.; Palmer, T. Letter: The high levels of lysine, homocitrulline, and homoarginine found in argininosuccinate synthetase deficiency. Pediatr. Res 1974, 8, 857–858. [Google Scholar]
- Ryan, W.L.; Johnson, R.J.; Dimari, S. Homoarginine synthesis by rat kidney. Arch. Biochem. Biophys 1969, 131, 521–526. [Google Scholar]
- Hernández-Guzmán, G.; Alvarez-Morales, A. Isolation and characterization of the gene coding for the amidinotransferase involved in the biosynthesis of phaseolotoxin in Pseudomonas syringae pv. phaseolicola. Mol. Plant. Microbe Interact 2001, 14, 545–554. [Google Scholar]
- Davids, M.; Ndika, J.D.; Salomons, G.S.; Blom, H.J.; Teerlink, T. Promiscuous activity of arginine:glycine amidinotransferase is responsible for the synthesis of the novel cardiovascular risk factor homoarginine. FEBS Lett 2012, 586, 3653–3657. [Google Scholar]
- März, W.; Meinitzer, A.; Drechsler, C.; Pilz, S.; Krane, V.; Kleber, M.E.; Fischer, J.; Winkelmann, B.R.; Böhm, B.O.; Ritz, E.; et al. Homoarginine, cardiovascular risk, and mortality. Circulation 2010, 122, 967–975. [Google Scholar]
- Valtonen, P.; Laitinen, T.; Lyyra-Laitinen, T.; Raitakari, O.T.; Juonala, M.; Viikari, J.S.; Heiskanen, N.; Vanninen, E.; Punnonen, K.; Heinonen, S. Serum l-homoarginine concentration is elevated during normal pregnancy and is related to flow-mediated vasodilatation. Circ. J 2008, 72, 1879–1884. [Google Scholar]
- Saarelainen, H.; Valtonen, P.; Punnonen, K.; Laitinen, T.; Raitakari, O.T.; Juonala, M.; Heiskanen, N.; Lyyra-Laitinen, T.; Viikari, J.S.; Vanninen, E.; et al. Subtle changes in ADMA and l-arginine concentrations in normal pregnancies are unlikely to account for pregnancy-related increased flow-mediated dilatation. Clin. Physiol. Funct. Imaging 2008, 28, 120–124. [Google Scholar]
- Sourij, H.; Meinitzer, A.; Pilz, S.; Grammer, T.B.; Winkelmann, B.R.; Boehm, B.O.; März, W. Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis 2011, 218, 220–225. [Google Scholar]
- Marescau, B.; Nagels, G.; Possemiers, I.; de Broe, M.E.; Becaus, I.; Billiouw, J.M.; Lornoy, W.; de Deyn, P.P. Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism 1997, 46, 1024–1031. [Google Scholar]
- Meinitzer, A.; Puchinger, M.; Winklhofer-Roob, B.M.; Rock, E.; Ribalta, J.; Roob, J.M.; Sundl, I.; Halwachs-Baumann, G.; März, W. Reference values for plasma concentrations of asymmetrical dimethylarginine (ADMA) and other arginine metabolites in men after validation of a chromatographic method. Clin. Chim. Acta 2007, 384, 141–148. [Google Scholar]
- Skilton, M.R.; Serusclat, A.; Sethu, A.H.A.U.; Brun, S.; Bernard, S.; Balkau, B.; Moulin, P.; Bonnet, F. Noninvasive measurement of carotid extra-media thickness: Associations with cardiovascular risk factors and intima-media thickness. JACC Cardiovasc. Imaging 2009, 2, 176–182. [Google Scholar]
- Skilton, M.R.; Sullivan, T.R.; Ayer, J.G.; Harmer, J.A.; Toelle, B.G.; Webb, K.; Marks, G.B.; Celermajer, D.S. Carotid extra-medial thickness in childhood: Early life effects on the arterial adventitia. Atherosclerosis 2012, 222, 478–482. [Google Scholar]
- Heistad, D.D.; Armstrong, M.L.; Marcus, M.L. Hyperemia of the aortic wall in atherosclerotic monkeys. Circ. Res 1981, 48, 669–675. [Google Scholar]
- Kwon, H.M.; Sangiorgi, G.; Ritman, E.L.; McKenna, C.; Holmes, D.R., Jr.; Schwartz, R.S.; Lerman, A. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J. Clin. Invest 1998, 101, 1551–1556. [Google Scholar]
- Herrmann, J.; Samee, S.; Chade, A.; Rodriguez Porcel, M.; Lerman, L.O.; Lerman, A. Differential effect of experimental hypertension and hypercholesterolemia on adventitial remodeling. Arterioscler. Thromb. Vasc. Biol 2005, 25, 447–453. [Google Scholar]
- Michel, J.B.; Thaunat, O.; Houard, X.; Meilhac, O.; Caligiuri, G.; Nicoletti, A. Topological determinants and consequences of adventitial responses to arterial wall injury. Arterioscler. Thromb. Vasc. Biol 2007, 27, 1259–1268. [Google Scholar]
- JaŸwińska-Kozuba, A.; Martens-Lobenhoffer, J.; Surdacki, A.; Kruszelnicka, O.; Rycaj, J.; Godula-Stuglik, U.; Bode-Böger, S.M. Associations between endogenous dimethylarginines and renal function in healthy children and adolescents. Int. J. Mol. Sci 2012, 13, 15464–15474. [Google Scholar]
- Atzler, D.; Mieth, M.; Maas, R.; Böger, R.H.; Schwedhelm, E. Stable isotope dilution assay for liquid chromatography-tandem mass spectrometric determination of l-homoarginine in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 2011, 879, 2294–2298. [Google Scholar]
- Gruber, H.J.; Mayer, C.; Meinitzer, A.; Almer, G.; Horejsi, R.; Möller, R.; Pilz, S.; März, W.; Gasser, R.; Truschnig-Wilders, M.; et al. Asymmetric dimethylarginine (ADMA) is tightly correlated with growth in juveniles without correlations to obesity related disorders. Exp. Clin. Endocrinol. Diabetes 2008, 116, 520–524. [Google Scholar]
- Lepage, N.; McDonald, N.; Dallaire, L.; Lambert, M. Age-specific distribution of plasma amino acid concentrations in a healthy pediatric population. Clin. Chem 1997, 43, 2397–2402. [Google Scholar]
- Hammarqvist, F.; Angsten, G.; Meurling, S.; Andersson, K.; Wernerman, J. Age-related changes of muscle and plasma amino acids in healthy children. Amino Acids 2010, 39, 359–366. [Google Scholar]
- Chobanyan-Jürgens, K.; Fuchs, A.J.; Tsikas, D.; Kanzelmeyer, N.; Das, A.M.; Illsinger, S.; Vaske, B.; Jordan, J.; Lücke, T. Increased asymmetric dimethylarginine (ADMA) dimethylaminohydrolase (DDAH) activity in childhood hypercholesterolemia type II. Amino Acids 2012, 43, 805–811. [Google Scholar]
- Böger, R.H.; Bode-Böger, S.M. The clinical pharmacology of l-arginine. Annu. Rev. Pharmacol. Toxicol 2001, 41, 79–99. [Google Scholar]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev 2000, 80, 1107–1213. [Google Scholar]
- McGuire, D.M.; Tormanen, C.D.; Segal, I.S.; Van Pilsum, J.F. The effect of growth hormone and thyroxine on the amount of l-arginine:glycine amidinotransferase in kidneys of hypophysectomized rats. Purification and some properties of rat kidney transamidinase. J. Biol. Chem 1980, 255, 1152–1159. [Google Scholar]
- Hoberman, H.D.; Sims, E.A.; Engstrom, W.W. The effect of methyltestosterone on the rate of synthesis of creatine. J. Biol. Chem 1948, 173, 111–116. [Google Scholar]
- Hrabák, A.; Bajor, T.; Temesi, A. Comparison of substrate and inhibitor specificity of arginase and nitric oxide (NO) synthase for arginine analogues and related compounds in murine and rat macrophages. Biochem. Biophys. Res. Commun 1994, 198, 206–212. [Google Scholar]
- Cittadini, D.; Pietropaolo, C.; Decristofaro, D.; D’Ayjello Caracciolo, M. In vivo effect of l-lysine on rat liver arginase. Nature 1964, 203, 643–644. [Google Scholar]
- Fuentes, J.M.; Campo, M.L.; Soler, G. Kinetics and inhibition by some aminoacids of lactating rat mammary gland arginase. Arch. Int. Physiol. Biochim. Biophys 1994, 102, 255–258. [Google Scholar]
- Santhanam, L.; Christianson, D.W.; Nyhan, D.; Berkowitz, D.E. Arginase and vascular aging. J. Appl. Physiol 2008, 105, 1632–1642. [Google Scholar]
- Pollock, J.S.; Förstermann, U.; Mitchell, J.A.; Warner, T.D.; Schmidt, H.H.H.W.; Nakane, M.; Murad, F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc. Natl. Acad. Sci. USA 1991, 88, 10480–10484. [Google Scholar]
- Baydoun, A.R.; Emery, P.W.; Pearson, J.D.; Mann, G.E. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem. Biophys. Res. Commun 1990, 173, 940–948. [Google Scholar]
- Böger, R.H.; Bode-Böger, S.M.; Tsao, P.S.; Lin, P.S.; Chan, J.R.; Cooke, J.P. An endogenous inhibitor of nitric oxide synthase regulates endothelial adhesiveness for monocytes. J. Am. Coll. Cardiol 2000, 36, 2287–2295. [Google Scholar]
- Surdacki, A. l-arginine analogs—Inactive markers or active agents in atherogenesis? Cardiovasc. Hematol. Agents Med. Chem 2008, 6, 302–311. [Google Scholar]
- Berkowitz, D.E.; White, R.; Li, D.; Minhas, K.M.; Cernetich, A.; Kim, S.; Burke, S.; Shoukas, A.A.; Nyhan, D.; Champion, H.C.; et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 2003, 108, 2000–2006. [Google Scholar]
- Knowles, R.G.; Palacios, M.; Palmer, R.M.; Moncada, S. Formation of nitric oxide from l-arginine in the central nervous system: A transduction mechanism for stimulation of the soluble guanylate cyclase. Proc. Natl. Acad. Sci. USA 1989, 86, 5159–5162. [Google Scholar]
- Hecker, M.; Walsh, D.T.; Vane, J.R. On the substrate specificity of nitric oxide synthase. FEBS Lett 1991, 294, 221–224. [Google Scholar]
- Moali, C.; Boucher, J.L.; Sari, M.A.; Stuehr, D.J.; Mansuy, D. Substrate specificity of NO synthases: Detailed comparison of l-arginine, homo-l-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-l-arginine. Biochemistry 1998, 37, 10453–10460. [Google Scholar]
- Davids, M.; Teerlink, T. Plasma concentrations of arginine and asymmetric dimethylarginine do not reflect their intracellular concentrations in peripheral blood mononuclear cells. Metabolism 2013, 62, 1455–1461. [Google Scholar]
- Morris, S.M., Jr. Recent advances in arginine metabolism: Roles and regulation of the arginases. Br. J. Pharmacol 2009, 157, 922–930. [Google Scholar]
- Ryoo, S.; Gupta, G.; Benjo, A.; Lim, H.K.; Camara, A.; Sikka, G.; Sohi, J.; Santhanam, L.; Soucy, K.; Tuday, E.; et al. Endothelial arginase II: A novel target for the treatment of atherosclerosis. Circ. Res 2008, 102, 923–932. [Google Scholar]
- Kim, J.H.; Bugaj, L.J.; Oh, Y.J.; Bivalacqua, T.J.; Ryoo, S.; Soucy, K.G.; Santhanam, L.; Webb, A.; Camara, A.; Sikka, G.; et al. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J. Appl. Physiol 2009, 107, 1249–1257. [Google Scholar]
- Shin, W.S.; Berkowitz, D.E.; Ryoo, S.W. Increased arginase II activity contributes to endothelial dysfunction through endothelial nitric oxide synthase uncoupling in aged mice. Exp. Mol. Med 2012, 44, 594–602. [Google Scholar]
- Scalera, F.; Borlak, J.; Beckmann, B.; Martens-Lobenhoffer, J.; Thum, T.; Täger, M.; Bode-Böger, S.M. Endogenous nitric oxide synthesis inhibitor asymmetric dimethyl l-arginine accelerates endothelial cell senescence. Arterioscler. Thromb. Vasc. Biol 2004, 24, 1816–1822. [Google Scholar]
- Shemyakin, A.; Kövamees, O.; Rafnsson, A.; Böhm, F.; Svenarud, P.; Settergren, M.; Jung, C.; Pernow, J. Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Circulation 2012, 126, 2943–2950. [Google Scholar]
- Dumont, J.; Zureik, M.; Bauters, C.; Grupposo, M.C.; Cottel, D.; Montaye, M.; Hamon, M.; Ducimetière, P.; Amouyel, P.; Brousseau, T. Association of OAZ1 gene polymorphisms with subclinical and clinical vascular events. Arterioscler. Thromb. Vasc. Biol 2007, 27, 2120–2126. [Google Scholar]
- Pilz, S.; Meinitzer, A.; Tomaschitz, A.; Kienreich, K.; Dobnig, H.; Schwarz, M.; Wagner, D.; Drechsler, C.; Piswanger-Sölkner, C.; März, W.; et al. Associations of homoarginine with bone metabolism and density, muscle strength and mortality: Cross-sectional and prospective data from 506 female nursing home patients. Osteoporos. Int 2013, 24, 377–381. [Google Scholar]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol 2009, 20, 629–637. [Google Scholar]
- Staples, A.; LeBlond, R.; Watkins, S.; Wong, C.; Brandt, J. Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr. Nephrol 2010, 25, 2321–2326. [Google Scholar]
- Martens-Lobenhoffer, J.; Bode-Böger, S.M. Quantification of l-arginine, asymmetric dimethylarginine and symmetric dimethylarginine in human plasma: A step improvement in precision by stable isotope dilution mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 2012, 904, 140–143. [Google Scholar]
- Martens-Lobenhoffer, J.; Bode-Böger, S.M. Fast and efficient determination of arginine, symmetric dimethylarginine, and asymmetric dimethylarginine in biological fluids by hydrophilic-interaction liquid chromatography-electrospray tandem mass spectrometry. Clin. Chem 2006, 52, 488–493. [Google Scholar]
- Martens-Lobenhoffer, J.; Postel, S.; Tröger, U.; Bode-Böger, S.M. Determination of ornithine in human plasma by hydrophilic interaction chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 2007, 855, 271–275. [Google Scholar]
- Touboul, P.-J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Fatar, M.; et al. Mannheim carotid intima-media thickness consensus (2004–2006). Cerebrovasc. Dis 2007, 23, 75–80. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jaźwińska-Kozuba, A.; Martens-Lobenhoffer, J.; Kruszelnicka, O.; Rycaj, J.; Chyrchel, B.; Surdacki, A.; Bode-Böger, S.M. Opposite Associations of Plasma Homoarginine and Ornithine with Arginine in Healthy Children and Adolescents. Int. J. Mol. Sci. 2013, 14, 21819-21832. https://doi.org/10.3390/ijms141121819
Jaźwińska-Kozuba A, Martens-Lobenhoffer J, Kruszelnicka O, Rycaj J, Chyrchel B, Surdacki A, Bode-Böger SM. Opposite Associations of Plasma Homoarginine and Ornithine with Arginine in Healthy Children and Adolescents. International Journal of Molecular Sciences. 2013; 14(11):21819-21832. https://doi.org/10.3390/ijms141121819
Chicago/Turabian StyleJaźwińska-Kozuba, Aleksandra, Jens Martens-Lobenhoffer, Olga Kruszelnicka, Jarosław Rycaj, Bernadeta Chyrchel, Andrzej Surdacki, and Stefanie M. Bode-Böger. 2013. "Opposite Associations of Plasma Homoarginine and Ornithine with Arginine in Healthy Children and Adolescents" International Journal of Molecular Sciences 14, no. 11: 21819-21832. https://doi.org/10.3390/ijms141121819
APA StyleJaźwińska-Kozuba, A., Martens-Lobenhoffer, J., Kruszelnicka, O., Rycaj, J., Chyrchel, B., Surdacki, A., & Bode-Böger, S. M. (2013). Opposite Associations of Plasma Homoarginine and Ornithine with Arginine in Healthy Children and Adolescents. International Journal of Molecular Sciences, 14(11), 21819-21832. https://doi.org/10.3390/ijms141121819




