Comparative Evaluation of Urinary PCA3 and TMPRSS2: ERG Scores and Serum PHI in Predicting Prostate Cancer Aggressiveness
Abstract
:1. Introduction
2. Results and Discussion
2.1. Baseline Characteristics of the Studied Patients
| Baseline Characteristics | Number of Patients (%) or Median (IQR) |
|---|---|
| Age (years) | 64 (58–66) |
| Gleason score at biopsy | 6: n = 63 (41%) 7: n = 79 (51%) 8: n = 12 (8%) |
| tPSA (ng/mL) | 6.5 (5.0–9.9) |
| fPSA (ng/mL) | 0.9 (0.6–1.2) |
| %fPSA (%) | 12.5 (10.5–16.1) |
| %p2PSA (%) | 13.8 (10.2–19.7) |
| PHI | 42.15 (33–56) |
| PCA3 score | 45 (23–87) |
| T2 score | 34 (5–138) |
| Pathological Characteristics Prostatectomy (Number of Patients) | Number of Patients (%) | Serum PSA | PCA3 Score | T2 Score | PHI | ||||
|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | p Value | Median (IQR) | p Value | Median (IQR) | p Value | Median (IQR) | p Value | ||
| Pathological T stage n = 154 | pT2 n = 106 (69%) | 6.5 (5.0–9.0) | 0.467 | 47 (23–82) | 0.724 | 27 (5–106) | 0.027 | 41 (32–53) | 0.086 |
| ≥pT3 n = 48 (31%) | 6.4 (5.2–10.9) | 40 (25–111) | 52 (10–352) | 47 (34–59) | |||||
| Gleason stage n = 154 | G6 n = 22 (14%) | 6.5 (4.6–11.0) | 0.739 | 47 (23–81) | 0.897 | 38 (6–148) | 0.123 | 34 (31–40) | 0.013 |
| G ≥ 7 n = 132 (86%) | 6.5 (5.2–9.3) | 43 (23–90) | 35 (7–151) | 44 (34–57) | |||||
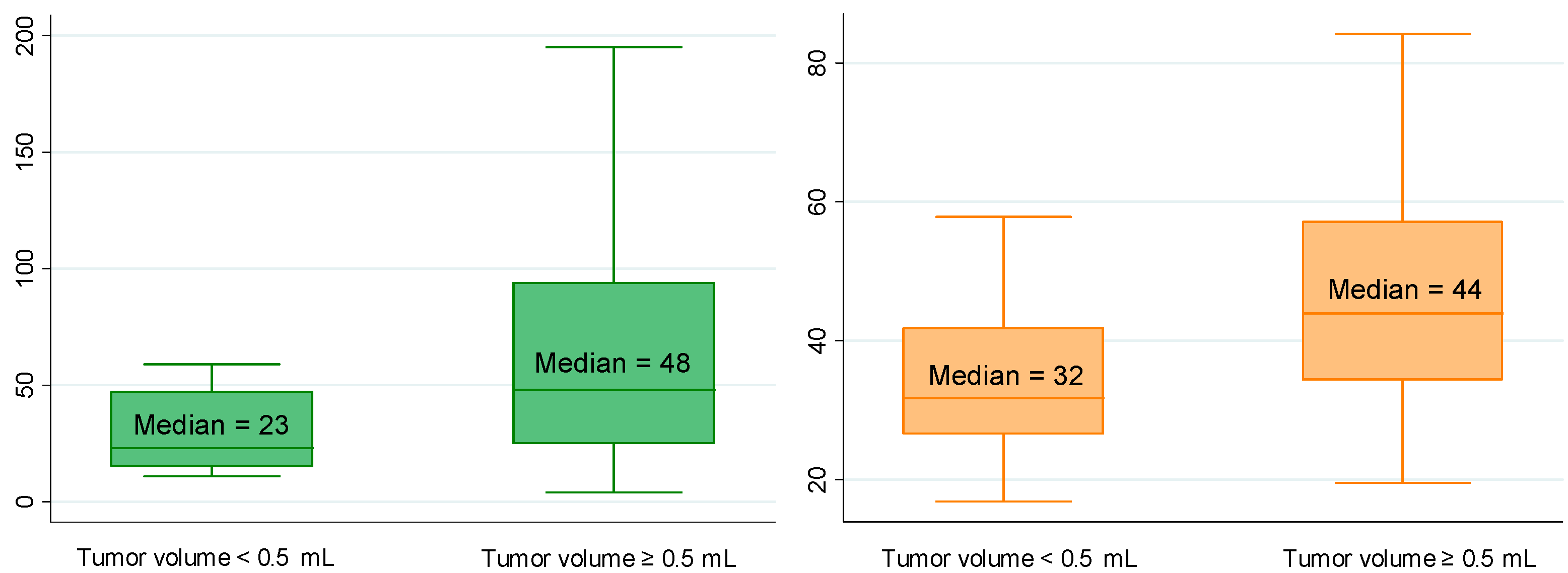
| Total tumor volume (mL) a n = 153 | <0.5 n = 19 (12%) | 6.2 (4.1–10.1) | 0.276 | 23 (15–47) | 0.004 | 14 (1–105) | 0.090 | 32 (27–42) | 0.002 |
| ≥0.5 n = 134 (88%) | 6.5 (5.1–9.3) | 48 (25–94) | 37 (6–154) | 44 (34–57) | |||||
| Number of tumor foci n = 154 | Uni n = 41 (27%) | 6.1 (4.3–11.0) | 0.230 | 27 (19–49) | 0.002 | 55 (8–178) | 0.299 | 41 (33–52) | 0.410 |
| Multi n = 113 (73%) | 6.6 (5.4–9.2) | 51 (27–94) | 33 (3–127) | 43 (33–57) | |||||
| Blood embols n = 154 | No n = 148 (96%) | 6.5 (5.0–9.1) | 0.030 | 44 (23–85) | 0.253 | 35 (5–138) | 0.709 | 42 (33–55) | 0.009 |
| Yes n = 6 (4%) | 12.9 (8.6–18.1) | 103 (25–148) | 20 (2–164) | 79 (46–140) | |||||
| Positive resection margins n = 154 | R0 n = 121 (79%) | 6.3 (5.0–8.8) | 0.029 | 42 (23–82) | 0.241 | 36 (5–137) | 0.620 | 40 (32–53) | 0.007 |
| R1 n = 33 (21%) | 7.8 (5.9–14.1) | 61 (26–110) | 29 (3–214) | 50 (41–65) | |||||
2.2. Correlations between Biological Biomarkers and Pathological Findings
| Pathological Characteristics | Serum PSA | Urinary PCA3 Score | Urinary T2 Score | PHI |
|---|---|---|---|---|
| Tumor volume | ||||
| Univariate analysis | p = 0.240 | p = 0.004 | p = 0.025 | p = 0.004 |
| Multivariate analysis | - | p = 0.076 | p = 0.249 | p = 0.020 |
| Gleason sum | ||||
| Univariate analysis | p = 0.658 | p = 0.922 | p = 0.168 | p = 0.028 |
| Multivariate analysis | - | - | - | - |
| pT stage | ||||
| Univariate analysis | p = 0.231 | p = 0.328 | p = 0.005 | p = 0.038 |
| Multivariate analysis | - | - | p = 0.026 | p = 0.042 |
| Multifocality | ||||
| Univariate analysis | p = 0.704 | p = 0.014 | p = 0.413 | p = 0.814 |
| Multivariate analysis | - | - | - | - |
| Positive margins | ||||
| Univariate analysis | p = 0.006 | p = 0.580 | p = 0.074 | p = 0.003 |
| Multivariate analysis | p = 0.357 | - | - | p = 0.131 |



2.3. Integration of Biological Biomarkers into Predictive Models
| Predictors | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| Base Model | Base Model + PHI | ||||
| OR (95% CI) | p Value | AUC ((95% CI) | OR ((95% CI) | OR ((95% CI) | |
| Age | 1.067 (0.989–1.153) | 0.099 | 62.5% (50.1–74.8) | 1.018 (0.935–1.110) | 1.047 (0.954–1.150) |
| DRE findings | 1.705 (0.471–6.177) | 0.393 | 53.8% (45.7–61.9) | 0.959 (0.215–4.275) | 1.413 (0.268–7.438) |
| Serum total PSA | 1.022 (0.926–1.127) | 0.658 | 52.2% (38.0–66.4) | 0.966 (0.856–1.091) | 0.867 (0.738–1.018) |
| Biopsy Gleason sum | 20.698 (4.626–92.615) | <0.0001 | 79.2% (71.8–86.5) | 21.505 (4.418–104.677) | 18.839 (4.018–88.335) |
| PHI | 1.030 (0.998–1.062) | 0.028 | 66.6% (54.4–78.7) | - | 1.049 (1.002–1.097) |
| AUC (multivariate models) | 81.3% (71.1–91.5) | 86.1% (79.0–93.1) | |||
| Gain in predictive accuracy * | - | +4.8 | |||
| p value * | - | NS | |||
| Predictors | Univariate Analysis | Multivariate Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Base Model | Base Model + PCA3 Score | Base Model + T2 Score | Base Model + PHI | Base Model + PCA3 and T2 Scores | Base Model + PCA3 Score + PHI | Base Model + T2 Score + PHI | Base Model + PCA3 and T2 Scores + PHI | ||||
| OR (95% CI) | p Value | AUC (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age | 1.091 (1.007–1.183) | 0.035 | 67.2% (55.3–79.2) | 1.096 (1.008–1.193) | 1.066 (0.977–1.164) | 1.091 (1.001–1.189) | 1.119 (1.021–1.227) | 1.070 (0.980–1.169) | 1.087 (0.988–1.196) | 1.110 (1.011–1.220) | 1.089 (0.988–1.198) |
| DRE findings | 1.409 (0.383–5.177) | 0.595 | 52.6% (43.4–61.7) | 1.433 (0.371–5.543) | 1.536 (0.391–6.036) | 1.229 (0.314–4.807) | 1.486 (0.361–6.121) | 1.270 (0.319–5.052) | 1.624 (0.382–6.905) | 1.237 (0.291–5.259) | 1.293 (0.295–5.664) |
| Serum total PSA | 1.072 (0.943–1.219) | 0.240 | 57.7% (42.2–73.3) | 1.075 (0.938–1.231) | 1.057 (0.926–1.208) | 1.082 (0.943–1.243) | 0.932 (0.789–1.102) | 1.068 (0.930–1.226) | 0.917 (0.774–1.087) | 0.949 (0.801–1.124) | 0.935 (0.787–1.112) |
| Biopsy Gleason sum | 1.375 (0.524–3.610) | 0.519 | 53.9% (41.6–66.2) | 0.932 (0.331–2.627) | 1.010 (0.354–2.883) | 0.931 (0.326–2.659) | 0.907 (0.322–2.553) | 0.986 (0.343–2.832) | 1.017 (0.354–2.925) | 0.903 (0.315–2.593) | 0.970 (0.334–2.818) |
| PCA3 score | 1.019 (1.002–1.036) | 0.004 | 70.8 (58.9–82.6) | - | 1.016 (0.999–1.034) | - | - | 1.013 (0.996–1.030) | 1.017 (0.999–1.035) | - | 1.014 (0.996–1.032) |
| T2 score | 1.004 (0.999–1.009) | 0.025 | 62.0% (49.0–75.1) | - | - | 1.004 (0.999–1.010) | - | 1.003 (0.998–1.009) | - | 1.004 (0.998–1.009) | 1.003 (0.997–1.008) |
| PHI | 1.050 (1.008–1.093) | 0.004 | 71.7% (58.7–84.8) | - | - | - | 1.068 (1.018–1.122) | - | 1.068 (1.017–1.121) | 1.066 (1.016–1.118) | 1.065 (1.015–1.118) |
| AUC of multivariate models | 68.9% (57.2–80.5) | 74.4% (63.5–85.4) | 71.5% (59.9–83.1) | 76.0% (62.6–89.4) | 75.2% (64.5–85.9) | 81.1% (69.2–93.0) | 79.2% (69.2–93.0) | 82.7% (71.6–93.8) | |||
| Gain in predictive accuracy * | - | +5.5 | +2.6 | +7.1 | +6.3 | +12.2 | +10.3 | +13.8 | |||
| p value * | - | 0.056 | 0.428 | 0.240 | 0.052 | 0.028 | 0.076 | 0.011 | |||



2.4. Discussion
3. Experimental Section
3.1. Patients and Study Design
3.2. Tissue Samples
3.3. Data Analysis and Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilt, T.J.; Ahmed, H.U. Prostate cancer screening and the management of clinically localized disease. BMJ 2013, 346, f325. [Google Scholar] [CrossRef]
- Bussemakers, M.J.; van Bokhoven, A.; Verhaegh, G.W.; Smit, F.P.; Karthaus, H.F.; Schalken, J.A.; Debruyne, F.M.; Ru, N.; Isaacs, W.B. DD3: A new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999, 59, 5975–5979. [Google Scholar]
- Hessels, D.; Klein Gunnewiek, J.M.; van Oort, I.; Karthaus, H.F.; van Leenders, G.J.; van Balken, B.; Kiemeney, L.A.; Witjes, J.A.; Schalken, J.A. DD3(PCA3)-based molecular urine analysis for the diagnosis of prostate cancer. Eur. Urol. 2003, 44, 8–15. [Google Scholar] [CrossRef]
- Groskopf, J.; Aubin, S.M.; Deras, I.L.; Blase, A.; Bodrug, S.; Clark, C.; Brentano, S.; Mathis, J.; Pham, J.; Meyer, T.; et al. APTIMA PCA3 molecular urine test: Development of a method to aid in the diagnosis of prostate cancer. Clin. Chem. 2006, 52, 1089–1095. [Google Scholar] [CrossRef]
- Auprich, M.; Bjartell, A.; Chun, F.K.; de la Taille, A.; Freedland, S.J.; Haese, A.; Schalken, J.; Stenzl, A.; Tombal, B.; van der Poel, H. Contemporary role of prostate cancer antigen 3 in the management of prostate cancer. Eur. Urol. 2011, 60, 1045–1054. [Google Scholar] [CrossRef]
- Vlaeminck-Guillem, V.; Ruffion, A.; Andre, J.; Devonec, M.; Paparel, P. Urinary prostate cancer 3 test: Toward the age of reason? Urology 2010, 75, 447–453. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Bjartell, A.; Chinnaiyan, A.M.; Jenster, G.; Nam, R.K.; Rubin, M.A.; Schalken, J.A. ETS gene fusions in prostate cancer: From discovery to daily clinical practice. Eur. Urol. 2009, 56, 275–286. [Google Scholar] [CrossRef]
- Pettersson, A.; Graff, R.E.; Bauer, S.R.; Pitt, M.J.; Lis, R.T.; Stack, E.C.; Martin, N.E.; Kunz, L.; Penney, K.L.; Ligon, A.H.; et al. The TMPRSS2:ERG rearrangement, ERG expression, and prostate cancer outcomes: A cohort study and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1497–1509. [Google Scholar] [CrossRef]
- Hessels, D.; Smit, F.P.; Verhaegh, G.W.; Witjes, J.A.; Cornel, E.B.; Schalken, J.A. Detection of TMPRSS2-ERG fusion transcripts and prostate cancer antigen 3 in urinary sediments may improve diagnosis of prostate cancer. Clin. Cancer Res. 2007, 13, 5103–5108. [Google Scholar] [CrossRef]
- Laxman, B.; Tomlins, S.A.; Mehra, R.; Morris, D.S.; Wang, L.; Helgeson, B.E.; Shah, R.B.; Rubin, M.A.; Wei, J.T.; Chinnaiyan, A.M. Noninvasive detection of TMPRSS2:ERG fusion transcripts in the urine of men with prostate cancer. Neoplasia 2006, 8, 885–888. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Aubin, S.M.; Siddiqui, J.; Lonigro, R.J.; Sefton-Miller, L.; Miick, S.; Williamsen, S.; Hodge, P.; Meinke, J.; Blase, A.; et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci. Transl. Med. 2011, 3, 94ra72. [Google Scholar] [CrossRef]
- Le, B.V.; Griffin, C.R.; Loeb, S.; Carvalhal, G.F.; Kan, D.; Baumann, N.A.; Catalona, W.J. [-2]Proenzyme prostate specific antigen is more accurate than total and free prostate specific antigen in differentiating prostate cancer from benign disease in a prospective prostate cancer screening study. J. Urol. 2010, 183, 1355–1359. [Google Scholar]
- Jansen, F.H.; van Schaik, R.H.; Kurstjens, J.; Horninger, W.; Klocker, H.; Bektic, J.; Wildhagen, M.F.; Roobol, M.J.; Bangma, C.H.; Bartsch, G. Prostate-specific antigen (PSA) isoform p2PSA in combination with total PSA and free PSA improves diagnostic accuracy in prostate cancer detection. Eur. Urol. 2010, 57, 921–927. [Google Scholar] [CrossRef]
- Guazzoni, G.; Nava, L.; Lazzeri, M.; Scattoni, V.; Lughezzani, G.; Maccagnano, C.; Dorigatti, F.; Ceriotti, F.; Pontillo, M.; Bini, V.; et al. Prostate-specific antigen (PSA) isoform p2PSA significantly improves the prediction of prostate cancer at initial extended prostate biopsies in patients with total PSA between 2.0 and 10 ng/mL: Results of a prospective study in a clinical setting. Eur. Urol. 2011, 60, 214–222. [Google Scholar] [CrossRef]
- Stephan, C.; Vincendeau, S.; Houlgatte, A.; Cammann, H.; Jung, K.; Semjonow, A. Multicenter evaluation of [-2]proprostate-specific antigen and the prostate health index for detecting prostate cancer. Clin. Chem. 2013, 59, 306–314. [Google Scholar] [CrossRef]
- Filella, X.; Foj, L.; Maria Auge, J.; Molina, R.; Alcover, J. Clinical utility of %p2PSA and prostate health index in the detection of prostate cancer. Clin. Chem. Lab. Med.: CCLM/FESCC 2014. [Google Scholar] [CrossRef]
- Ferro, M.; Bruzzese, D.; Perdona, S.; Marino, A.; Mazzarella, C.; Perruolo, G.; D’Esposito, V.; Cosimato, V.; Buonerba, C.; di Lorenzo, G.; et al. Prostate Health Index (Phi) and Prostate Cancer Antigen 3 (PCA3) significantly improve prostate cancer detection at initial biopsy in a total PSA range of 2–10 ng/mL. PLoS One 2013, 8, e67687. [Google Scholar]
- Ferro, M.; Bruzzese, D.; Perdona, S.; Mazzarella, C.; Marino, A.; Sorrentino, A.; di Carlo, A.; Autorino, R.; di Lorenzo, G.; Buonerba, C.; et al. Predicting prostate biopsy outcome: Prostate health index (phi) and prostate cancer antigen 3 (PCA3) are useful biomarkers. Clin. Chim. Acta 2012, 413, 1274–1278. [Google Scholar] [CrossRef] [Green Version]
- Perdona, S.; Bruzzese, D.; Ferro, M.; Autorino, R.; Marino, A.; Mazzarella, C.; Perruolo, G.; Longo, M.; Spinelli, R.; di Lorenzo, G.; et al. Prostate health index (phi) and prostate cancer antigen 3 (PCA3) significantly improve diagnostic accuracy in patients undergoing prostate biopsy. Prostate 2013, 73, 227–235. [Google Scholar] [CrossRef]
- Scattoni, V.; Lazzeri, M.; Lughezzani, G.; de Luca, S.; Passera, R.; Bollito, E.; Randone, D.; Abdollah, F.; Capitanio, U.; Larcher, A.; et al. Head-to-head comparison of prostate health index and urinary PCA3 for predicting cancer at initial or repeat biopsy. J. Urol. 2013, 190, 496–501. [Google Scholar] [CrossRef]
- Stephan, C.; Jung, K.; Semjonow, A.; Schulze-Forster, K.; Cammann, H.; Hu, X.; Meyer, H.A.; Bogemann, M.; Miller, K.; Friedersdorff, F. Comparative assessment of urinary prostate cancer antigen 3 and TMPRSS2:ERG gene fusion with the serum [-2]proprostate-specific antigen-based prostate health index for detection of prostate cancer. Clin. Chem. 2013, 59, 280–288. [Google Scholar] [CrossRef]
- Nakanishi, H.; Groskopf, J.; Fritsche, H.A.; Bhadkamkar, V.; Blase, A.; Kumar, S.V.; Davis, J.W.; Troncoso, P.; Rittenhouse, H.; Babaian, R.J. PCA3 molecular urine assay correlates with prostate cancer tumor volume: Implication in selecting candidates for active surveillance. J. Urol. 2008, 179, 1804–1809. [Google Scholar] [CrossRef]
- Auprich, M.; Chun, F.K.; Ward, J.F.; Pummer, K.; Babaian, R.; Augustin, H.; Luger, F.; Gutschi, S.; Budaus, L.; Fisch, M.; et al. Critical assessment of preoperative urinary prostate cancer antigen 3 on the accuracy of prostate cancer staging. Eur. Urol. 2011, 59, 96–105. [Google Scholar] [CrossRef]
- Durand, X.; Xylinas, E.; Radulescu, C.; Haus-Cheymol, R.; Moutereau, S.; Ploussard, G.; Forgues, A.; Robert, G.; Vacherot, F.; Loric, S.; et al. The value of urinary prostate cancer gene 3 (PCA3) scores in predicting pathological features at radical prostatectomy. BJU Int. 2012, 110, 43–49. [Google Scholar] [CrossRef]
- Whitman, E.J.; Groskopf, J.; Ali, A.; Chen, Y.; Blase, A.; Furusato, B.; Petrovics, G.; Ibrahim, M.; Elsamanoudi, S.; Cullen, J.; et al. PCA3 score before radical prostatectomy predicts extracapsular extension and tumor volume. J. Urol. 2008, 180, 1975–1978. [Google Scholar] [CrossRef]
- Vlaeminck-Guillem, V.; Devonec, M.; Colombel, M.; Rodriguez-Lafrasse, C.; Decaussin-Petrucci, M.; Ruffion, A. Urinary PCA3 score predicts prostate cancer multifocality. J. Urol. 2011, 185, 1234–1239. [Google Scholar] [CrossRef]
- Augustin, H.; Mayrhofer, K.; Pummer, K.; Mannweiler, S. Relationship between prostate cancer gene 3 (PCA3) and characteristics of tumor aggressiveness. Prostate 2013, 73, 203–210. [Google Scholar] [CrossRef]
- Hessels, D.; van Gils, M.P.; van Hooij, O.; Jannink, S.A.; Witjes, J.A.; Verhaegh, G.W.; Schalken, J.A. Predictive value of PCA3 in urinary sediments in determining clinico-pathological characteristics of prostate cancer. Prostate 2010, 70, 10–16. [Google Scholar] [CrossRef]
- Leyten, G.H.; Hessels, D.; Jannink, S.A.; Smit, F.P.; de Jong, H.; Cornel, E.B.; de Reijke, T.M.; Vergunst, H.; Kil, P.; Knipscheer, B.C.; et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur. Urol. 2014, 65, 534–542. [Google Scholar] [CrossRef]
- Van Gils, M.P.; Hessels, D.; Hulsbergen-van de Kaa, C.A.; Witjes, J.A.; Jansen, C.F.; Mulders, P.F.; Rittenhouse, H.G.; Schalken, J.A. Detailed analysis of histopathological parameters in radical prostatectomy specimens and PCA3 urine test results. Prostate 2008, 68, 1215–1222. [Google Scholar] [CrossRef]
- Young, A.; Palanisamy, N.; Siddiqui, J.; Wood, D.P.; Wei, J.T.; Chinnaiyan, A.M.; Kunju, L.P.; Tomlins, S.A. Correlation of urine TMPRSS2:ERG and PCA3 to ERG+ and total prostate cancer burden. Am. J. Clin. Pathol. 2012, 138, 685–696. [Google Scholar] [CrossRef]
- Van Poppel, H.; Haese, A.; Graefen, M.; de la Taille, A.; Irani, J.; de Reijke, T.; Remzi, M.; Marberger, M. The relationship between Prostate CAncer gene 3 (PCA3) and prostate cancer significance. BJU Int. 2012, 109, 360–366. [Google Scholar] [CrossRef]
- Liss, M.A.; Santos, R.; Osann, K.; Lau, A.; Ahlering, T.E.; Ornstein, D.K. PCA3 molecular urine assay for prostate cancer: Association with pathologic features and impact of collection protocols. World J. Urol. 2011, 29, 683–688. [Google Scholar] [CrossRef]
- Rostad, K.; Hellwinkel, O.J.; Haukaas, S.A.; Halvorsen, O.J.; Oyan, A.M.; Haese, A.; Budaus, L.; Albrecht, H.; Akslen, L.A.; Schlomm, T.; et al. TMPRSS2:ERG fusion transcripts in urine from prostate cancer patients correlate with a less favorable prognosis. APMIS 2009, 117, 575–582. [Google Scholar] [CrossRef]
- Guazzoni, G.; Lazzeri, M.; Nava, L.; Lughezzani, G.; Larcher, A.; Scattoni, V.; Gadda, G.M.; Bini, V.; Cestari, A.; Buffi, N.M.; et al. Preoperative prostate-specific antigen isoform p2PSA and its derivatives, %p2PSA and prostate health index, predict pathologic outcomes in patients undergoing radical prostatectomy for prostate cancer. Eur. Urol. 2012, 61, 455–466. [Google Scholar] [CrossRef]
- Epstein, J.I. Prognostic significance of tumor volume in radical prostatectomy and needle biopsy specimens. J. Urol. 2011, 186, 790–797. [Google Scholar] [CrossRef]
- Ploussard, G.; Durand, X.; Xylinas, E.; Moutereau, S.; Radulescu, C.; Forgue, A.; Nicolaiew, N.; Terry, S.; Allory, Y.; Loric, S.; et al. Prostate cancer antigen 3 score accurately predicts tumour volume and might help in selecting prostate cancer patients for active surveillance. Eur. Urol. 2011, 59, 422–429. [Google Scholar] [CrossRef]
- Mouraviev, V.; Villers, A.; Bostwick, D.G.; Wheeler, T.M.; Montironi, R.; Polascik, T.J. Understanding the pathological features of focality, grade and tumour volume of early-stage prostate cancer as a foundation for parenchyma-sparing prostate cancer therapies: Active surveillance and focal targeted therapy. BJU Int. 2011, 108, 1074–1085. [Google Scholar] [CrossRef]
- Leyten, G.H.; Wierenga, E.A.; Sedelaar, J.P.; van Oort, I.M.; Futterer, J.J.; Barentsz, J.O.; Schalken, J.A.; Mulders, P.F. Value of PCA3 to predict biopsy outcome and its potential role in selecting patients for multiparametric MRI. Int. J. Mol. Sci. 2013, 14, 11347–11355. [Google Scholar] [CrossRef]
- Semjonow, A.; Kopke, T.; Eltze, E.; Pepping-Schefers, B.; Burgel, H.; Darte, C. Pre-analytical in vitro stability of [-2]proPSA in blood and serum. Clin. Biochem. 2010, 43, 926–928. [Google Scholar] [CrossRef]
- Chen, M.E.; Johnston, D.; Reyes, A.O.; Soto, C.P.; Babaian, R.J.; Troncoso, P. A streamlined three-dimensional volume estimation method accurately classifies prostate tumors by volume. Am. J. Surg. Pathol. 2003, 27, 1291–1301. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M. Traditional statistical methods for evaluating prediction models are uninformative as to clinical value: Towards a decision analytic framework. Semin. Oncol. 2010, 37, 31–38. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Begg, C.B. One statistical test is sufficient for assessing new predictive markers. BMC Med. Res. Methodol. 2011, 11, 13. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tallon, L.; Luangphakdy, D.; Ruffion, A.; Colombel, M.; Devonec, M.; Champetier, D.; Paparel, P.; Decaussin-Petrucci, M.; Perrin, P.; Vlaeminck-Guillem, V. Comparative Evaluation of Urinary PCA3 and TMPRSS2: ERG Scores and Serum PHI in Predicting Prostate Cancer Aggressiveness. Int. J. Mol. Sci. 2014, 15, 13299-13316. https://doi.org/10.3390/ijms150813299
Tallon L, Luangphakdy D, Ruffion A, Colombel M, Devonec M, Champetier D, Paparel P, Decaussin-Petrucci M, Perrin P, Vlaeminck-Guillem V. Comparative Evaluation of Urinary PCA3 and TMPRSS2: ERG Scores and Serum PHI in Predicting Prostate Cancer Aggressiveness. International Journal of Molecular Sciences. 2014; 15(8):13299-13316. https://doi.org/10.3390/ijms150813299
Chicago/Turabian StyleTallon, Lucile, Devillier Luangphakdy, Alain Ruffion, Marc Colombel, Marian Devonec, Denis Champetier, Philippe Paparel, Myriam Decaussin-Petrucci, Paul Perrin, and Virginie Vlaeminck-Guillem. 2014. "Comparative Evaluation of Urinary PCA3 and TMPRSS2: ERG Scores and Serum PHI in Predicting Prostate Cancer Aggressiveness" International Journal of Molecular Sciences 15, no. 8: 13299-13316. https://doi.org/10.3390/ijms150813299
APA StyleTallon, L., Luangphakdy, D., Ruffion, A., Colombel, M., Devonec, M., Champetier, D., Paparel, P., Decaussin-Petrucci, M., Perrin, P., & Vlaeminck-Guillem, V. (2014). Comparative Evaluation of Urinary PCA3 and TMPRSS2: ERG Scores and Serum PHI in Predicting Prostate Cancer Aggressiveness. International Journal of Molecular Sciences, 15(8), 13299-13316. https://doi.org/10.3390/ijms150813299




