The Role of Survivin in Podocyte Injury Induced by Puromycin Aminonucleoside
Abstract
:Objective
Methods
Results
Conclusion
1. Introduction
2. Results
2.1. Survivin Expression Was Increased in Podocytes after PAN Treatment
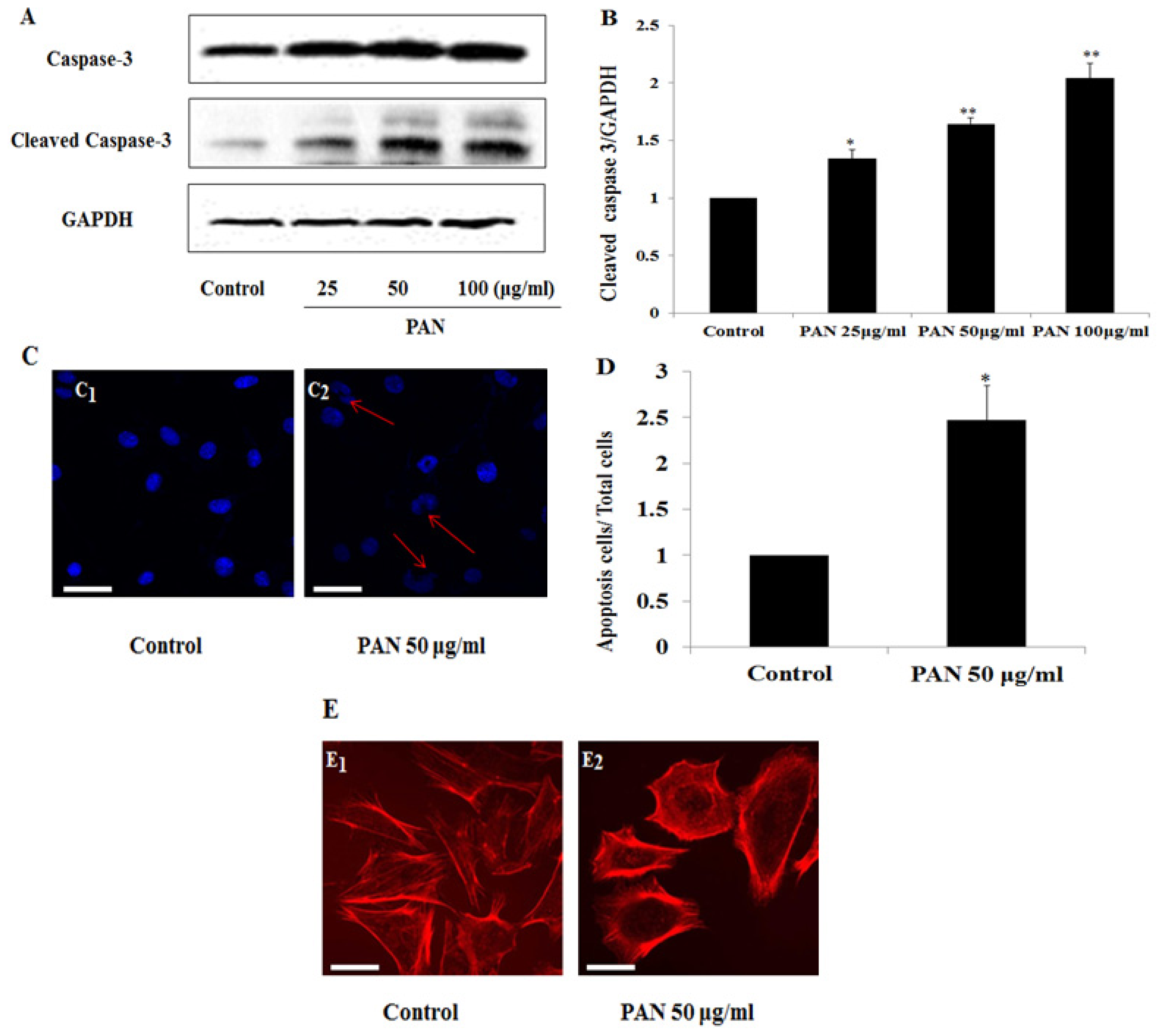
2.2. PAN Induced Podocyte Apoptosis with Significant Rearrangement of F-Actin
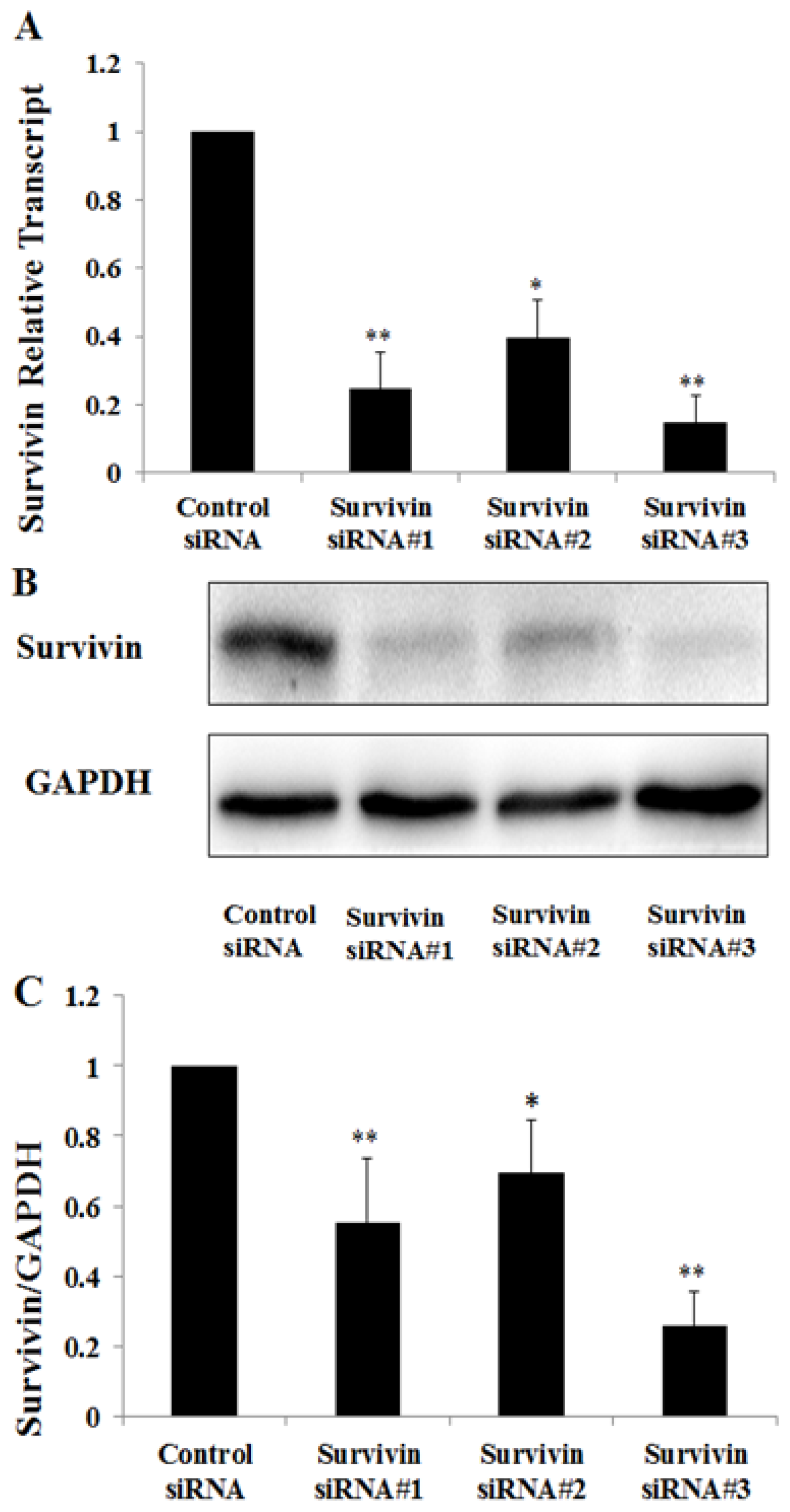
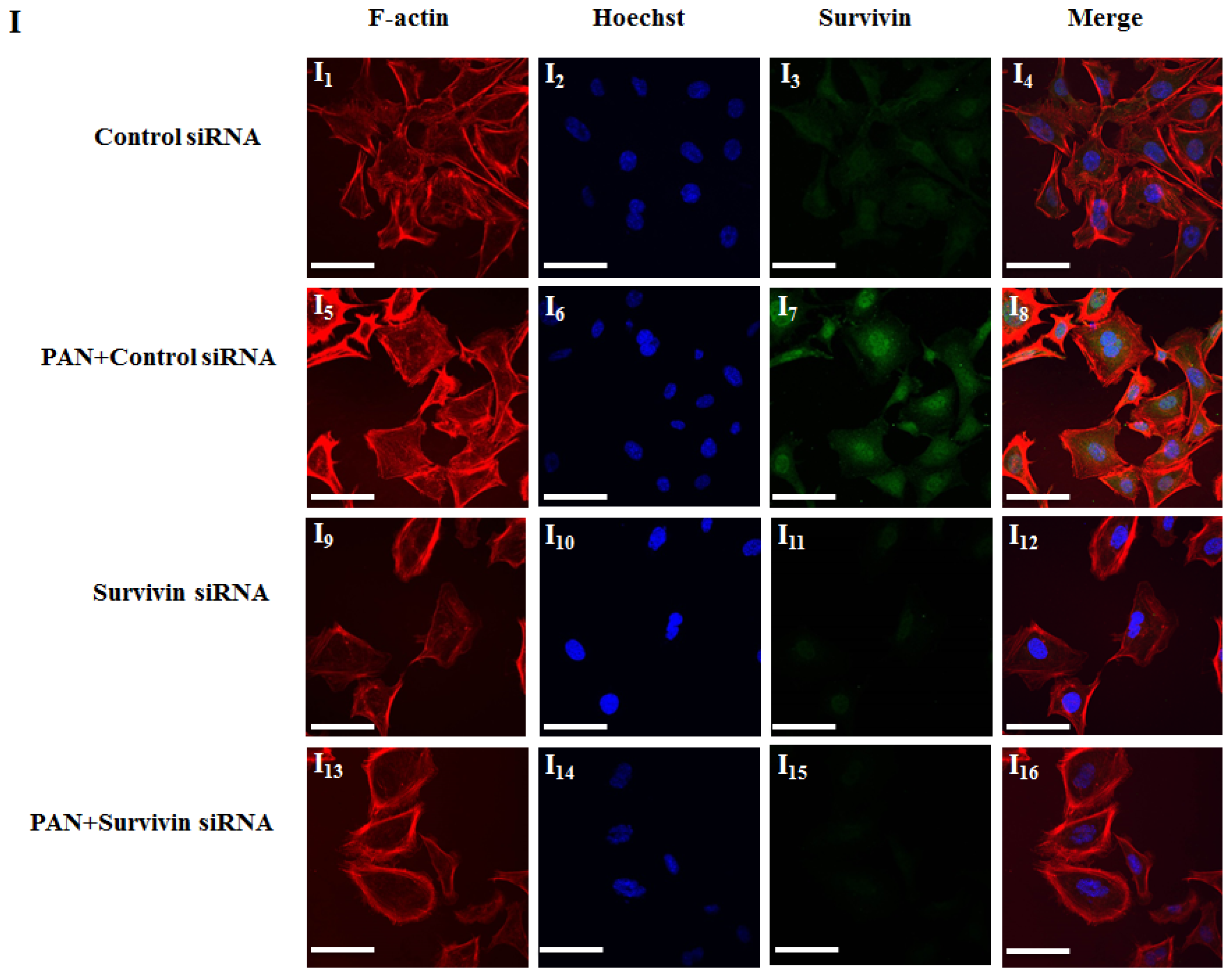
2.3. Knockdown of Survivin Expression Exacerbated PAN-Induced Injury of Podocytes
2.4. Over-Expression of Survivin Expression Ameliorated PAN-Induced Injury of Podocyte
3. Discussion
4. Experimental Section
4.1. Podocyte Culture
4.2. Quantitative Real-Time PCR
4.3. Western Blot
4.4. Small Interfering RNA (siRNA) Experiment
4.5. Survivin Over-Expression Experiment
4.6. Cell Viability Assay
4.7. Hoechst 33258 Staining
4.8. Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL)
4.9. Fluorescence Confocal Microscopy
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsXuejuan Li performed the whole experiments related to this study, drafted the manuscript and did the whole revision process. Xiaoyan Zhang did the partial study design and the manuscript revision. Xiaoyan Li and Fangrui Ding participated in partial experiments. Jie Ding acted as corresponding author and did the whole study design and the manuscript revision.
References
- Li, F. Survivin study: What is the next wave? J. Cell Physiol 2003, 197, 8–29. [Google Scholar]
- Li, F.; Ling, X. Survivin study: An update of “What is the next wave?”. J. Cell Physiol 2006, 208, 476–486. [Google Scholar]
- Ambrosini, G.; Adida, C.; Altieri, D.C. A noble anti-apoptotic gene, survivin, is expressed in cancer and lymphoma. Nat. Med 1997, 3, 917–921. [Google Scholar]
- Shariat, S.F.; Lotan, Y.; Saboorian, H.; Khoddami, S.M.; Roehrborn, C.G.; Slawin, K.M.; Ashfaq, R. Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer 2004, 100, 751–757. [Google Scholar]
- Sohn, D.M.; Kim, S.Y.; Baek, M.J.; Lim, C.W.; Lee, M.H.; Cho, M.S.; Kim, T.Y. Expression of survivin and clinical correlation in patients with breast cancer. Biomed. Pharmacother 2006, 60, 289–292. [Google Scholar]
- Sarela, A.I.; Verveke, C.S.; Ramsdale, J.; Davis, C.I.; Markham, A.F.; Guillou, P.J. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br. J. Cancer 2002, 86, 886–892. [Google Scholar]
- Lu, C.D.; Altieri, D.C.; Tanigawa, N. Expression of novel antiapoptotic gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res 1998, 58, 1808–1812. [Google Scholar]
- Okada, E.; Murai, Y.; Matsui, K.; Isizawa, S.; Cheng, C.; Masuda, M.; Takano, Y. Survivin expression in tumor cell nuclei is predictive of favorable prognosis in gastric cancer patients. Cancer Lett 2001, 163, 109–116. [Google Scholar]
- Li, F.; Ling, X.; Huang, H.; Brattain, L.; Apontes, P.; Wu, J.; Binderup, L.; Brattain, M.G. Differential expression of survivin and apoptosis by vitamin D3 in two isogenic MCF-7 breast cancer cell sublines. Oncogene 2005, 24, 1385–1395. [Google Scholar]
- Tamm, I.; Wang, Y.; Sausville, E.; Scudiero, D.A.; Vigna, N.; Oltersdorf, T.; Reed, J.C. IAP-family protein surviving inhibitoes caspase activity and apoptosis induced by Fas (CD95), Bax, caspase, and anticancer drugs. Cancer Res 1998, 58, 5315–5320. [Google Scholar]
- Shin, S.; Sung, B.J.; Cho, Y.S.; Kim, H.J.; Ha, N.C.; Hwang, J.I.; Chung, C.W.; Jung, Y.K.; Oh, B.H. An antiapoptotic protein human surviving is a direct inhibitor of caspase 3 and 7. Biochemistry 2001, 40, 1117–1123. [Google Scholar]
- Dohi, T.; Beltrami, E.; Wall, N.R.; Plescia, J.; Altieri, D.C. Mitochondrial surviving inhibors apoptosis and promotes tumorigenesis. J. Clin. Investig 2004, 114, 1117–1127. [Google Scholar]
- Altieri, D.C. Survivin and IAP proteins in cell-death mechanisms. Biochem. J 2010, 430, 199–205. [Google Scholar]
- Altieri, D.C. Survivin in apoptosis control and cell cycleregulation in cancer. Prog. Cell Cycle Res 2003, 3, 447–452. [Google Scholar]
- Waligórska-Stachura, J.; Jankowska, A.; Waśko, R.; Liebert, W.; Biczysko, M.; Czarnywojtek, A.; Baszko-Błaszyk, D.; Shimek, V.; Ruchała, M. Survivin—Prognostic tumor biomarker in human neoplasms—Review. Ginekol. Pol 2012, 83, 537–540. [Google Scholar]
- Rödel, F.; Sprenger, T.; Kaina, B.; Liersch, T.; Rödel, C.; Fulda, S.; Hehlgans, S. Survivin as a prognostic/predictive marker and molecular target in cancer therapy. Curr. Med. Chem 2012, 19, 3679–3688. [Google Scholar]
- Uren, A.G.; Wong, L.; Pakusch, M.; Fowler, K.J.; Burrows, F.J.; Vaux, D.L.; Choo, K.H. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr. Biol 2000, 10, 1319–1328. [Google Scholar]
- Li, F.; Ambrosini, G.; Chu, E.Y.; Plescia, J.; Tognin, S.; Marchisio, P.C.; Altieri, D.C. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998, 396, 580–584. [Google Scholar]
- Dallaglio, K.; Marconi, A.; Pincelli, C. Survivin: A dual player in healthy and diseased skin. J. Investig. Dermatol 2012, 132, 18–27. [Google Scholar]
- Xing, Z.; Conway, E.M.; Kang, C.; Winoto, A. Essential role of survivin, an inhibitor of apoptosis protein, in T cell development, maturation, and homeostasis. J. Exp. Med 2004, 199, 69–80. [Google Scholar]
- Okada, H.; Bakal, C.; Shahinian, A.; Elia, A.; Wakeham, A.; Suh, W.K.; Duncan, G.S.; Ciofani, M.; Rottapel, R.; Zuniga-Pflucker, J.C.; et al. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J. Exp. Med 2004, 199, 399–410. [Google Scholar]
- Chiou, S.K.; Moon, W.S.; Jones, M.K.; Tarnawski, A.S. Survivin expression in the stomach: Implications for mucosal integrity and protection. Biochem. Biophys. Res. Commun 2003, 305, 374–379. [Google Scholar]
- Kindt, N.; Menzebach, A.; van de Wouwer, M.; Betz, I.; de Vriese, A.; Conway, E.M. Protective role of the inhibitor of apoptosis protein, survivin, in toxin-induced acute renal failure. FASEB. J 2008, 22, 510–521. [Google Scholar]
- Lechler, P.; Wu, X.; Bernhardt, W.; Campean, V.; Gastiger, S.; Hackenbeck, T.; Klanke, B.; Weidemann, A.; Warnecke, C.; Amann, K.; et al. The tumor gene survivin is highly expressed in adult renal tubular cells: Implications for a pathophysiological role in the kidney. Am. J. Pathol 2007, 171, 1483–1498. [Google Scholar]
- Santini, D.; Abbate, A.; Scarpa, S.; Vasaturo, F.; Biondi-Zoccai, G.G.; Bussani, R.; de Giorgio, F.; Bassan, F.; Camilot, D.; di Marino, M.P.; et al. Surviving acute myocardial infarction: Survivin expression in viable cardiomyocytes after infarction. J. Clin. Pathol 2004, 57, 1321–1324. [Google Scholar]
- Abbate, A.; Scarpa, S.; Santini, D.; Palleiro, J.; Vasaturo, F.; Miller, J.; Morales, C.; Vetrovec, G.W.; Baldi, A. Myocardial expression of survivin, an apoptosis inhibitor, in aging and heart failure: An experimental study in the spontaneously hypertensive rat. Int. J. Cardiol 2006, 113, 371–376. [Google Scholar]
- Conway, E.M.; Zwerts, F.; van Eygen, V.; DeVriese, A.; Nagai, N.; Luo, W.; Collen, D. Survivin-dependent angiogenesis in ischemic brain: Molecular mechanisms of hypoxia-induced up-regulation. Am. J. Pathol 2003, 163, 935–946. [Google Scholar]
- Jones, M.K.; Padilla, O.R.; Webb, N.A.; Norng, M. The anti-apoptosis protein, survivin, mediates gastric epithelial cell cytoprotection against ethanol-induced injury via activation of the p34 (CDC2) cyclin dependent kinase. J. Cell. Physiol 2008, 215, 750–764. [Google Scholar]
- Terasaki, Y.; Terasaki, M.; Urushiyama, H.; Nagasaka, S.; Takahashi, M.; Kunugi, S.; Ishikawa, A.; Wakamatsu, K.; Kuwahara, N.; Miyake, K.; et al. Role of survivin in acute lung injury: Epithelial cells of mice and humans. Lab. Investig 2013, 93, 1147–1163. [Google Scholar]
- Chen, J.; Wu, W.; Tahir, S.K.; Kroeger, P.E.; Rosenberg, S.H.; Cowsert, L.M.; Bennett, F.; Krajewski, S.; Krajewska, M.; Welsh, K.; et al. Down-regulation of survivin by antisense oligonucleotides increases apoptosis, inhibits cytokinesis and anchorage-independent growth. Neoplasia 2000, 2, 235–241. [Google Scholar]
