Transplantation of Human Neural Stem Cells in a Parkinsonian Model Exerts Neuroprotection via Regulation of the Host Microenvironment
Abstract
:1. Introduction
2. Results
2.1. Behavioral Tests

2.2. hNSC (Human Neural Stem Cells) Transplantation Protects both Cell Bodies and Axons of the Nigrostriatal Dopaminergic Pathway

2.3. Survival, Migration and Phenotypic Fate of Grafted hNSCs


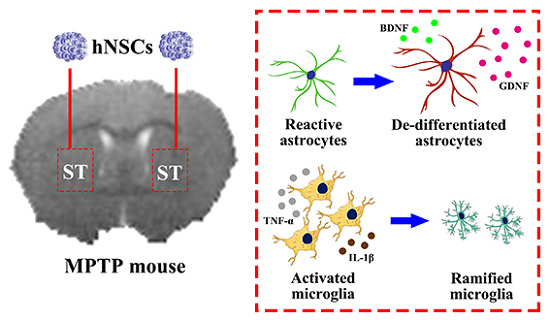
2.4. Reactive Astrocytes Response to hNSCs Transplantation
2.5. Quantitative Analysis of Neurotrophic Factors



2.6. Inhibition of Microglia and Proinflammatory Cytokines after Transplantation



3. Discussion
3.1. Survival of Grafted hNSCs in the Striatum
3.2. Distribution of Local Astrocytes Response to hNSCs Transplantation
3.3. EDAs as a Promising Source for Endogenous Repair
3.4. Secretion of Neurotrophic Factors within Local Microenvironment
3.5. Inhibition of Microglia and Proinflammatory Cytokines
4. Experimental Section
4.1. Preparation of hNSCs
4.2. MPTP Administration
4.3. Behavioral Tests
4.3.1. Rotarod
4.3.2. Pole Test
4.4. Cell Transplantation
4.5. Fixation and Immunohistochemistry
4.5.1. Immunofluorescence Staining
4.5.2. Peroxidase Immunohistochemistry
4.6. QRT-PCR Analysis of Gene Expression in the Striatum and VM
4.7. ELISA Analysis
4.8. Quantification
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Hung, A.Y.; Schwarzschild, M.A. Treatment of Parkinson’s disease: What’s in the non-dopaminergic pipeline? J. Am. Soc. Exp. Neurother. 2014, 11, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Arenas, E. Towards stem cell replacement therapies for Parkinsonʼs disease. Biochem. Biophys. Res. Commun. 2010, 396, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Neurogenesis in the adult brain. J. Neurosci. 2002, 22, 612–613. [Google Scholar] [PubMed]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, L.; Daley, B.F.; Sortwell, C.E.; Collier, T.J. Endogenous neural precursors influence grafted neural stem cells and contribute to neuroprotection in the parkinsonian rat. Eur. J. Neurosci. 2012, 35, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Ourednik, J.; Ourednik, V.; Lynch, W.P.; Schachner, M.; Snyder, E.Y. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat. Biotechnol. 2002, 20, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, T.; Matsukawa, N.; Hara, K.; Yu, G.; Xu, L.; Maki, M.; Kim, S.U.; Borlongan, C.V. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinsonʼs disease. J. Neurosci. 2006, 26, 12497–12511. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Bao, X.J.; Wang, R.Z. Potential of neural stem cell-based therapies for Alzheimer’s disease. J. Neurosci. Res. 2015, 93, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Chen, D.F. Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells 2008, 26, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Harrower, T.P.; Tyers, P.; Hooks, Y.; Barker, R.A. Long-term survival and integration of porcine expanded neural precursor cell grafts in a rat model of Parkinsonʼs disease. Exp. Neurol. 2006, 197, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Inyushin, M.Y.; Huertas, A.; Kucheryavykh, Y.V.; Kucheryavykh, L.Y.; Tsydzik, V.; Sanabria, P.; Eaton, M.J.; Skatchkov, S.N.; Rojas, L.V.; Wessinger, W.D. L-DOPA uptake in astrocytic endfeet enwrapping blood vessels in rat brain. Parkinson’s Dis. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Arcuino, G.; Takano, T.; Liu, Q.S.; Nedergaard, M. Signaling at the gliovascular interface. J. Neurosci. 2003, 23, 9254–9262. [Google Scholar] [PubMed]
- Lois, C.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Chain migration of neuronal precursors. Science 1996, 271, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.C.; Matesic, D.F.; Marvin, M.; McKay, R.D.; Brüstle, O. Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol. Dis. 1995, 2, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Wachter, B.; Schurger, S.; Rolinger, J.; von Ameln-Mayerhofer, A.; Berg, D.; Wagner, H.J.; Kueppers, E. Effect of 6-hydroxydopamine (6-OHDA) on proliferation of glial cells in the rat cortex and striatum: Evidence for de-differentiation of resident astrocytes. Cell Tissue Res. 2010, 342, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Gomide, V.C.; Silveira, G.A.; Chadi, G. Transient and widespread astroglial activation in the brain after a striatal 6-OHDA-induced partial lesion of the nigrostriatal system. Int. J. Neurosci. 2005, 115, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Zang, T.; Zou, Y.; Fang, S.; Smith, D.K.; Bachoo, R.; Zhang, C.L. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 2013, 15, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimerʼs disease model. Cell Stem Cell 2014, 14, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Seyedzadeh, M.H.; Safari, Z.; Zare, A.; Gholizadeh Navashenaq, J.; Razavi, S.A.; Kardar, G.A.; Khorramizadeh, M.R. Study of curcumin immunomodulatory effects on reactive astrocyte cell function. Int. Immunopharmacol. 2014, 22, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Deng, M. Furin mediates brain-derived neurotrophic factor upregulation in cultured rat astrocytes exposed to oxygen-glucose deprivation. J. Neurosci. Res. 2015, 93, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Akerud, P.; Canals, J.M.; Snyder, E.Y.; Arenas, E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J. Neurosci. 2001, 21, 8108–8118. [Google Scholar] [PubMed]
- Niranjan, R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: Focus on astrocytes. Mol. Neurobiol. 2014, 49, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Eve, D.J.; Musso, J., 3rd; Klasko, S.K.; Cruz, E.; Borlongan, C.V.; Sanberg, P.R. Inflammation and stem cell migration to the injured brain in higher organisms. Stem Cells Dev. 2009, 18, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Burnstein, R.M.; Foltynie, T.; He, X.; Menon, D.K.; Svendsen, C.N.; Caldwell, M.A. Differentiation and migration of long term expanded human neural progenitors in a partial lesion model of Parkinson’s disease. Int. J. Biochem. Cell Biol. 2004, 36, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Liker, M.A.; Petzinger, G.M.; Nixon, K.; McNeill, T.; Jakowec, M.W. Human neural stem cell transplantation in the MPTP-lesioned mouse. Brain Res. 2003, 971, 168–177. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.A.; Ito, S.; Corfas, G. Extracellular signals that regulate the tangential migration of olfactory bulb neuronal precursors: Inducers, inhibitors, and repellents. J. Neurosci. 2001, 21, 7654–7663. [Google Scholar] [PubMed]
- Uchida, N.; Buck, D.W.; He, D.; Reitsma, M.J.; Masek, M.; Phan, T.V.; Tsukamoto, A.S.; Gage, F.H.; Weissman, I.L. Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. USA 2000, 97, 14720–14725. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, B.; Abbracchio, M.P. To be or not to be (inflamed)—Is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol. Sci. 2005, 26, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, L.; Daley, B.F.; Paumier, K.L.; Collier, T.J. Transplantation of subventricular zone neural precursors induces an endogenous precursor cell response in a rat model of Parkinson’s disease. J. Comp. Neurol. 2009, 515, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Panickar, K.S.; Norenberg, M.D. Astrocytes in cerebral ischemic injury: Morphological and general considerations. Glia 2005, 50, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, D.; Pang, H.; Caudle, W.M.; Li, Y.; Gao, H.; Liu, Y.; Qian, L.; Wilson, B.; di Monte, D.A.; et al. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. J. Immunol. 2008, 181, 7194–7204. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Uchida, H.; Kuroiwa, H.; Kasahara, J.; Araki, T. Role of glial cells in neurotoxin-induced animal models of Parkinson’s disease. Neurol. Sci. 2011, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Episcopo, F.L.; Tirolo, C.; Testa, N.; Caniglia, S.; Morale, M.C.; Marchetti, B. Reactive astrocytes are key players in nigrostriatal dopaminergic neurorepair in the MPTP mouse model of Parkinson’s disease: Focus on endogenous neurorestoration. Curr. Aging Sci. 2013, 6, 45–55. [Google Scholar] [CrossRef]
- Aoki, E.; Yano, R.; Yokoyama, H.; Kato, H.; Araki, T. Role of nuclear transcription factor kappa B (NF-κB) for MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine)-induced apoptosis in nigral neurons of mice. Exp. Mol. Pathol. 2009, 86, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Denaro, F.J. Astrocytic responses to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in cat and mouse brain. J. Neuropathol. Exp. Neurol. 1988, 47, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Chadi, G.; Gomide, V.C. FGF-2 and S100β immunoreactivities increase in reactive astrocytes, but not in microglia, in ascending dopamine pathways following a striatal 6-OHDA-induced partial lesion of the nigrostriatal system. Cell Biol. Int. 2004, 28, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.J.; Moon, J.H.; Yoon, B.S.; Hyeon, S.; Jun, E.K.; Park, G.; Yun, W.; Park, J.; Park, M.; Kim, A.; et al. Reprogramming of mouse somatic cells into pluripotent stem-like cells using a combination of small molecules. Biomaterials 2014, 35, 7336–7345. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Hida, H.; Shimano, Y.; Fujimoto, I.; Hashitani, T.; Kumazaki, M.; Sakurai, T.; Nishino, H. GDNF is a major component of trophic activity in DA-depleted striatum for survival and neurite extension of DAergic neurons. Brain Res. 2001, 916, 76–84. [Google Scholar] [CrossRef]
- Niles, L.P.; Armstrong, K.J.; Rincón Castro, L.M.; Dao, C.V.; Sharma, R.; McMillan, C.R.; Doering, L.C.; Kirkham, D.L. Neural stem cells express melatonin receptors and neurotrophic factors: Colocalization of the MT1 receptor with neuronal and glial markers. BMC Neurosci. 2004, 5. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.C.; Ferron, S.R.; Vicente, D.; Porlan, E.; Perez-Villalba, A.; Trujillo, C.M.; D'Ocón, P.; Fariñas, I. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron 2014, 83, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Emborg, M.E.; Bloch, J.; Ma, S.Y.; Chu, Y.; Leventhal, L.; McBride, J.; Chen, E.Y.; Palfi, S.; Roitberg, B.Z.; et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 2000, 290, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Iravani, M.M.; Sadeghian, M.; Leung, C.C.; Jenner, P.; Rose, S. Lipopolysaccharide-induced nigral inflammation leads to increased IL-1β tissue content and expression of astrocytic glial cell line-derived neurotrophic factor. Neurosci. Lett. 2012, 510, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Redmond, D.E.; Bjugstad, K.B.; Teng, Y.D.; Ourednik, V.; Ourednik, J.; Wakeman, D.R.; Parsons, X.H.; Gonzalez, R.; Blanchard, B.C.; Kim, S.U.; et al. Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 12175–12180. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Q.W.; Xu, M.; Guo, J.J.; Shen, S.W.; Wang, Y.Q.; Sun, F.Y. New striatal neurons form projections to substantia nigra in adult rat brain after stroke. Neurobiol Dis. 2012, 45, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.D.; Dass, B.; Gasmi, M.; Bakay, R.; Stansell, J.E.; Tuszynski, M.; Bankiewicz, K.; Chen, E.Y.; Chu, Y.; Bishop, K.; et al. Transgene expression, bioactivity, and safety of CERE-120 (AAV2-neurturin) following delivery to the monkey striatum. Mol. Ther. 2008, 16, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Belmadani, A.; Tran, P.B.; Ren, D.; Miller, R.J. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J. Neurosci. 2006, 26, 3182–3191. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Burdett, T.C.; Desjardins, C.A.; Logan, R.; Cipriani, S.; Xu, Y.; Schwarzschild, M.A. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 300–305. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, F.-X.; Bao, X.-J.; Sun, X.-C.; Wu, J.; Bai, Q.-R.; Chen, G.; Li, X.-Y.; Zhou, Q.-Y.; Yang, Y.-F.; Shen, Q.; et al. Transplantation of Human Neural Stem Cells in a Parkinsonian Model Exerts Neuroprotection via Regulation of the Host Microenvironment. Int. J. Mol. Sci. 2015, 16, 26473-26492. https://doi.org/10.3390/ijms161125966
Zuo F-X, Bao X-J, Sun X-C, Wu J, Bai Q-R, Chen G, Li X-Y, Zhou Q-Y, Yang Y-F, Shen Q, et al. Transplantation of Human Neural Stem Cells in a Parkinsonian Model Exerts Neuroprotection via Regulation of the Host Microenvironment. International Journal of Molecular Sciences. 2015; 16(11):26473-26492. https://doi.org/10.3390/ijms161125966
Chicago/Turabian StyleZuo, Fu-Xing, Xin-Jie Bao, Xi-Cai Sun, Jun Wu, Qing-Ran Bai, Guo Chen, Xue-Yuan Li, Qiang-Yi Zhou, Yuan-Fan Yang, Qin Shen, and et al. 2015. "Transplantation of Human Neural Stem Cells in a Parkinsonian Model Exerts Neuroprotection via Regulation of the Host Microenvironment" International Journal of Molecular Sciences 16, no. 11: 26473-26492. https://doi.org/10.3390/ijms161125966
APA StyleZuo, F.-X., Bao, X.-J., Sun, X.-C., Wu, J., Bai, Q.-R., Chen, G., Li, X.-Y., Zhou, Q.-Y., Yang, Y.-F., Shen, Q., & Wang, R.-Z. (2015). Transplantation of Human Neural Stem Cells in a Parkinsonian Model Exerts Neuroprotection via Regulation of the Host Microenvironment. International Journal of Molecular Sciences, 16(11), 26473-26492. https://doi.org/10.3390/ijms161125966