The Fab Fragment of a Humanized Anti-Toll Like Receptor 4 (TLR4) Monoclonal Antibody Reduces the Lipopolysaccharide Response via TLR4 in Mouse Macrophage
Abstract
:1. Introduction
2. Results
2.1. Purification of the Humanized Anti-TLR4 Antibody Fab Fragment

2.2. Specific Binding of the Humanized Anti-TLR4 Antibody Fab with TLR4

2.3. Optimization of the Appropriate Concentration of Humanized Anti-TLR4 Antibody Fab for Inhibition of Lipopolysaccharides (LPS)-Stimulated Mouse Macrophages


2.4. Inhibitory Effects of the Humanized Anti-TLR4 Antibody Fab on Cytokine Expression after LPS Stimulation of Mouse Macrophages

2.5. Inhibitory Effects of the Humanized Anti-TLR4 Antibody Fab on Cytokine Expression in Cell Culture Supernatants after LPS Stimulation of Mouse Macrophages

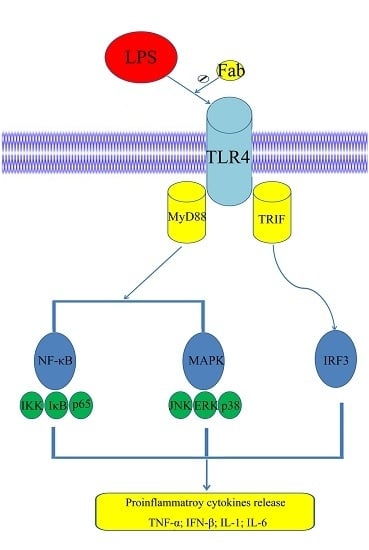
2.6. Inhibition of LPS-Induced TLR4 Signaling Using the Humanized Anti-TLR4 Antibody Fab




3. Discussion
4. Experimental Section
4.1. Reagents and Mice
4.2. Cells and Cell Culture
4.3. Flow Cytometry Analysis
4.4. qPCR (Real Time Quantitative PCR)
4.5. Measurement of Cytokine Levels in Culture Supernatants
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [PubMed]
- Medvedev, A.E. Toll-like receptor polymorphisms, inflammatory and infectious diseases, allergies, and cancer. J. Interferon Cytokine Res. 2013, 33, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C. Endogenous ligands of TLR2 and TLR4: Agonists or assistants. J. Leukoc. Biol. 2010, 87, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Mullen, L.M.; Chamberlain, G.; Sacre, S. Pattern recognition receptors as potential therapeutic targets in inflammatory rheumatic disease. Arthritis Res. Ther. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, T.; Mocsai, A. The role of neutrophils in autoimmune diseases. Immunol. Lett. 2012, 143, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Legein, B.; Temmerman, L.; Biessen, E.A.; Lutgens, E. Inflammation and immune system interactions in atherosclerosis. Cell. Mol. Life Sci. 2013, 70, 3847–3869. [Google Scholar] [CrossRef] [PubMed]
- Thada, S.; Valluri, V.L.; Gaddam, S.L. Influence of Toll-like receptor gene polymorphisms to tuberculosis susceptibility in humans. Scand. J. Immunol. 2013, 78, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Plociennikowska, A.; Hromada-Judycka, A.; Borzecka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [PubMed]
- O’Neill, L.A.; Bryant, C.E.; Doyle, S.L. Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol. Rev. 2009, 61, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xia, T.; Yu, X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm. Res. 2015, 64, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Chuenchor, W.; Jin, T.; Ravilious, G.; Xiao, T.S. Structures of pattern recognition receptors reveal molecular mechanisms of autoinhibition, ligand recognition and oligomerization. Curr. Opin. Immunol. 2014, 26, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Martinez-Juarez, A.; Ibarra-Sanchez, A.; Gonzalez-Espinosa, C. Lyn kinase controls TLR4-dependent IKK and MAPK activation modulating the activity of TRAF-6/TAK-1 protein complex in mast cells. Innate Immun. 2012, 18, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Anton, L.; Brown, A.G.; Parry, S.; Elovitz, M.A. Lipopolysaccharide induces cytokine production and decreases extravillous trophoblast invasion through a mitogen-activated protein kinase-mediated pathway: Possible mechanisms of first trimester placental dysfunction. Hum. Reprod. 2012, 27, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Lacatus, M. Innate immunity in surgical patients. Chirurgia (Bucur.) 2013, 108, 18–25. [Google Scholar] [PubMed]
- Lin, Y.C.; Huang, D.Y.; Chu, C.L.; Lin, Y.L.; Lin, W.W. The tyrosine kinase Syk differentially regulates Toll-like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3. Sci. Signal. 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ge, P.; Zhu, Y. TLR2 and TLR4 in the brain injury caused by cerebral ischemia and reperfusion. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Misharin, A.V.; Saber, R.; Perlman, H. Eosinophil contamination of thioglycollate-elicited peritoneal macrophage cultures skews the functional readouts of in vitro assays. J. Leukoc. Biol. 2012, 92, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; An, H.; Yao, M.; Hou, J.; Yu, Y.; Feng, G.; Cao, X. Scaffolding adaptor protein Gab1 is required for TLR3/4- and RIG-I-mediated production of proinflammatory cytokines and type I IFN in macrophages. J. Immunol. 2010, 184, 6447–6456. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, X.; Li, N.; Chen, T.; Yang, M.; Yao, M.; Liu, X.; Jin, B.; Wang, X.; Cao, X. CMRF-35-like molecule 3 preferentially promotes TLR9-triggered proinflammatory cytokine production in macrophages by enhancing TNF receptor-associated factor 6 ubiquitination. J. Immunol. 2011, 187, 4881–4889. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, B.; Wang, M.; Zhu, X.; Xu, J.; Zheng, W.; Zhang, Y.; Zheng, F.; Feng, Z.; Zhu, J. The Fab Fragment of a Humanized Anti-Toll Like Receptor 4 (TLR4) Monoclonal Antibody Reduces the Lipopolysaccharide Response via TLR4 in Mouse Macrophage. Int. J. Mol. Sci. 2015, 16, 25502-25515. https://doi.org/10.3390/ijms161025502
Cai B, Wang M, Zhu X, Xu J, Zheng W, Zhang Y, Zheng F, Feng Z, Zhu J. The Fab Fragment of a Humanized Anti-Toll Like Receptor 4 (TLR4) Monoclonal Antibody Reduces the Lipopolysaccharide Response via TLR4 in Mouse Macrophage. International Journal of Molecular Sciences. 2015; 16(10):25502-25515. https://doi.org/10.3390/ijms161025502
Chicago/Turabian StyleCai, Binggang, Maorong Wang, Xuhui Zhu, Jing Xu, Wenkai Zheng, Yiqing Zhang, Feng Zheng, Zhenqing Feng, and Jin Zhu. 2015. "The Fab Fragment of a Humanized Anti-Toll Like Receptor 4 (TLR4) Monoclonal Antibody Reduces the Lipopolysaccharide Response via TLR4 in Mouse Macrophage" International Journal of Molecular Sciences 16, no. 10: 25502-25515. https://doi.org/10.3390/ijms161025502
APA StyleCai, B., Wang, M., Zhu, X., Xu, J., Zheng, W., Zhang, Y., Zheng, F., Feng, Z., & Zhu, J. (2015). The Fab Fragment of a Humanized Anti-Toll Like Receptor 4 (TLR4) Monoclonal Antibody Reduces the Lipopolysaccharide Response via TLR4 in Mouse Macrophage. International Journal of Molecular Sciences, 16(10), 25502-25515. https://doi.org/10.3390/ijms161025502