Isolation of a Pluripotent Neural Stem Cell from the Embryonic Bovine Brain
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation, Culture, and Morphology of NSCs

2.2. Optimization of NSCs
2.3. Characterization of NSCs
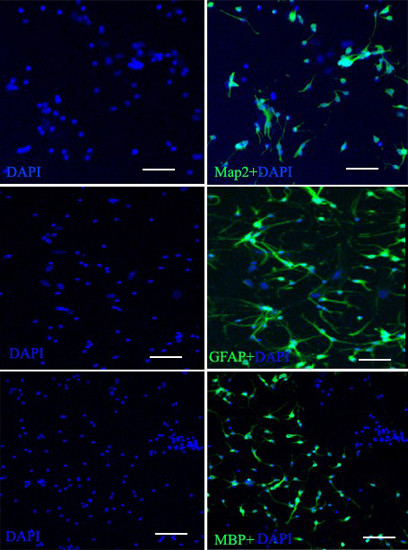
2.3.1. Markers of NSCs

2.3.2. Neurogenic Differentiation of NSCs


2.4. Discussion
3. Experimental Section
3.1. Reagents and Animals
3.2. Isolation of NSCs from Bovine Brain Embryo
3.3. Optimization of Cell Culture Systems for NSCs
3.4. Marker of NSCs
3.5. RT-PCR Assays
| Gene | Primer Sequence | Tm (°C) | Fragment Size (bp) |
|---|---|---|---|
| Sox 2 | F: 5'-TCCTATTCTCAGCAGGGCAC-3' | 61 | 217 |
| R: 5'-AGTGCTGGGACATGTGAAGT-3' | |||
| GAPDH | F: 5'-GGCAAGTTCAACGGCACAGTCA-3' | 58 | 364 |
| R: 5'-TAAGTCCCTCCACGATGCCAAAG-3' | |||
| GFAP | F: 5'-GTCGTGGGTGAGCAGTTACA-3' | 58 | 341 |
| R: 5'-CTAAAACACGGGGAGGTGGG-3' | |||
| MBP | F: 5'-CAGAGACACTGGCATCCTCG-3' | 60 | 350 |
| R: 5'-CAGACGCTCTGCCTCCATAG-3' | |||
| MAP2 | F: 5'-CGCCAATGGATTCCCCTACA-3' | 54 | 322 |
| R: 5'-TTCCTCCACTGGGACAGTCT-3' | |||
| Musashi | F: 5'-GTCTCGAGTCATGCCCTACG-3' | 55 | 223 |
| F: 5'-CATGGGTCCATAAGCGGTGA-3' |
3.6. Differentiation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J. Neurosci. 1996, 16, 7599–7609. [Google Scholar] [PubMed]
- Imura, T.; Kornblum, H.I.; Sofroniew, M.V. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J. Neurosci. 2003, 23, 2824–2832. [Google Scholar] [PubMed]
- Wen, C.M.; Cheng, Y.H.; Huang, Y.F.; Wang, C.S. Isolation and characterization of a neural progenitor cell line from tilapia brain. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 149, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Shah, N.M.; Anderson, D.J. Regulatory mechanisms in stem cell biology. Cell 1997, 88, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Lois, C.; Alvarez-Buylla, A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl. Acad. Sci. USA 1993, 90, 2074–2077. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.D.; Takahashi, J.; Gage, F.H. The adult rat hippocampus contains primordial neural stem cells. Mol. Cell Neurosci. 1997, 8, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Van Strien, M.E.; Sluijs, J.A.; Reynolds, B.A.; Steindler, D.A.; Aronica, E.; Hol, E.M. Isolation of neural progenitor cells from the human adult subventricular zone based on expression of the cell surface marker CD271. Stem Cells Transl. Med. 2014, 3, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, F.; Liu, W.; Cui, L.; Shang, Q.; Xia, W.; Wang, J.; Cui, Y.; Yang, G.; