Comparative Analysis of Flower Volatiles from Nine Citrus at Three Blooming Stages
Abstract
:1. Introduction
2. Result and Discussion
2.1. Analysis of Citrus Flower Volatiles by HS-SPME-GC-MS
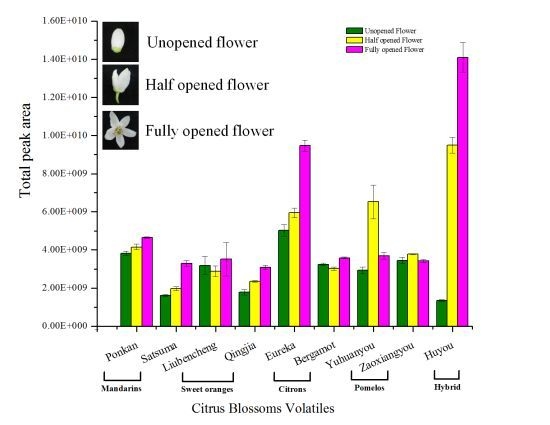
2.2. Variation in Total Flower Volatile Amount
2.3. Changes in Chemical Classes of Volatiles Produced during Flower Development
2.4. Variation in Volatile Compounds from Nine Citrus Cultivars
2.5. Changes in Volatile Abundance during Flower Blooming in Nine Cultivars
2.6. Multivariate Analysis of Flower Volatiles during Development from Nine Cultivars
3. Experimental Section
3.1. Materials
3.2. Headspace Extraction of Flower Volatiles
3.3. Gas Chromatography with Mass Spectrometry (GC-MS) Analysis
3.4. Identification of Volatiles
3.5. Electronic Nose Measurements
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
ijms-14-22346-s001.pdf




| Citrus types | Common name | Cultivars | Code | |
|---|---|---|---|---|
| 1 | C. reticulata Blanco | Mandarin | Ponkan | PK |
| 2 | C. unshiu Marc. | Mandarin | Satsuma | ST |
| 3 | C. sinensis. (L.) Osbeck | Sweet orange | Liubencheng | LBC |
| 4 | C. sinensis. (L.) Osbeck | Sweet orange | Qingjia | QJ |
| 5 | C. limon. (L.) Burm. | Lemon | Eureka | ERK |
| 6 | C. medica. (L.) | Citron | Bergamot | BM |
| 7 | C. grandis (L.) Osbeck | Pomelo | Yuhuanyou | YHY |
| 8 | C. grandis (L.) Osbeck | Pomelo | Zaoxiangyou | ZXY |
| 9 | C. changshanensis Chen et. Fu (C. aurantium × C. grandis) | Citrus hybrid | Huyou | HY |
| RI a | Compound name | FC b | Ponkan | Satsuma | Qingjia | Liubencheng | Eureka | Bergamot | Yuhuanyou | Zaoxiangyou | Huyou | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uc | H | F | U | H | F | U | H | F | U | H | F | U | H | F | U | H | F | U | H | F | U | H | F | U | H | F | |||
| Aldehydes | |||||||||||||||||||||||||||||
| 809 | Hexanal | A1 | M | M | -d | - | - | - | - | - | - | - | - | M | M | M | M | - | - | M | M | M | M | M | M | M | 3.44 | M | M |
| 858 | 2-Hexenal * | A2 | M | - | M | 2.84 | 1.84 | M | 2.36 | 1.68 | 1.19 | 1.51 | 1.20 | 1.86 | 1.33 | 1.45 | M | M | M | M | M | M | 1.68 | M | M | 1.24 | 5.95 | 1.52 | M |
| 977 | Benzaldehyde | A3 | T | T | M | T | M | T | T | T | T | - | - | M | T | T | T | - | - | - | - | T | T | - | - | - | M | T | - |
| 1048 | Benzene acetaldehyde * | A4 | M | M | 1.08 | - | - | - | M | M | M | - | M | 1.31 | - | M | M | - | - | - | - | - | 1.34 | - | - | - | M | M | M |
| 1109 | Nonanal | A5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | M | M | M | - | - | - | - | - | - | - | - | - |
| 1154 | Lilac aldehyde B * | A6 | - | - | - | - | - | T | - | T | T | - | T | - | - | - | - | - | - | - | M | T | - | M | T | T | - | - | T |
| 1202 | Myrtenal | A7 | - | - | - | M | M | - | - | - | - | T | - | - | - | - | - | - | - | - | - | T | - | T | - | T | - | T | - |
| 1214 | Decanal | A8 | T | T | T | T | T | - | - | - | - | T | T | - | M | M | T | - | - | - | M | M | - | T | - | - | - | T | M |
| 1311 | Undecanal | A9 | - | - | - | - | - | - | - | - | - | - | - | - | T | - | M | T | T | T | - | - | - | - | - | - | - | - | - |
| Monoterpene Hydrocarbons | |||||||||||||||||||||||||||||
| 939 | α-thujene | Mh1 | M | M | M | M | M | M | M | M | M | M | M | M | T | T | M | M | M | M | - | T | 2.18 | T | T | T | M | M | M |
| 945 | α-pinene | Mh2 | M | M | M | 1.08 | 2.80 | M | M | M | M | M | M | 1.42 | M | M | M | M | M | M | M | M | M | M | M | M | 1.57 | 2.28 | M |
| 962 | Camphene | Mh3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | T | 4.48 | - | - | T | - |
| 979 | Sabinene | Mh4 | - | - | - | - | - | - | 11.15 | 6.07 | 6.11 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 991 | β-pinene | Mh5 | 9.20 | 7.83 | 6.59 | 2.92 | 3.91 | 6.51 | - | - | - | 11.88 | 7.98 | 3.53 | 2.07 | M | 1.11 | M | M | M | 3.38 | 1.67 | 2.42 | 7.49 | - | 4.90 | 5.89 | 8.08 | 6.84 |
| 999 | β-myrcene | Mh6 | 1.46 | 1.38 | 1.11 | - | 2.55 | 1.53 | 2.53 | 1.82 | 1.48 | 2.46 | 1.73 | 1.62 | 2.01 | 2.02 | 2.42 | 1.40 | 1.50 | 1.53 | 8.08 | 4.15 | 1.55 | M | M | M | 2.04 | 1.95 | 15.50 |
| 1018 | α-terpinene | Mh7 | M | M | M | M | M | M | M | - | M | 1.62 | - | M | - | - | - | M | M | M | T | T | M | - | - | - | M | M | M |