- Levkau, B.; Schäfers, M.; Wohlschlaeger, J.; von Wnuck Lipinski, K.; Keul, P.; Hermann, S.; Kawaguchi, N.; Kirchhof, P.; Fabritz, L.; Stypmann, J.; et al. Survivin determines cardiac function by controlling total cardiomyocyte number. Circulation 2008, 117, 1583–1593. [Google Scholar]
- Pavenstadt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev 2003, 83, 253–307. [Google Scholar]
- Kriz, W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc. Res. Tech 2002, 57, 189–195. [Google Scholar]
- D’Agati, V.D. The spectrum of focal segmental glomerulosclerosis: New insights. Curr. Opin. Nephrol. Hypertens 2008, 17, 271–281. [Google Scholar]
- Pollak, M.R. Focal segmental glomerulosclerosis: Recent advances. Curr. Opin. Nephrol. Hypertens 2008, 17, 138–142. [Google Scholar]
- Miao, J.; Fan, Q.; Cui, Q.; Zhang, H.; Chen, L.; Wang, S.; Guan, N.; Guan, Y.; Ding, J. Newly identified cytoskeletal components are associated with dynamic changes of podocyte foot processes. Nephrol. Dial. Transplant 2009, 24, 3297–3305. [Google Scholar]
- Shiiki, H.; Sasaki, Y.; Nishino, T.; Kimura, T.; Kurioka, H.; Fujimoto, S.; Dohi, K. Cell proliferation and apoptosis of the glomerular epithelial cells in rats with puromycin aminonucleoside nephrosis. Pathobiology 1998, 66, 221–229. [Google Scholar]
- Kim, Y.H.; Goyal, M.; Kurnit, D.; Wharram, B.; Wiggins, J.; Holzman, L.; Kershaw, D.; Wiggins, R. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 2002, 60, 957–968. [Google Scholar]
- Zheng, C.X.; Chen, Z.H.; Zeng, C.H.; Qin, W.S.; Li, L.S.; Liu, Z.H. Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Kidney Int. 2008, 74, 596–612. [Google Scholar]
- Wada, T.; Pippin, J.W.; Marshall, C.B.; Griffin, S.V.; Shankland, S.J. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: Role of p53 and Bcl-2-related family proteins. J. Am. Soc. Nephrol 2005, 16, 2615–2625. [Google Scholar]
- Liu, S.F.; Ding, J.; Fan, Q.F.; Zhang, H. Establishment of a podocyte cell injury model induced by puromycin aminonucleoside. Beijing Da Xue Xue Bao 2008, 40, 586–589. (In Chinese) [Google Scholar]
- Suzuki, T.; Takemura, H.; Noiri, E.; Nosaka, K.; Toda, A.; Taniguchi, S.; Uchida, K.; Fujita, T.; Kimura, S.; Nakao, A. Puromycin aminonucleoside induces apoptosis and increases HNE in cultured glomerular epithelial cells(1). Free Radic. Biol. Med 2001, 31, 615–623. [Google Scholar]
- Koshikawa, M.; Mukoyama, M.; Mori, K.; Suganami, T.; Sawai, K.; Yoshioka, T.; Nagae, T.; Yokoi, H.; Kawachi, H.; Shimizu, F.; et al. Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J. Am. Soc. Nephrol 2005, 16, 2690–2701. [Google Scholar]
- Liu, S.; Shi, W.; Xiao, H.; Liang, X.; Deng, C.; Ye, Z.; Mei, P.; Wang, S.; Liu, X.; Shan, Z.; et al. Receptor activator of NF-kappaB and podocytes: Towards a function of a novel receptor-ligand pair in the survival response of podocyte injury. PLoS One 2012, 7, e41331. [Google Scholar]
- Ma, J.; Matsusaka, T.; Yang, H.C.; Zhong, J.; Takagi, N.; Fogo, A.B.; Kon, V.; Ichikawa, I. Induction of podocyte-derived VEGF ameliorates podocyte injury and subsequent abnormal glomerular development caused by puromycin aminonucleoside. Pediatr. Res 2011, 70, 83–89. [Google Scholar]
