Sunflower Oil but Not Fish Oil Resembles Positive Effects of Virgin Olive Oil on Aged Pancreas after Life-Long Coenzyme Q Addition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Body Weight Evolution and Adaptation to the Diet
2.2. Circulating Hormone Levels and Biochemical Parameters
| Parameter | VOO | SO | FO |
|---|---|---|---|
| Insulin (pg/mL) | 302.7 ± 84.6 | 212.3 ± 58.7 | 170.7 ± 47.9 * |
| Leptin (pg/mL) | 14,211.4 ± 2966.2 | 13,462.9 ± 2275.2 | 21,580.5 ± 4152.9 |
| Glucose (mM) | 6.8 ± 0.5 | 6.4 ± 0.6 | 6.4 ± 0.5 |
| HOMA | 2.3 ± 0.7 | 1.6 ± 0.5 | 1.2 ± 0.4 * |
| Triglycerides (mM) | 1.5 ± 0.3 | 1.4 ± 0.3 | 1.1 ±0.1 |
| Cholesterol (mM) | 2.2 ± 0.4 | 2.5 ± 0.1 | 1.6 ± 0.1 |
| Phospholipids (mM) | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 |
| Total lipids (mg/dL) | 387.1 ± 61.5 | 438.0 ± 56.4 | 252.3 ± 33.7 * |
2.3. Histological Study
2.3.1. Pancreatic Parenchyma


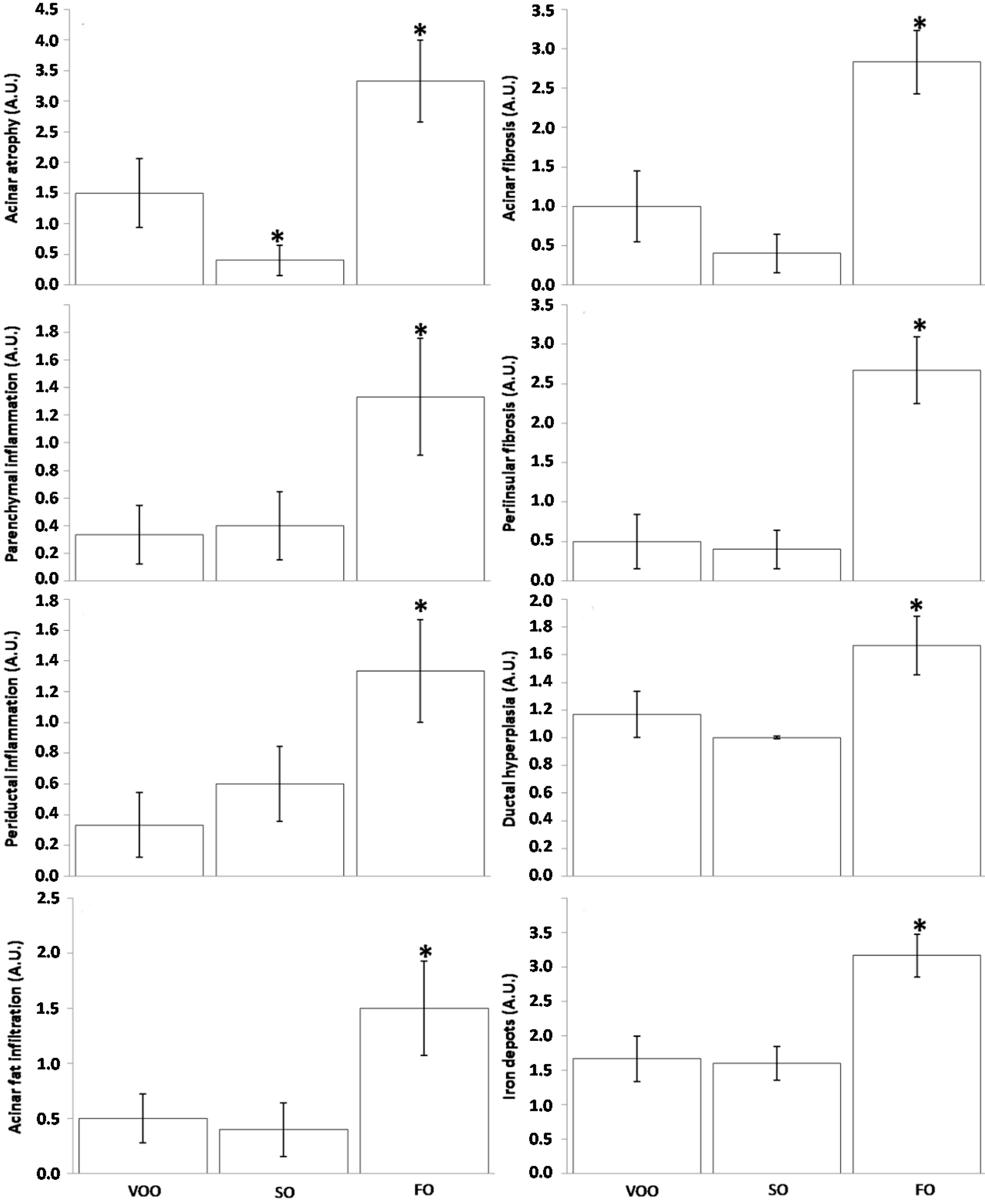
2.3.2. Immunohistochemical Assessment of Pancreatic Islets

2.4. Pancreatic Contents of Insulin and Glucagon
| Parameter | VOO | SO | FO |
|---|---|---|---|
| Insulin (pg/μg protein) | 18,902.5 ± 6989.5 | 14,144 ± 4588.5 | 19,576 ± 3720.3 |
| Glucagon (pg/μg protein) | 151.8 ± 12.8 | 143 ± 37.2 | 129.6 ± 23.8 |
3. Experimental Section
3.1. Animals and Diet
3.2. Histological Assessments
3.3. Immunohistochemistry for Insulin and Glucagon Expression
3.4. Protein, Hormone and Metabolite Determinations
3.5. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, S.K.; MacDonald, R.J. Signaling and transcriptional control of pancreatic organogenesis. Curr. Opin. Genet. Dev. 2002, 12, 540–547. [Google Scholar] [CrossRef]
- Murtaugh, L.C.; Melton, D.A. Genes, signals, and lineages in pancreas development. Ann. Rev. Cell Dev. Biol. 2003, 19, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Czako, L.; Hegyi, P.; Rakonczay, Z., Jr.; Wittmann, T.; Otsuki, M. Interactions between the endocrine and exocrine pancreas and their clinical relevance. Pancreatology 2009, 9, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Braganza, J.M.; Lee, S.H.; McCloy, R.F.; McMahon, M.J. Chronic pancreatitis. Lancet 2011, 377, 1184–1197. [Google Scholar] [CrossRef]
- Keller, J.; Layer, P. Human pancreatic exocrine response to nutrients in health and disease. Gut 2005, 54, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Nolan, C.J. Islet β cell failure in type 2 diabetes. J. Clin. Investig. 2006, 116, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Prentki, M. The islet β-cell: Fuel responsive and vulnerable. Trends Endocrinol. Metab. 2008, 19, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Fair, J.M.; Haskell, W.L. Saturated fat intake and insulin resistance in men with coronary artery disease. The Stanford Coronary Risk Intervention Project Investigators and Staff. Circulation 1991, 84, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.J.; Newman, B.; Quesenberry, C.P.; Selby, J.V. Usual dietary fat intake and insulin concentrations in healthy women twins. Diabetes Care 1993, 16, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A.; Bessesen, D.H.; Hamman, R.F. High saturated fat and low starch and fibre are associated with hyperinsulinaemia in a non-diabetic population: The san luis valley diabetes study. Diabetologia 1997, 40, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; McInerney, D.; Owens, D.; Collins, P.; Johnson, A.; Tomkin, G.H. Diabetes and the Mediterranean diet: A beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium-dependent vasoreactivity. QJM 2000, 93, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Soriguer, F.; Esteva, I.; Rojo-Martinez, G.; de Adana, M.R.; Dobarganes, M.C.; Garcia-Almeida, J.M.; Tinahones, F.; Beltran, M.; Gonzalez-Romero, S.; Olveira, G.; et al. Oleic acid from cooking oils is associated with lower insulin resistance in the general population (Pizarra study). Eur. J. Endocrinol. 