| 1025 | p-cymene | Mh8 | M | M | M | 8.56 | 6.53 | - | - | - | - | - | - | M | - | - | - | - | - | - | - | - | 1.01 | - | - | - | 2.91 | - | - |
| 1029 | Limonene | Mh9 | 1.48 | 1.15 | 1.07 | - | - | 1.69 | 3.25 | 1.75 | 1.54 | 4.64 | 2.69 | 2.41 | 51.99 | 44.95 | 52.53 | 29.30 | 27.25 | 36.17 | 3.32 | 2.19 | 3.68 | 4.92 | 2.54 | 3.32 | 6.68 | 10.09 | 7.05 |
| 1040 | (Z)-ocimene | Mh10 | T | - | - | - | - | - | M | - | 1.71 | M | M | - | 1.01 | 1.10 | 1.25 | M | M | - | M | M | M | T | 6.78 | 16.52 | |||
| 1044 | (E)-ocimene | Mh11 | 3.03 | 2.29 | 2.16 | 6.37 | 6.32 | 2.27 | 1.62 | 1.18 | 1.39 | 2.95 | 1.54 | 8.40 | 6.14 | 5.35 | 6.35 | 2.74 | 2.56 | 2.96 | 6.41 | 3.23 | 1.97 | 9.14 | 5.45 | 8.33 | 4.90 | - | - |
| 1060 | γ-terpinene | Mh12 | 1.90 | 1.44 | 1.63 | 13.79 | 8.91 | M | M | M | M | M | M | M | 2.60 | 1.97 | 3.17 | 3.68 | 3.71 | 4.29 | M | T | 11.06 | T | T | T | 18.07 | 22.83 | T |
| 1084 | β-cymene * | Mh13 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | T |
| 1090 | Terpinolene | Mh14 | - | - | - | - | - | - | M | M | M | M | M | M | M | M | - | M | M | M | - | - | - | - | - | - | 1.07 | 1.59 | M |
| 1128 | 1,3,8-p-menthatriene * | Mh15 | - | - | - | - | - | - | - | - | - | - | - | - | T | T | T | - | - | - | - | - | - | T | - | T | - | - | T |
| 1131 | 2,4,6-octatriene,3,4-dimethyl * | Mh16 | M | M | M | M | M | T | M | M | T | M | M | M | T | M | M | 1.16 | 1.05 | 1.35 | M | M | T | M | M | M | M | M | M |
| Oxygenated Monoterpenes | |||||||||||||||||||||||||||||
| 1031 | 1,8-Cineol | Om1 | - | - | - | 6.05 | 3.15 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1068 | cis-β-terpineol * | Om2 | 1.99 | 1.65 | 1.08 | M | M | M | 3.60 | 1.81 | M | 2.93 | 1.77 | - | M | T | T | M | M | M | T | T | M | T | T | T | M | M | T |
| 1076 | cis-linalol oxide * | Om3 | - | - | - | - | - | - | - | T | - | - | - | M | - | - | - | - | - | - | M | M | T | M | M | M | - | - | - |
| 1108 | Linalool | Om4 | 46.76 | 50.43 | 47.74 | 22.67 | 17.41 | 42.76 | 44.74 | 42.91 | 46.98 | 29.15 | 36.11 | 24.95 | 7.95 | 6.88 | 3.94 | 1.45 | 1.72 | 1.59 | 54.41 | 56.16 | 21.59 | 36.44 | 53.55 | 48.43 | 18.83 | 10.10 | 30.11 |
| 1137 | Limonene oxide, cis | Om5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | T | 1.15 | - | - | - | - | - | T |
| 1139 | Limonene oxide, trans | Om6 | - | - | - | - | - | - | - | - | - | - | - | - | 2.58 | - | 3.04 | 1.63 | 2.19 | 2.78 | - | - | - | - | - | - | - | - | - |
| 1155 | Citronellal | Om7 | M | M | M | - | - | M | M | M | M | M | M | M | 1.05 | M | M | M | M | M | M | M | M | T | T | T | M | M | T |
| 1176 | Umbellulone * | Om8 | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1177 | Terpinen-4-ol | Om9 | M | M | M | M | M | M | M | M | M | M | M | M | M | - | M | T | T | T | M | M | M | M | M | M | M | M | T |
| 1189 | p-cymen-8-ol * | Om10 | M | M | T | M | M | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1192 | α-terpineol | Om11 | 3.83 | 2.94 | 1.96 | 5.45 | 5.58 | 1.01 | 4.59 | 2.60 | 1.12 | 4.37 | 2.83 | M | 1.54 | - | - | 2.39 | 2.72 | 1.97 | - | M | - | M | M | - | 2.73 | M | M |
| 1205 | trans-dihydrocarvone * | Om12 | - | - | - | - | - | - | - | - | - | - | - | - | M | M | M | - | - | - | - | - | - | - | - | - | - | - | - |
| 1208 | Carvone * | Om13 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | T | T | T | - | - | - | - | - | - | - | - | T |
| 1210 | trans-piperitol * | Om14 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | T |
| 1215 | p-Menth-1-en-9-al * | Om15 | T | T | M | M | - | - | - | - | - | - | - | - | - | M | M | - | - | - | - | - | - | M | M | M | - | - | - |
| 1219 | cis-carveol | Om16 | - | - | - | 1.00 | M | M | - | - | - | T | - | - | M | M | M | M | M | 1.15 | T | T | - | T | T | T | - | T | T |
| 1230 | cis-geraniol | Om17 | - | - | - | - | - | M | M | M | M | M | M | M | M | T | M | M | - | - | 1.19 | 2.15 | M | 1.37 | 2.84 | 2.46 | M | M | 1.01 |
| 1233 | β-citronellol | Om18 | T | M | M | - | - | M | M | M | T | M | M | M | T | T | - | - | - | - | - | - | M | - | - | - | T | M | M |
| 1236 | Methyl thymyl ether | Om19 | 1.93 | 1.19 | 1.07 | - | - | - | - | - | - | M | 5.74 | T | T | T | T | - | - | - | - | - | - | - | - | - | - | - | - |
| 1237 | Isogeraniol * | Om20 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | T | T | - | - | T |
| 1242 | β-citral | Om21 | T | T | M | - | - | M | M | M | M | M | M | 2.55 | M | M | M | 17.75 | 17.10 | 13.27 | 1.52 | 2.28 | 1.64 | M | 1.03 | M | M | M | - |
| 1258 | trans-geraniol | Om22 | M | 1.12 | 2.47 | M | - | - | T | - | - | T | M | 6.80 | - | - | - | M | M | M | M | 1.82 | 6.52 | M | 1.20 | 1.24 | M | 2.45 | M |
| 1273 | α-citral | Om23 | M | T | T | - | - | M | M | M | M | M | 11.17 | 3.30 | 1.24 | M | M | 25.81 | 24.50 | 20.07 | 1.83 | 3.07 | 2.40 | M | 1.19 | M | M | 1.38 | M |
| 1282 | α-thujenal | Om24 | - | - | - | M | 1.33 | M | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1299 | Carvacrol * | Om25 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | M | - | - | - | - | - | - | - | - | - | - | - | - |
| 1733 | E,E-farnesal * | Om26 | - | - | - | - | - | M | - | - | - | - | - | - | T | - | M | T | M | T | - | - | M | M | M | M | - | M | M |
| Sesquiterpene Hydrocarbons | |||||||||||||||||||||||||||||
| 1335 | δ-elemene | Sh1 | 1.36 | M | 1.36 | - | - | - | - | - | - | - | - | M | M | - | - | - | - | - | 1.02 | M | 1.23 | M | M | M | 1.14 | M | 1.05 |
| 1336 | α-cubebene * | Sh2 | T | T | T | - | - | - | - | - | - | - | - | - | M | T | T | - | - | - | - | - | - | - | - | - | - | T | T |
| 1371 | Copaene * | Sh3 | - | - | - | - | - | - | - | - | - | - | - | - | - | M | M | - | - | - | - | - | - | - | - | - | M | - | - |
| 1392 | β-elemene | Sh4 | M | - | T | 5.40 | 4.76 | 2.13 | 1.93 | M | 1.47 | 19.43 | 3.09 | 5.73 | - | 7.53 | 6.02 | - | - | - | - | M | 4.98 | M | M | 2.25 | M | 4.48 | M |
| 1398 | Zingiberene * | Sh5 | - | - | M | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | M | - | - | - | - | M | T |
| 1407 | Bergamotene * | Sh6 | - | - | - | - | - | - | - | - | - | T | T | M | T | - | - | - | - | - | - | - | - | M | - | - | M | - | - |
| 1408 | trans-α-bergamotene | Sh7 | M | T | - | - | - | M | M | M | T | - | - | - | - | T | T | - | - | - | - | - | - | - | - | - | - | - | - |
| 1412 | Caryophyllene | Sh8 | M | M | M | 2.11 | 2.61 | - | M | M | M | 1.02 | M | 1.34 | 3.50 | 3.93 | 3.14 | 1.83 | 1.99 | 1.79 | 1.13 | 0.70 | 2.18 | T | - | T | 2.31 | 2.98 | 2.00 |
| 1417 | α-santalene * | Sh9 | - | - | - | - | - | - | - | - | - | - | - | - | - | T | T | T | T | T | - | - | - | - | - | - | - | - | - |
| 1422 | β-cubebene | Sh10 | T | T | T | - | M | M | - | - | - | - | - | 0.56 | T | - | T | T | T | T | 0.08 | 0.06 | 0.25 | - | 1.64 | - | M | M | M |
| 1431 | γ-elemene | Sh11 | 1.16 | M | 1.06 | M | M | M | - | - | - | - | - | 1.37 | T | T | T | - | - | - | M | M | 1.77 | - | T | M | 2.48 | 1.44 | M |
| 1435 | α-bergamotene | Sh12 | - | - | - | - | - | 4.00 | - | - | - | - | - | - | - | 2.22 | 1.78 | M | M | M | - | - | T | - | - | - | - | T | - |
| 1445 | α-caryophyllene | Sh13 | - | - | - | - | - | - | M | - | - | - | - | - | - | M | M | - | - | - | - | - | - | - | - | - | - | - | - |
| 1456 | Bicyclosesquiphellandrene * | Sh14 | M | M | M | - | - | - | M | M | - | M | M | M | - | - | - | T | T | T | - | T | - | M | M | T | - | - | T |
| 1461 | β-farnesene | Sh15 | 3.53 | 2.24 | 3.04 | - | - | - | 3.89 | 3.56 | 2.69 | 2.03 | 2.25 | 3.43 | 1.88 | 2.26 | 1.64 | M | M | M | T | M | 5.16 | T | - | - | M | 4.60 | T |
| 1464 | α-gurjunene * | Sh16 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | T | - |
| 1470 | α-elemene * | Sh17 | - | - | - | - | - | - | - | - | - | - | - | - | - | T | T | - | - | - | - | - | M | - | - | - | - | - | T |
| 1474 | Germacrene D | Sh18 | M | M | T | M | M | T | - | - | - | - | - | 1.36 | M | M | M | M | M | M | M | M | 1.17 | - | M | M | 2.24 | 1.52 | 1.14 |
| 1475 | β-eudesmene * | Sh19 | - | - | - | - | - | - | - | - | - | - | T | - | M | M | M | - | - | - | - | - | - | - | - | - | - | M | - |
| 1478 | α-selinene * | Sh20 | - | - | - | T | M | - | - | - | - | - | - | M | - | - | M | - | - | - | - | - | M | - | - | - | 2.04 | - | - |
| 1481 | Allo-aromadendrene | Sh21 | - | - | - | - | - | - | - | T | - | - | - | - | M | M | M | - | - | - | - | - | - | - | - | - | - | - | T |
| 1489 | Bicyclogermacrene | Sh22 | - | - | - | M | M | T | - | - | - | T | - | - | - | - | - | - | - | - | T | M | - | M | M | M | - | - | - |
| 1491 | α-muurolene * | Sh23 | - | M | M | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | M | - | - | T | - | - | - |
| 1496 | α-bulnesene * | Sh24 | T | - | M | - | - | - | - | T | - | - | - | - | M | M | M | - | - | - | - | - | T | - | - | - | - | T | - |
| 1499 | (Z,E)-α-farnesene | Sh25 | - | - | - | - | - | - | - | - | - | - | - | - | M | M | - | - | - | - | - | T | - | M | M | - | - | - | T |
| 1502 | γ-muurolene | Sh26 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | T | T | - | - | - | M | M |
| 1503 | α-farnesene * | Sh27 | - | - | M | - | - | - | - | - | - | - | - | T | M | M | M | - | - | - | - | - | - | - | - | - | - | M | M |
| 1504 | β-bisabolene | Sh28 | - | - | - | - | - | - | - | - | - | - | - | - | 2.42 | 2.82 | 2.34 | 1.54 | 1.63 | 1.47 | - | - | - | - | - | - | - | - | - |
| 1506 | δ-cadinene | Sh29 | 2.17 | 1.30 | 1.67 | - | - | - | M | 1.07 | M | M | M | - | - | - | - | - | - | - | M | M | - | 1.13 | M | M | - | - | M |
| 1507 | β-sesquiphellandrene | Sh30 | M | - | M | 4.31 | 6.41 | - | M | M | M | M | M | - | T | T | T | - | - | - | - | - | M | - | - | - | M | M | - |
| 1510 | Eudesma-3,7(11)-diene * | Sh31 | - | - | - | - | - | - | - | - | - | - | - | M | - | - | - | - | - | - | - | - | - | - | - | - | - | T | T |
| 1520 | cis-α-bisabolene * | Sh32 | - | - | - | - | - | - | - | - | - | - | - | - | T | - | T | M | M | M | - | - | T | - | - | - | - | - | - |
| Oxygenated Monoterpenes | |||||||||||||||||||||||||||||
| 1530 | Nerolidol | Os1 | M | M | T | - | - | M | M | 3.04 | 3.64 | M | 1.74 | - | - | M | T | M | 1.44 | M | 3.57 | 4.04 | M | 6.94 | 7.47 | 8.75 | M | M | T |
| 1532 | Caryophyllene oxide | Os2 | - | - | - | - | - | M | - | - | - | - | - | - | - | - | - | T | M | T | - | - | - | - | - | - | - | T | 4.81 |
| 1658 | β-eudesmol * | Os3 | T | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | T | T | - |
| 1696 | Tetradecanal * | Os4 | - | - | - | - | - | - | - | - | - | - | - | M | - | - | - | - | - | - | - | - | - | - | - | - | - | - | T |
| 1734 | Farnesol | Os5 | - | M | M | M | 1.07 | M | M | 1.54 | M | 1.41 | 1.11 | - | M | M | M | M | M | M | 1.32 | 1.73 | M | 2.38 | 2.28 | 2.00 | M | M | - |
| 1765 | α-sinensal | Os6 | M | M | M | - | - | - | - | - | - | M | M | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Ketones | |||||||||||||||||||||||||||||
| 1125 | Chrysanthenone * | K1 | - | - | - | M | M | - | M | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1395 | cis-jasmone | K2 | M | M | M | M | 1.44 | M | M | M | M | M | M | M | T | M | M | - | - | - | - | - | M | - | - | - | M | M | - |
| Esters | |||||||||||||||||||||||||||||
| 1306 | Methyl geranate | E1 | - | - | - | M | - | M | 1.79 | 8.26 | 15.81 | - | - | - | M | M | - | - | - | - | M | M | M | M | M | M | - | - | T |
| 1347 | Citronellyl acetate * | E2 | - | - | - | - | - | - | - | - | - | - | - | - | - | T | T | - | - | - | - | - | - | - | - | - | - | T | T |
| 1370 | Nerol acetate | E3 | - | - | - | - | - | T | T | T | T | T | T | - | - | - | - | T | T | T | M | M | M | M | M | M | - | - | - |
| 1390 | Geranyl acetate | E4 | - | - | - | T | M | - | - | - | - | - | - | - | - | - | - | T | M | M | - | - | - | - | - | - | - | - | - |
| Miscellaneous | |||||||||||||||||||||||||||||
| 1298 | p-thymol | P1 | 4.96 | 4.21 | 4.03 | - | M | - | - | - | - | - | - | - | M | 1.05 | - | - | - | - | - | - | T | - | - | - | - | - | - |
| 1665 | Methyl jasmonate * | DFG | - | - | - | - | - | - | - | - | - | - | - | T | - | - | - | - | - | - | T | T | - | - | - | - | - | - | - |
| 1075 | 1-octanol | Ac1 | - | - | - | - | - | - | - | - | - | - | - | - | T | T | T | T | - | T | - | - | - | - | - | - | T | T | T |
| 1116 | Phenylethyl alcohol | Ac2 | T | M | M | M | M | M | - | M | M | - | T | M | - | - | - | - | - | - | M | M | M | M | M | M | - | M | - |
| 897 | Styrene * | Ah1 | - | - | - | - | - | - | - | - | - | - | - | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1091 | α,p-dimethylstyrene | Ah2 | M | M | M | 2.17 | 1.33 | - | - | - | - | - | - | - | - | - | - | - | - | - | M | M | M | - | - | - | - | - | - |
| 1160 | Naphthalene * | Ah3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | M | - | - | - | - | - | - | - | - | - | - | - | - |
| 1140 | Benzyl nitrile | N1 | M | 2.14 | 4.61 | 1.99 | 3.43 | 1.20 | 1.02 | 1.38 | 1.25 | M | M | 2.49 | - | - | - | - | - | - | M | M | - | M | M | M | M | - | M |
| 1292 | Indole | N2 | 2.28 | 3.96 | 4.99 | 3.69 | 5.00 | 4.90 | 5.27 | 10.41 | 4.86 | 4.45 | 6.91 | 8.43 | M | 1.01 | M | T | M | M | 4.79 | 5.51 | 8.84 | 8.16 | 7.18 | 7.88 | 3.72 | 4.52 | 2.83 |
| 1341 | Methyl anthranilate | N3 | - | M | M | 1.12 | 4.87 | 17.91 | - | - | - | - | - | 1.77 | M | 2.47 | T | - | - | - | - | 4.20 | 2.79 | 8.21 | - | - | - | - | 2.17 |
| 1535 | Pentadecane, 3-methyl-* | Ak1 | - | - | - | - | - | 2.56 | - | - | - | M | T | 1.14 | - | T | T | - | - | - | - | - | - | - | - | - | - | - | - |
| 1680 | Hexadecane, 2-methyl-* | Ak2 | - | - | - | - | - | T | M | M | M | T | M | - | - | - | - | - | - | - | - | - | - | - | - | - | - | T | - |
| 1689 | 8-heptadecene | Ak3 | 2.23 | 2.62 | 1.70 | M | M | 1.26 | 1.54 | 2.43 | 1.74 | M | 2.76 | T | M | 1.65 | M | M | M | M | - | - | 1.03 | - | - | - | 1.59 | M | - |
| 1809 | Octadecane, 2-methyl * | Ak4 | T | T | T | - | - | - | M | M | T | M | T | T | - | - | - | - | - | - | - | M | - | M | M | M | - | - | T |
| Compounds | FC a | PK b | ST | LBC | QJ | ERK | BM | YHY | ZXY | HY | Mean rank |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Linalool | Om4 | 46.76 1st | 22.67 1st | 44.74 1st | 29.15 1st | 7.95 2nd | 1.45 10th | 54.41 1st | 36.44 1st | 18.83 1st | 29.16 1st |
| Limonene | Mh9 | 1.48 13th | - c | 3.25 7th | 4.64 4th | 51.99 1st | 29.30 1st | 3.32 7th | 4.92 7th | 6.68 3rd | 11.73 2nd |
| β-pinene | Mh5 | 9.20 2nd | 2.92 10th | - | 11.88 3rd | 2.07 8th | 0.54 18th | 3.38 6th | 7.49 5th | 5.89 5th | 4.82 3rd |
| (E)-ocimene | Mh11 | 3.03 6th | 6.37 4th | 1.62 12th | 2.95 7th | 6.14 3rd | 2.74 5th | 6.14 3rd | 9.14 3rd | 4.90 6th | 4.81 4th |
| γ-terpinene | Mh12 | 1.90 12th | 13.79 2nd | 0.53 22nd | 0.53 21st | 2.60 5th | 3.68 4th | 0.11 34th | 0.09 39th | 18.07 2nd | 4.59 5th |
| Indole | N2 | 2.28 7th | 3.69 9th | 5.27 3rd | 4.45 5th | 0.63 19th | 0.08 41st | 4.79 4th | 8.16 4th | 3.72 7th | 3.67 6th |
| α-citral | Om23 | 0.12 41st | - | 0.68 19th | 0.59 18th | 1.24 14th | 25.81 2nd | 1.83 8th | 0.92 14th | 0.45 28th | 3.52 7th |
| β-elemene | Sh4 | 0.39 27th | 5.40 7th | 1.93 10th | 19.43 2nd | - | - | - | 0.26 30th | 0.42 29th | 3.09 8th |
| α-terpineol | Om11 | 3.83 4th | 5.45 6th | 4.59 4th | 4.37 6th | 1.54 12th | 2.39 6th | - | 0.42 23th | 2.73 10th | 2.81 9th |
| β-citral | Om21 | 0.04 53th | - | 0.48 23rd | 0.43 23rd | 0.92 17th | 17.75 3rd | 1.52 9th | 0.93 13th | 0.30 33rd | 2.49 10th |
| β-myrcene | Mh6 | 1.46 14th | - | 2.53 8th | 2.46 9th | 2.01 9th | 1.40 11th | 8.08 2nd | 0.98 11th | 2.04 14th | 2.33 11th |
| 2-hexenal | A2 | 0.62 20th | 2.84 11th | 2.36 9th | 1.51 12th | 1.33 13th | 0.64 17th | 0.93 14th | 0.88 15th | 5.95 4th | 1.90 12th |
| Caryophyllene | Sh8 | 0.48 23rd | 2.11 13th | 0.65 20th | 1.02 14th | 3.50 4th | 1.83 7th | 1.13 12th | 0.07 43rd | 2.31 12th | 1.46 13th |
| p-cymene | Mh8 | 0.56 21st | 8.56 3rd | - | - | - | - | - | 2.91 9th | 1.34 14th | |
| β-farnesene | Sh15 | 0.13 40th | - | 0.29 39th | 0.26 45th | - | 0.27 31th | 3.57 5th | 0.06 45th | 0.10 42th | 1.31 15th |
| Compounds | FC a | PK b | ST | LBC | QJ | ERK | BM | YHY | ZXY | HY | Mean rank |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Linalool | Om4 | 50.43 1st | 17.41 1st | 42.91 1st | 36.11 1st | 6.88 3rd | 1.72 9th | 56.16 1st | 53.55 1st | 10.10 2nd | 30.58 1st |
| Limonene | Mh9 | 1.151 5th | - c | 1.75 11th | 2.69 9th | 44.95 1st | 27.25 1st | 2.19 9th | 2.54 7th | 10.09 3rd | 10.29 2nd |
| Indole | N2 | 3.96 4th | 5.00 6th | 10.41 2nd | 6.91 4th | 1.01 15th | 0.16 32nd | 5.51 2nd | 7.18 3rd | 4.52 7th | 4.96 3rd |
| α-citral | Om23 | 0.045 1st | - | 0.66 19th | 11.17 2nd | 0.911 8th | 24.50 2nd | 3.07 7th | 1.19 11th | 1.38 17th | 4.77 4th |
| γ-terpinene | Mh12 | 1.44 11th | 8.91 2nd | 0.30 27th | 0.32 29th | 1.97 11th | 3.71 4th | 0.05 43rd | 0.05 42nd | 22.83 1st | 4.40 5th |
| β-pinene | Mh5 | 7.83 2nd | 3.91 9th | - | 7.98 3rd | 0.81 19th | 0.68 18th | 1.67 13th | - | 8.08 4th | 3.44 6th |
| β-citral | Om21 | 0.094 3rd | - | 0.33 26th | 0.26 31th | 0.65 22nd | 17.10 3rd | 2.28 8th | 1.03 12th | 0.841 9th | 2.51 7th |
| β-elemene | Sh4 | - | 4.76 8th | 0.12 38th | 3.09 6th | 7.53 2nd | - | 0.14 29th | 0.19 29th | 4.48 8th | 2.26 8th |
| Methyl anthranilate | N3 | 0.22 34th | 4.87 7th | 8.26 3rd | - | 2.47 7th | - | 4.20 3rd | - | - | 2.22 9th |
| Nerolidol | Os4 | 0.24 32nd | - | 3.04 6th | 1.74 12th | 0.15 38th | 1.44 12th | 4.04 5th | 7.47 2nd | 0.40 30th | 2.06 10th |
| β-Myrcene | Mh6 | 1.38 12th | 2.55 16th | 1.82 9th | 1.73 13th | 2.02 10th | 1.50 11th | 4.15 4th | 0.89 13th | 1.95 12th | 2.00 11th |
| α-terpineol | Om11 | 2.94 5th | 5.58 5th | 2.60 7th | 2.83 7th | - | 2.72 5th | 0.42 17th | 0.19 28th | 0.50 27th | 1.98 12th |
| (E)-ocimene | Mh11 | 2.29 7th | 2.81 13th | 1.18 15th | 1.54 14th | - | 2.56 6th | - | 5.45 4th | - | 1.76 13th |
| β-farnesene | Sh15 | 2.24 8th | - | 3.56 5th | 2.25 10th | 2.26 8th | 0.13 37th | 0.10 39th | - | 4.60 6th | 1.68 14th |
| Caryophyllene | Sh8 | 0.33 31th | 2.61 15th | 0.56 21th | 0.93 17th | 3.93 5th | 1.99 8th | 0.70 14th | - | 2.98 9th | 1.56 15th |
| Compounds | FC a | PK b | ST | LBC | QJ | ERK | BM | YHY | ZXY | HY | Mean rank |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Linalool | Om4 | 47.74 1st | 42.76 1st | 46.98 1st | 24.95 1st | 3.94 4th | 1.59 9th | 21.59 1st | 48.43 1st | 30.11 1st | 29.79 1st |
| Limonene | Mh9 | 1.071 8th | 1.69 8th | 1.54 8th | 2.41 11th | 52.53 1st | 36.17 1st | 3.68 7th | 3.32 6th | 7.05 4th | 12.16 2nd |
| Indole | N2 | 4.99 3rd | 4.90 4th | 4.86 4th | 8.43 2nd | 0.73 16th | 0.14 33th | 8.84 3rd | 7.88 4th | 2.83 7th | 4.84 3rd |
| Methyl anthranilate | N3 | 0.342 9th | 17.91 2nd | 15.81 2nd | 1.77 13th | 0.04 53th | - c | 2.79 8th | - | 2.17 8th | 4.54 4th |
| β-pinene | Mh5 | 6.59 2nd | 6.51 3rd | - | 3.53 6th | 1.11 12th | 0.60 17th | 2.42 9th | 4.90 5th | 6.84 5th | 3.61 5th |
| α-citral | Om23 | 0.074 8th | 0.43 23th | 0.78 18th | 3.30 8th | 0.50 24th | 20.07 2nd | 2.40 10th | 0.76 14th | 0.84 14th | 3.24 6th |
| β-myrcene | Mh6 | 1.11 14th | 1.53 9th | 1.48 9th | 1.62 15th | 2.42 8th | 1.53 10th | 1.55 17th | 0.99 12th | 15.50 3rd | 3.08 7th |
| (E)-ocimene | Mh11 | 2.16 8th | 0.93 15th | 1.39 11th | 8.40 3rd | - | 2.96 15th | - | 8.33 3rd | - | 2.69 8th |
| β-elemene | Sh5 | 0.02 58th | 2.13 7th | 1.47 10th | 5.73 5th | 6.02 3rd | - | 4.98 6th | 2.25 8th | 0.34 23rd | 2.55 9th |
| γ-terpinene | Mh12 | 1.63 12th | 0.18 34th | 0.16 29th | 0.12 42th | 3.17 5th | 4.29 4th | 11.06 2nd | 0.04 41th | 0.08 35th | 2.30 10th |
| (Z)-ocimene | Mh10 | - | - | - | 1.71 14th | - | 1.25 13th | - | 0.22 26th | 16.52 2nd | 2.19 11th |
| β-citral | Om21 | 0.15 39th | 0.29 28th | 0.28 25th | 2.55 9th | 0.52 23th | 13.27 3rd | 1.64 16th | 0.72 15th | - | 2.16 12th |
| β-farnesene | Sh15 | 3.04 6th | - | 2.69 6th | 3.43 7th | 1.64 11th | 0.11 36th | 5.16 5th | - | 0.09 34th | 1.80 13th |
| Nerolidol | Os1 | 0.06 49th | 0.25 29th | 3.64 5th | - | 0.064 5th | 0.51 19th | 0.92 24th | 8.75 2nd | 0.02 59th | 1.58 14th |
| Caryophyllene | Sh8 | 0.42 25th | - | 0.43 21st | 1.34 20th | 3.14 6th | 1.79 8th | 2.18 11th | 0.05 40th | 2.00 9th | 1.26 15th |
Acknowledgments
Conflicts of Interest
References
- Colquhoun, T.A.; Clark, D.G. Unraveling the regulation of floral fragrance biosynthesis. Plant Signal Behav 2011, 6, 378–381. [Google Scholar]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett 2010, 13, 643–656. [Google Scholar]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst 2008, 39, 549–569. [Google Scholar]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol 2002, 5, 237–243. [Google Scholar]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol 2009, 12, 479–485. [Google Scholar]
- Dudareva, N.; Andersson, S.; Orlova, I.; Gatto, N.; Reichelt, M.; Rhodes, D.; Boland, W.; Gershenzon, J. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc. Natl. Acad. Sci. USA 2005, 102, 933–938. [Google Scholar]
- Chrubasik, C.; Roufogalis, B.D.; Muller Ladner, U.; Chrubasik, S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother. Res 2008, 22, 725–733. [Google Scholar]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Sci. Signal 2006, 311, 808. [Google Scholar]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev 2006, 72, 1–120. [Google Scholar]
- Knudsen, J.T.; Tollsten, L.; Bergström, L.G. Floral scents-a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar]
- Buckinham, J. Dictionary of natural products. Available online: http://dmnp.chemnetbase.com/ (accessed on 4 October 2013).
- Liang, P.H.; Ko, T.P.; Wang, A.H.J. Structure, mechanism and function of prenyltransferases. Eur. J. Biochem 2002, 269, 3339–3354. [Google Scholar]
- Okada, K.; Kasahara, H.; Yamaguchi, S.; Kawaide, H.; Kamiya, Y.; Nojiri, H.; Yamane, H. Genetic evidence for the role of isopentenyl diphosphate isomerases in the mevalonate pathway and plant development in Arabidopsis. Plant Cell Physiol 2008, 4, 604–616. [Google Scholar]
- Allemann, R.K. Chemical wizardry? The generation of diversity interpenoid biosynthesis. Pure Appl. Chem 2008, 80, 1791–1798. [Google Scholar]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar]
- Schmidt, M.R.; Wei, W. Loss of agro–biodiversity, uncertainty, and perceived control: A comparative risk perception study in Austria and China. Risk Anal 2006, 26, 455–470. [Google Scholar]
- Swingle, W. The botany of citrus and its wild relatives of the orange subfamily. Available online: http://websites.lib.ucr.edu/agnic/webber/Vol1/Chapter3.html (accessed on 7 October 2013).
- Liu, C.; Jiang, D.; Cheng, Y.; Deng, X.; Chen, F.; Fang, L.; Ma, Z.; Xu, J. Chemotaxonomic study of Citrus, Poncirus and Fortunella genotypes based on peel oil volatile compounds-deciphering the genetic origin of Mangshanyegan (Citrus nobilis Lauriro). PLoS One 2013, 8, e58411. [Google Scholar]
- Tanaka, T. Species problem in citrus: A critical study of wild and cultivated units of citrus based upon field studies in their native homes (Revisio Aurantiacearum IX). Jpn. Soc. Promot. Sci. Tokyo Jpn. 1954, 3, 141–152. [Google Scholar]
- Moore, G.A. Oranges and lemons: Clues to the taxonomy of Citrus from molecular markers. Trends Genet 2001, 17, 536–540. [Google Scholar]
- Azam, M.; Qian, J.; Bo, Z.; Changjie, X.; Kunsong, C. Citrus leaf volatiles as affected by developmental stage and genotype. Int. J. Mol. Sci 2013, 14, 17744–17766. [Google Scholar]
- Boussaada, O.; Chemli, R. Seasonal variation of essential oil composition of Citrus aurantium L. var. amara. J. Essent. Oil Bear Plants 2007, 10, 109–120. [Google Scholar]
- González-Mas, M.C.; Rambla, J.L.; Alamar, M.C.; Gutiérrez, A.; Granell, A. Comparative analysis of the volatile fraction of fruit juice from different Citrus species. PLoS One 2011, 6, e22016. [Google Scholar]
- Jabalpurwala, F.A.; Smoot, J.M.; Rouseff, R.L. A comparison of citrus blossom volatiles. Phytochemistry 2009, 70, 1428–1434. [Google Scholar]
- Hou, Y.; Liang, W.; Zhang, L.; Cheng, S.; He, F.; Wu, Z. Fresh water algae chemotaxonomy by high-performance liquid chromatographic (HPLC) analysis. Front. Environ. Sci. Engin. China 2011, 5, 84–91. [Google Scholar]
- Li, R.; Luo, G.; Meyers, P.A.; Gu, Y.; Wang, H.; Xie, S. Leaf wax n-alkane chemotaxonomy of bamboo from a tropical rain forest in Southwest China. Plant Syst. Evol 2012, 298, 731–738. [Google Scholar]
- Ozel, M.; Gogus, F.; Hamilton, J.; Lewis, A. The essential oil of Pista ciavera L. at various temperatures of direct thermal desorption using comprehensive gas chromatography coupled with time-of-flight mass spectrometry. Chromatographia 2004, 60, 79–83. [Google Scholar]
- Pellati, F.; Benvenuti, S.; Melegari, M. Chromatographic performance of a new polar poly(ethylene glycol) bonded phase for the phytochemical analysis of Hypericum perforatum L. J. Chromatogr 2005, 1088, 205–217. [Google Scholar]
- Kim, N.-S.; Lee, D.-S. Comparison of different extraction methods for the analysis of fragrances from Lavandula species by gas chromatography-mass spectrometry. J. Chromatogr 2002, 982, 31–47. [Google Scholar]
- Li, Z.-G.; Lee, M.-R.; Shen, D.-L. Analysis of volatile compounds emitted from fresh Syringa oblata flowers in different florescence by headspace solid-phase microextraction–gas chromatography-mass spectrometry. Anal. Chim. Acta 2006, 576, 43–49. [Google Scholar]
- Bartak, P.; Bednář, P.; Čáp, L.; Ondrakova, L.; Stránský, Z. SPMEA valuable tool for investigation of flower scent. J. Sep. Sci 2003, 26, 715–721. [Google Scholar]
- Flamini, G.; Cioni, P.L.; Morelli, I. Use of solid-phase micro-extraction as a sampling technique in the determination of volatiles emitted by flowers, isolated flower parts and pollen. J. Chromatogr. A 2003, 998, 229–233. [Google Scholar]
- Raguso, R.A. Why are some floral nectars scented? Ecology 2004, 85, 1486–1494. [Google Scholar]
- Miyazaki, T.; Plotto, A.; Goodner, K.; Gmitter, F.G. Distribution of aroma volatile compounds in tangerine hybrids and proposed inheritance. J. Sci. Food Agric 2011, 91, 449–460. [Google Scholar]
- Alissandrakis, E.; Daferera, D.; Tarantilis, P.A.; Polissiou, M.; Harizanis, P.C. Ultrasound-assisted extraction of volatile compounds from citrus flowers and citrus honey. Food Chem 2003, 82, 575–582. [Google Scholar]