- Yu, S.Y.; Qi, R. Role of bad in podocyte apoptosis induced by puromycin aminonucleoside. Transplant. Proc 2013, 45, 569–573. [Google Scholar]
- Rincon, J.; Romero, M.; Viera, N.; Pedreañez, A.; Mosquera, J. Increased oxidative stress and apoptosis in acute puromycin aminonucleoside nephrosis. Int. J. Exp. Path 2004, 85, 25–33. [Google Scholar]
- George, J.; Banik, N.L.; Ray, S.K. Survivin knockdown and concurrent 4-HPR treatment controlled human glioblastoma in vitro and in vivo. Neuro Oncol 2010, 12, 1088–1101. [Google Scholar]
- Watanabe, A.; Taniguchi, F.; Izawa, M.; Suou, K.; Uegaki, T.; Takai, E.; Terakawa, N. The role of survivin in the resistance of endometriotic stromal cells to drug-induced apoptosis. Hum. Reprod 2009, 24, 3172–3179. [Google Scholar]
- Chen, J.; Chen, J.K.; Conway, E.M.; Harris, R.C. Survivin mediates renal proximal tubule recovery from AKI. J. Am. Soc. Nephrol 2013, 24, 2023–2033. [Google Scholar]
- Altieri, D.C. Targeting survivin in cancer. Cancer Lett 2013, 332, 225–228. [Google Scholar]
- Croci, D.O.; Cogno, I.S.; Vittar, N.B.; Salvatierra, E.; Trajtenberg, F.; Podhajcer, O.; Osinaga, E.; Rabinovich, G.A.; Rivarola, V.A. Silencing survivin gene expression promotes apoptosis of human breast cancer cells through a caspase independent pathway. J. Cell. Biochem 2008, 105, 381–390. [Google Scholar]
- Cheung, C.H.; Chen, H.H.; Kuo, C.C.; Chang, C.Y.; Coumar, M.S.; Hsieh, H.P.; Chang, J.Y. Survivin counteracts the therapeutic effect of microtubule de-stabilizers by stabilizing tubulin polymers. Mol. Cancer 2009, 8, 1–15. [Google Scholar]
- Shimo, T.; Adachi, Y.; Yamanouchi, S.; Tsuji, S.; Kimata, T.; Umezawa, K.; Okigaki, M.; Takaya, J.; Ikehara, S.; Kaneko, K. A novel nuclear factor κB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates puromycin aminonucleoside-induced nephrosis in mice. Am. J. Nephrol 2013, 37, 302–309. [Google Scholar]
- Eto, N.; Wada, T.; Inagi, R.; Takano, H.; Shimizu, A.; Kato, H.; Kurihara, H.; Kawachi, H.; Shankland, S.J.; Fujita, T.; et al. Podocyte protection by darbepoetin: Preservation of the cytoskeleton and nephrin expression. Kidney Int 2007, 72, 455–463. [Google Scholar]




© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, X.; Zhang, X.; Li, X.; Ding, F.; Ding, J. The Role of Survivin in Podocyte Injury Induced by Puromycin Aminonucleoside. Int. J. Mol. Sci. 2014, 15, 6657-6673. https://doi.org/10.3390/ijms15046657
Li X, Zhang X, Li X, Ding F, Ding J. The Role of Survivin in Podocyte Injury Induced by Puromycin Aminonucleoside. International Journal of Molecular Sciences. 2014; 15(4):6657-6673. https://doi.org/10.3390/ijms15046657
Chicago/Turabian StyleLi, Xuejuan, Xiaoyan Zhang, Xiaoyan Li, Fangrui Ding, and Jie Ding. 2014. "The Role of Survivin in Podocyte Injury Induced by Puromycin Aminonucleoside" International Journal of Molecular Sciences 15, no. 4: 6657-6673. https://doi.org/10.3390/ijms15046657
APA StyleLi, X., Zhang, X., Li, X., Ding, F., & Ding, J. (2014). The Role of Survivin in Podocyte Injury Induced by Puromycin Aminonucleoside. International Journal of Molecular Sciences, 15(4), 6657-6673. https://doi.org/10.3390/ijms15046657