2004, 150, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Bonanome, A.; Grundy, S.M.; Zhang, Z.-J.; Unger, R.H. Comparison of a high-carbohydrate diet with a high-monounsaturated-fat diet in patients with non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1988, 319, 829–834. [Google Scholar] [CrossRef]
- Rojo-Martínez, G.; Esteva, I.; Ruiz de Adana, M.S.; García-Almeida, J.M.; Tinahones, F.; Cardona, F.; Morcillo, S.; García-Escobar, E.; García-Fuentes, E.; Soriguer, F. Dietary fatty acids and insulin secretion: A population-based study. Eur. J. Clin. Nutr. 2006, 60, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Mañas, M.; Yago, M.D.; Martínez-Victoria, E.; Quiles, J.L.; Ramírez-Tortosa, M.C.; Yaqoob, P. Others olive oil and regulation of gastrointestinal function. In Olive Oil and Health; Quiles, J.L., Ramírez-Tortosa, M.C., Yaqoob, P., Eds.; CABI Publishing: London, UK, 2006; pp. 284–308. [Google Scholar]
- Ballesta, M.C.; Mañas, M.; Mataix, F.J.; Martínez-Victoria, E.; Seiquer, I. Long-term adaptation of pancreatic response by dogs to dietary fats of different degrees of saturation: Olive and sunflower oil. Br. J. Nutr. 1990, 64, 487–496. [Google Scholar] [CrossRef]
- Yago, M.D.; Martinez-Victoria, E.; Huertas, J.R.; Mañas, M. Effects of the amount and type of dietary fat on exocrine pancreatic secretion in dogs after different periods of adaptation. Arch. Physiol. Biochem. 1997, 105, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Díaz, R.J.; Yago, M.D.; Martínez-Victoria, E.; Naranjo, J.A.; Martínez, M.A.; Mañas, M. Comparison of the effects of dietary sunflower oil and virgin olive oil on rat exocrine pancreatic secretion in vivo. Lipids 2003, 38, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Roche, E.; Ramírez-Tortosa, C.L.; Arribas, M.I.; Ochoa, J.J.; Sirvent-Belando, J.E.; Battino, M.; Ramírez-Tortosa, M.C.; González-Alonso, A.; Pérez-López, M.P.; Quiles, J.L. Comparative analysis of pancreatic changes in aged rats fed life long with sunflower, fish, or olive oils. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Ferri, E.; Gorini, A.; Villa, R.F.; Huertas, J.F.R.; Fiorella, P.; Genova, M.L.; Lenaz, G.; Marchetti, M. natural distribution and occurrence of coenzyme Q homologues. Mol. Membr. Biol. 1990, 9, 179–190. [Google Scholar] [CrossRef]
- Nohl, H.; Gille, L.; Kozlov, A.V. Antioxidant-derived prooxidant formation from ubiquinol. Free Radic. Biol. Med. 1998, 25, 666–675. [Google Scholar] [CrossRef]
- Budavari, S. The Merck Index, 12th ed.; Merck & Co: Hunterdon County, NJ, USA, 1996; p. 1606. [Google Scholar]
- Ibrahim, W.H.; Bhagavan, H.N.; Chopra, R.K.; Chow, C.K. Dietary coenzyme Q10 and vitamin E alter the status of these compounds in rat tissues and mitochondria. J. Nutr. 2000, 130, 2343–2348. [Google Scholar] [PubMed]
- Lenaz, G. The mitochondrial production of reactive oxygen species: Mechanisms and implications in human pathology. IUBMB Life 2001, 52, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Ramasarma, T. Natural occurrence and distribution of coenzyme Q. Coenzyme Q 1985, 67–81. [Google Scholar]
- Grossi, G.; Bargossi, A.M.; Fiorella, P.L.; Piazzi, S.; Battino, M.; Bianchi, G.P. Improved high-performance liquid chromatographic method for the determination of coenzyme Q10 in plasma. J. Chromatogr. A 1992, 593, 217–226. [Google Scholar] [CrossRef]
- Lass, A.; Kwong, L.; Sohal, R.S. Mitochondrial coenzyme Q content and aging. Biofactors 1999, 9, 199–205. [Google Scholar] [CrossRef]
- Ernster, L.; Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta 1995, 1271, 195–204. [Google Scholar] [CrossRef]
- Pepping, J. Coenzyme Q10. Am. J. Health Syst. Pharm. 1999, 56, 519–521. [Google Scholar] [PubMed]
- Forsmark-Andrée, P.; Lee, C.-P.; Dallner, G.; Ernster, L. Lipid peroxidation and changes in the ubiquinone content and the respiratory chain enzymes of submitochondrial particles. Free Radic. Biol. Med. 1997, 22, 391–400. [Google Scholar] [CrossRef]
- Aberg, F.; Appelkvist, E.L.; Dallner, G.; Ernster, L. Distribution and redox state of ubiquinones in rat and human tissues. Arch. Biochem. Biophys. 1992, 295, 230–234. [Google Scholar] [CrossRef]
- Okamoto, T.; Fukunaga, Y.; Ida, Y.; Kishi, T. Determination of reduced and total ubiquinones in biological materials by liquid chromatography with electrochemical detection. J. Chromatogr. B 1988, 430, 11–19. [Google Scholar] [CrossRef]
- Turrens, J.F.; Alexandre, A.; Lehninger, A.L. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 1985, 237, 408–414. [Google Scholar] [CrossRef]
- Kitano, M.; Watanabe, D.; Oda, S.; Kubo, H.; Kishida, H.; Fujii, K.; Kitahara, M.; Hosoe, K. Subchronic oral toxicity of ubiquinol in rats and dogs. Int. J. Toxicol. 2008, 27, 189–215. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Gorini, A.; Villa, R.F.; Genova, M.L.; Bovina, C.; Sassi, S.; Littarru, G.P.; Lenaz, G. Coenzyme Q content in synaptic and non-synaptic mitochondria from different brain regions in the ageing rat. Mech. Ageing Dev. 1995, 78, 173–187. [Google Scholar] [CrossRef]
- Onur, S.; Niklowitz, P.; Fischer, A.; Metges, C.C.; Grune, T.; Menke, T.; Rimbach, G.; Döring, F. A comparative study into alterations of coenzyme Q redox status in ageing pigs, mice, and worms. Biofactors 2014, 40, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, C.; Cocchi, M.; Weiss, H. Coenzyme Q10 levels in rat heart of different age. Biochem. Exp. Biol. 1980, 16, 39–42. [Google Scholar] [PubMed]
- Kalén, A.; Appelkvist, E.L.; Dallner, G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989, 24, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Lipid compositions of different regions of the human brain during aging. J. Neurochem. 1990, 54, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, G.; Matera, M.; Casanova, G.; Ruggiero, F.M.; Paradies, G. Mitochondrial dysfunction in rat brain with aging: Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem. Int. 2008, 53, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kwong, L.K.; Sohal, R.S. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch. Biochem. Biophys. 2000, 373, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Marcoff, L.; Thompson, P.D. The role of coenzyme Q10 in statin-associated myopathy. J. Am. Coll. Cardiol. 2007, 49, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, F.; Hilton, D.; Pepe, S.; Krum, H. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. Biofactors 2003, 18, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, F.L.; Haas, S.J.; Krum, H.; Hadj, A.; Ng, K.; Leong, J.-Y.; Watts, G.F. Coenzyme Q10 in the treatment of hypertension: A meta-analysis of the clinical trials. J. Hum. Hypertens. 2007, 21, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Shults, C.W. Therapeutic role of coenzyme Q10 in Parkinson’s disease. Pharmacol. Ther. 2005, 107, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Bello, R.I.; Gómez-Díaz, C.; Burón, M.I.; Alcaín, F.J.; Navas, P.; Villalba, J.M. Enhanced anti-oxidant protection of liver membranes in long-lived rats fed on a coenzyme Q10-supplemented diet. Exp. Gerontol. 2005, 40, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.J.; Pamplona, R.; Ramirez-Tortosa, M.C.; Granados-Principal, S.; Perez-Lopez, P.; Naudí, A.; Portero-Otin, M.; López-Frías, M.; Battino, M.; Quiles, J.L. Age-related changes in brain mitochondrial DNA deletion and oxidative stress are differentially modulated by dietary fat type and coenzyme Q10. Free Radic. Biol. Med. 2011, 50, 1053–1064. [Google Scholar]
- Huertas, J.R.; Martinez-Velasco, E.; Ibáñez, S.; López-Frias, M.; Ochoa, J.J.; Quiles, J.; Parenti Castelli, G.; Mataix, J.; Lenaz, G. Virgin olive oil and coenzyme Q10 protect heart mitochondria from peroxidative damage during aging. Biofactors 1999, 9, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Pamplona, R.; Ramirez-Tortosa, M.C.; Naudí, A.; Portero-Otin, M.; Araujo-Nepomuceno, E.; López-Frías, M.; Battino, M.; Ochoa, J.J. Coenzyme Q addition to an n-6 PUFA-rich diet resembles benefits on age-related mitochondrial DNA deletion and oxidative stress of a MUFA-rich diet in rat heart. Mech. Ageing Dev. 2010, 131, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.J.; Quiles, J.L.; López-Frías, M.; Huertas, J.R.; Mataix, J. Effect of lifelong coenzyme Q10 supplementation on age-related oxidative stress and mitochondrial function in liver and skeletal muscle of rats fed on a polyunsaturated fatty acid (PUFA)-rich diet. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Varela-Lopez, A.; Bullon, P.; Battino, M.; Ramirez-Tortosa, M.C.; Ochoa, J.J.; Cordero, M.D.; Ramirez-Tortosa, C.L.; Rubini, C.; Zizzi, A.; Quiles, J.L. Coenzyme Q protects against age-related alveolar bone loss associated to n-6 PUFA rich-diets by modulating mitochondrial mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Ochoa, J.J.; Huertas, J.R.; Mataix, J. Coenzyme Q supplementation protects from age-related DNA double-strand breaks and increases lifespan in rats fed on a PUFA-rich diet. Exp. Gerontol. 2004, 39, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Battino, M.; Varela-Lopez, A.; Perez-Lopez, P.; Granados-Principal, S.; Ramirez-Tortosa, M.C.; Ochoa, J.J.; Cordero, M.D.; Gonzalez-Alonso, A.; Ramirez-Tortosa, C.L.; et al. Diets based on virgin olive oil or fish oil but not on sunflower oil prevent age-related alveolar bone resorption by mitochondrial-related mechanisms. PLoS ONE 2013, 8, e74234. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.J.; Quiles, J.L.; Ibáñez, S.; Martínez, E.; López-Frías, M.; Huertas, J.R.; Mataix, J. Aging-related oxidative stress depends on dietary lipid source in rat postmitotic tissues. J. Bioenerg. Biomembr. 2003, 35, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, C.M.; Mesa, M.D.; Ramirez-Tortosa, M.C.; Nestares, M.T.; Ros, E.; Gil, A. Sunflower oil does not protect against LDL oxidation as virgin olive oil does in patients with peripheral vascular disease. Clin. Nutr. 2004, 23, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.B.; Nugent, D.; Jenkins, R. Variation in characteristics of islets of Langerhans in insulin-resistant, diabetic and non-diabetic-rat strains. Int. J. Exp. Pathol. 2010, 91, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.C.; Roche, H.M. The potential role of olive oil-derived MUFA in insulin sensitivity. Mol. Nutr. Food Res. 2007, 51, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Role of Insulin Resistance in Human Disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Kiechl, S.; Willeit, J.; Oberhollenzer, F.; Egger, G.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Muggeo, M. Prevalence of insulin resistance in metabolic disorders: The bruneck study. Diabetes 1998, 47, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Matute, P.; Marti, A.; Martínez, J.A.; Fernández-Otero, M.P.; Stanhope, K.L.; Havel, P.J.; Moreno-Aliaga, M.J. Eicosapentaenoic fatty acid increases leptin secretion from primary cultured rat adipocytes: Role of glucose metabolism. Am J. Physiol. 2005, 288, R1682–R1688. [Google Scholar] [CrossRef]
- Zethelius, B.; Lithell, H.; Hales, C.N.; Berne, C. Insulin sensitivity, proinsulin and insulin as predictors of coronary heart disease. A population-based 10-year, follow-up study in 70-year old men using the euglycaemic insulin clamp. Diabetologia 2005, 48, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Wannamethee, S.G.; Forouhi, N.G. Novel biochemical risk factors for type 2 diabetes: Pathogenic insights or prediction possibilities? Diabetologia 2008, 51, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; Caro, J.F. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S. Leptin expression and action: New experimental paradigms. Proc. Natl. Acad. Sci. USA 1997, 94, 4242–4245. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, G.; Detlefsen, S.; Feyerabend, B. Fibrosis of the pancreas: The initial tissue damage and the resulting pattern. Virchows Arch. 2004, 445, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.L.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. A high-fish-oil diet prevents adiposity and modulates white adipose tissue inflammation pathways in mice. J. Nutr. Biochem. 2015, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Claycombe, K.; Newman, S.J.; Stewart, T.; Siriwardhana, N.; Matthan, N.; Lichtenstein, A.H.; Moustaid-Moussa, N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J. Nutr. 2010, 140, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Claycombe, K.J.; Moustaid-Moussa, N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: Mechanistic insights. Adv. Nutr. 2011, 2, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Hutchison, E.; Hudson, J.; Kawashima, Y.; Komori, N.; Singh, A.; Brush, R.S.; Anderson, R.E.; Sonntag, W.E.; Matsumoto, H.; et al. Metabolic enrichment of omega-3 polyunsaturated fatty acids does not reduce the onset of idiopathic knee osteoarthritis in mice. Osteoarthr. Cartil. 2014, 22, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Boulis, T.S.; Rochelson, B.; Novick, O.; Xue, X.; Chatterjee, P.K.; Gupta, M.; Solanki, M.H.; Akerman, M.; Metz, C.N. Omega-3 polyunsaturated fatty acids enhance cytokine production and oxidative stress in a mouse model of preterm labor. J. Perinat. Med. 2014, 42, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Mollica, M.P.; Sica, R.; Donizzetti, I.; Gifuni, G.; Pignalosa, A.; Cavaliere, G.; Putti, R. Differential effects of high-fish oil and high-lard diets on cells and cytokines involved in the inflammatory process in rat insulin-sensitive tissues. Int. J. Mol. Sci. 2014, 15, 3040–3063. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Ho, C.-Y.; Chaung, H.-C.; Tain, Y.-L.; Hsieh, C.-S.; Kuo, F.-Y.; Yang, C.-Y.; Huang, L.-T. Fish omega-3 fatty acids induce liver fibrosis in the treatment of bile duct-ligated rats. Dig. Dis. Sci. 2013, 58, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Church, M.W.; Jen, K.-L.C.; Anumba, J.I.; Jackson, D.A.; Adams, B.R.; Hotra, J.W. Excess omega-3 fatty acid consumption by mothers during pregnancy and lactation caused shorter life span and abnormal ABRs in old adult offspring. Neurotoxicol. Teratol. 2010, 32, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, D.; Wu, Y.; Ji, B. Docosahexaenoic acid aggravates photooxidative damage in retinal pigment epithelial cells via lipid peroxidation. J. Photochem. Photobiol. B Biol. 2014, 140, 85–93. [Google Scholar] [CrossRef]
- Spindler, S.R.; Mote, P.L.; Flegal, J.M. Dietary supplementation with Lovaza and krill oil shortens the life span of long-lived F1 mice. Age 2014, 36, 9659. [Google Scholar] [CrossRef] [PubMed]
- Valencak, T.G.; Ruf, T. n-3 polyunsaturated fatty acids impair lifespan but have no role for metabolism. Aging Cell 2007, 6, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Haber, P.S.; Applegate, T.L.; Norton, I.D.; McCaughan, G.W.; Korsten, M.A.; Pirola, R.C.; Wilson, J.S. Periacinar stellate shaped cells in rat pancreas: Identification, isolation, and culture. Gut 1998, 43, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Bachem, M.G.; Schneider, E.; Gross, H.; Weidenbach, H.; Schmid, R.M.; Menke, A.; Siech, M.; Beger, H.; Grünert, A.; Adler, G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998, 115, 421–432. [Google Scholar] [CrossRef]
- Shek, F.W.-T.; Benyon, R.C.; Walker, F.M.; McCrudden, P.R.; Pender, S.L.F.; Williams, E.J.; Johnson, P.A.; Johnson, C.D.; Bateman, A.C.; Fine, D.R.; et al. Expression of transforming growth factor-β1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am. J. Pathol. 2002, 160, 1787–1798. [Google Scholar] [CrossRef]
- Apte, M.V.; Haber, P.S.; Darby, S.J.; Rodgers, S.C.; McCaughan, G.W.; Korsten, M.A.; Pirola, R.C.; Wilson, J.S. Pancreatic stellate cells are activated by proinflammatory cytokines: Implications for pancreatic fibrogenesis. Gut 1999, 44, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Haber, P.S.; Keogh, G.W.; Apte, M.V.; Moran, C.S.; Stewart, N.L.; Crawford, D.H.G.; Pirola, R.C.; McCaughan, G.W.; Ramm, G.A.; Wilson, J.S. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am. J. Pathol. 1999, 155, 1087–1095. [Google Scholar] [CrossRef]
- Luttenberger, T.; Schmid-Kotsas, A.; Menke, A.; Siech, M.; Beger, H.; Adler, G.; Grünert, A.; Bachem, M.G. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: Implications in pathogenesis of pancreas fibrosis. Lab. Investig. J. Tech. Methods Pathol. 2000, 80, 47–55. [Google Scholar] [CrossRef]
- Mews, P.; Phillips, P.; Fahmy, R.; Korsten, M.; Pirola, R.; Wilson, J.; Apte, M. Pancreatic stellate cells respond to inflammatory cytokines: Potential role in chronic pancreatitis. Gut 2002, 50, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Schmid-Kotsas, A.; Zhao, J.; Weidenbach, H.; Schmid, R.M.; Menke, A.; Adler, G.; Waltenberger, J.; Grünert, A.; Bachem, M.G. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am. J. Physiol. 2001, 281, C532–C543. [Google Scholar]
- Shimizu, K. Mechanisms of pancreatic fibrosis and applications to the treatment of chronic pancreatitis. J. Gastroenterol. 2008, 43, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Saurer, L.; Reber, P.; Schaffner, T.; Büchler, M.W.; Buri, C.; Kappeler, A.; Walz, A.; Friess, H.; Mueller, C. Differential expression of chemokines in normal pancreas and in chronic pancreatitis. Gastroenterology 2000, 118, 356–367. [Google Scholar] [CrossRef]
- Andoh, A.; Takaya, H.; Saotome, T.; Shimada, M.; Hata, K.; Araki, Y.; Nakamura, F.; Shintani, Y.; Fujiyama, Y.; Bamba, T. Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology 2000, 119, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, H.; Mizushima, T.; Shirahige, A.; Matsushita, K.; Ochi, K.; Ichimura, M.; Matsumura, N.; Shinji, T.; Tanimoto, M.; Koide, N. Xanthine oxidase-derived free radicals directly activate rat pancreatic stellate cells. J. Gastroenterol. Hepatol. 2006, 21, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Kikuta, K.; Masamune, A.; Satoh, M.; Suzuki, N.; Shimosegawa, T. 4-hydroxy-2,3-nonenal activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. World J. Gastroenterol. 2004, 10, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Kikuta, K.; Masamune, A.; Satoh, M.; Suzuki, N.; Satoh, K.; Shimosegawa, T. Hydrogen peroxide activates activator protein-1 and mitogen-activated protein kinases in pancreatic stellate cells. Mol. Cell. Biochem. 2006, 291, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Asaumi, H.; Watanabe, S.; Taguchi, M.; Tashiro, M.; Otsuki, M. Externally applied pressure activates pancreatic stellate cells through the generation of intracellular reactive oxygen species. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G972–G978. [Google Scholar] [CrossRef] [PubMed]
- Masamune, A.; Watanabe, T.; Kikuta, K.; Satoh, K.; Shimosegawa, T. NADPH oxidase plays a crucial role in the activation of pancreatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G99–G108. [Google Scholar] [CrossRef] [PubMed]
- Masamune, A.; Satoh, M.; Kikuta, K.; Suzuki, N.; Satoh, K.; Shimosegawa, T. Ellagic acid blocks activation of pancreatic stellate cells. Biochem. Pharmacol. 2005, 70, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Masamune, A.; Kikuta, K.; Satoh, M.; Suzuki, N.; Shimosegawa, T. Green tea polyphenol epigallocatechin-3-gallate blocks PDGF-induced proliferation and migration of rat pancreatic stellate cells. World J. Gastroenterol. 2005, 11, 3368–3374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-H.; Li, L.; Zhu, X.-Y.; Tang, W.; Hu, D.-M.; Dong, Y.; Li, L.-Y.; Wang, S.-F. Protective effects of edaravone on experimental chronic pancreatitis induced by dibutyltin dichloride in rats. Pancreatology 2013, 13, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Liao, Z.; Hu, L.-H.; Du, Y.-Q.; Man, X.-H.; Gu, J.-J.; Gao, J.; Gong, Y.-F.; Li, Z.-S. Comparison of antioxidative and antifibrotic effects of α-tocopherol with those of tocotrienol-rich fraction in a rat model of chronic pancreatitis. Pancreas 2011, 40, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- González, A.M.; Garcia, T.; Samper, E.; Rickmann, M.; Vaquero, E.C.; Molero, X. Assessment of the protective effects of oral tocotrienols in arginine chronic-like pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G846–G855. [Google Scholar] [CrossRef] [PubMed]
- Steil, G.M.; Trivedi, N.; Jonas, J.-C.; Hasenkamp, W.M.; Sharma, A.; Bonner-Weir, S.; Weir, G.C. Adaptation of β-cell mass to substrate oversupply: Enhanced function with normal gene expression. Am. J. Physiol. 2001, 280, E788–E796. [Google Scholar]
- Liu, Y.Q.; Jetton, T.L.; Leahy, J.L. β-Cell Adaptation to insulin resistance increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of non-diabetic Zucker fatty rats. J. Biol. Chem. 2002, 277, 39163–39168. [Google Scholar] [CrossRef] [PubMed]
- Jetton, T.L.; Lausier, J.; LaRock, K.; Trotman, W.E.; Larmie, B.; Habibovic, A.; Peshavaria, M.; Leahy, J.L. Mechanisms of compensatory β-cell growth in insulin-resistant rats: Roles of Akt kinase. Diabetes 2005, 54, 2294–2304. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Morton, N.M.; Hansson, A.; Emilsson, V. Rat insulinoma-derived pancreatic β-cells express a functional leptin receptor that mediates a proliferative response. Biochem. Biophys. Res. Commun. 1997, 238, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Sjöholm, A.; Emilsson, V. Fetal pancreatic islets express functional leptin receptors and leptin stimulates proliferation of fetal islet cells. Int. J. Obes. 2000, 24, 1246–1253. [Google Scholar] [CrossRef]
- Tanabe, K.; Okuya, S.; Tanizawa, Y.; Matsutani, A.; Oka, Y. Leptin induces proliferation of pancreatic β cell line MIN6 through activation of mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 1997, 241, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Khalaileh, A.; Gonen-Gross, T.; Magenheim, J.; Nir, T.; Porat, S.; Salpeter, S.; Stolovich-Rain, M.; Swisa, A.; Weinberg, N.; Dor, Y. Determinants of pancreatic β-cell regeneration. Diabetes Obes. Metab. 2008, 10, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [PubMed]
- Ochoa, J.J.; Quiles, J.L.; Huertas, J.R.; Mataix, J. Coenzyme Q10 protects from aging-related oxidative stress and improves mitochondrial function in heart of rats fed a polyunsaturated fatty acid (PUFA)-rich diet. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.; Zimliki, C.L.; Gautam, D.; Cui, Y.; Mears, D.; Wess, J. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor-deficient mice. Diabetes 2004, 53, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Trinder, P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 1969, 22, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, G.; David, H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973, 19, 476–482. [Google Scholar] [PubMed]
- Naito, H.K.; David, J.A. Laboratory considerations: Determination of cholesterol, triglyceride, phospholipid, and other lipids in blood and tissues. Lab. Res. Methods Biol. Med. 1984, 10, 1–76. [Google Scholar] [PubMed]
- Wallace, T.M.; Matthews, D.R. The assessment of insulin resistance in man. Diabet. Med. 2002, 19, 527–534. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Alonso, A.; Ramírez-Tortosa, C.L.; Varela-López, A.; Roche, E.; Arribas, M.I.; Ramírez-Tortosa, M.C.; Giampieri, F.; Ochoa, J.J.; Quiles, J.L. Sunflower Oil but Not Fish Oil Resembles Positive Effects of Virgin Olive Oil on Aged Pancreas after Life-Long Coenzyme Q Addition. Int. J. Mol. Sci. 2015, 16, 23425-23445. https://doi.org/10.3390/ijms161023425
González-Alonso A, Ramírez-Tortosa CL, Varela-López A, Roche E, Arribas MI, Ramírez-Tortosa MC, Giampieri F, Ochoa JJ, Quiles JL. Sunflower Oil but Not Fish Oil Resembles Positive Effects of Virgin Olive Oil on Aged Pancreas after Life-Long Coenzyme Q Addition. International Journal of Molecular Sciences. 2015; 16(10):23425-23445. https://doi.org/10.3390/ijms161023425
Chicago/Turabian StyleGonzález-Alonso, Adrián, César L. Ramírez-Tortosa, Alfonso Varela-López, Enrique Roche, María I. Arribas, M. Carmen Ramírez-Tortosa, Francesca Giampieri, Julio J. Ochoa, and José L. Quiles. 2015. "Sunflower Oil but Not Fish Oil Resembles Positive Effects of Virgin Olive Oil on Aged Pancreas after Life-Long Coenzyme Q Addition" International Journal of Molecular Sciences 16, no. 10: 23425-23445. https://doi.org/10.3390/ijms161023425
APA StyleGonzález-Alonso, A., Ramírez-Tortosa, C. L., Varela-López, A., Roche, E., Arribas, M. I., Ramírez-Tortosa, M. C., Giampieri, F., Ochoa, J. J., & Quiles, J. L. (2015). Sunflower Oil but Not Fish Oil Resembles Positive Effects of Virgin Olive Oil on Aged Pancreas after Life-Long Coenzyme Q Addition. International Journal of Molecular Sciences, 16(10), 23425-23445. https://doi.org/10.3390/ijms161023425