- Choi, H.-S. Characterization of Citrus unshiu (C. unshiu Marcov. forma Miyagawa-wase) blossom aroma by solid-phase microextraction in conjunction with an electronic nose. J. Agric. Food Chem 2003, 51, 418–423. [Google Scholar]
- Flamini, G.; Tebano, M.; Cioni, P.L. Volatiles emission patterns of different plant organs and pollen of Citrus limon. Anal. Chim. Acta 2007, 589, 120–124. [Google Scholar]
- Darjazi, B.B. Comparison of volatile components of flower, leaf, peel and juice of ‘Page’ mandarin [(Citrus reticulata var ‘Dancy’ × Citrus paradisi var ‘Duncan’) × Citrus clementina]. Afr. J. Biotech 2011, 10, 10437–10446. [Google Scholar]
- Darjazi, B.B. A comparison of volatile components of flower, leaf and peel of Citrus reticulata Blanco (Citrus nobilis Lour var. deliciosa Swingle). J. Med. Plants Res 2012, 6, 2365–2372. [Google Scholar]
- Xu, C.; Bao, L.; Zhang, B.; Bei, Z.; Ye, X.; Zhang, S.; Chen, K. Parentage analysis of Huyou (Citrus changshanensis) based on internal transcribed spacer sequences. Plant Breed 2006, 125, 519–522. [Google Scholar]
- Hosni, K.; Hassen, I.; Sebei, H.; Casabianca, H. Secondary metabolites from Chrysanthemum coronarium (Garland) flower heads: Chemical composition and biological activities. Ind. Crop Prod 2013, 44, 263–271. [Google Scholar]
- Flamini, G.; Cioni, P.L. Odour gradients and patterns in volatile emission of different plant parts and developing fruits of grapefruit (Citrus paradisi L.). Food Chem 2010, 120, 984–992. [Google Scholar]
- Lin, S.Y.; Roan, S.F.; Lee, C.L.; Chen, I.Z. Volatile organic components of fresh leaves as indicators of indigenous and cultivated citrus Species in Taiwan. Biosci. Biotechnol. Biochem 2010, 74, 806–811. [Google Scholar]
- Shalit, M.; Guterman, I.; Volpin, H.; Bar, E.; Tamari, T.; Menda, N.; Adam, Z.; Zamir, D.; Vainstein, A.; Weiss, D. Volatile ester formation in roses. Identification of an acetyl-coenzyme A. Geraniol/Citronellol acetyl transferase in developing rose petals. Plant Physiol 2003, 131, 1868–1876. [Google Scholar]
- Tanaka, T. Misunderstanding with regards citrus classification and nomenclature. Bull. Univ. Osaka Prefect Ser. B 1969, 21, 139–145. [Google Scholar]
- Coletta Filho, H.; Machado, M.; Targon, M.; Moreira, M.; Pompeu, J., Jr. Analysis of the genetic diversity among mandarins (Citrus spp.) using RAPD markers. Euphytica 1998, 102, 133–139. [Google Scholar]
- Federici, C.; Fang, D.; Scora, R.; Roose, M. Phylogenetic relationships within the genus Citrus (Rutaceae) and related genera as revealed by RFLP and RAPD analysis. Theor. Appl. Genet 1998, 96, 812–822. [Google Scholar]
- Kitajima, A.; Yamasaki, A.; Habu, T.; Preedasuttijit, B.; Hasegawa, K. Chromosome identification and karyotyping of Satsuma mandarin by genomic in situ hybridization. J. Am. Soc. Hort. Sci 2007, 132, 836–841. [Google Scholar]
- Luro, F.L.; Costantino, G.; Terol, J.; Argout, X.; Allario, T.; Wincker, P.; Talon, M.; Ollitrault, P.; Morillon, R. Transferability of the EST-SSRs developed on Nules clementine (Citrus clementina Hort ex Tan) to other Citrus species and their effectiveness for genetic mapping. BMC Genomics 2008, 9, 287. [Google Scholar]
- Zhang, B.; Xi, W.; Wei, W.; Shen, J.; Ferguson, I.; Chen, K. Changes in aroma-related volatiles and gene expression during low temperature storage and subsequent shelf-life of peach fruit. Postharvest. Biol. Technol 2011, 60, 7–16. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Azam, M.; Song, M.; Fan, F.; Zhang, B.; Xu, Y.; Xu, C.; Chen, K. Comparative Analysis of Flower Volatiles from Nine Citrus at Three Blooming Stages. Int. J. Mol. Sci. 2013, 14, 22346-22367. https://doi.org/10.3390/ijms141122346
Azam M, Song M, Fan F, Zhang B, Xu Y, Xu C, Chen K. Comparative Analysis of Flower Volatiles from Nine Citrus at Three Blooming Stages. International Journal of Molecular Sciences. 2013; 14(11):22346-22367. https://doi.org/10.3390/ijms141122346
Chicago/Turabian StyleAzam, Muhammad, Min Song, Fangjuan Fan, Bo Zhang, Yaying Xu, Changjie Xu, and Kunsong Chen. 2013. "Comparative Analysis of Flower Volatiles from Nine Citrus at Three Blooming Stages" International Journal of Molecular Sciences 14, no. 11: 22346-22367. https://doi.org/10.3390/ijms141122346
APA StyleAzam, M., Song, M., Fan, F., Zhang, B., Xu, Y., Xu, C., & Chen, K. (2013). Comparative Analysis of Flower Volatiles from Nine Citrus at Three Blooming Stages. International Journal of Molecular Sciences, 14(11), 22346-22367. https://doi.org/10.3390/ijms141122346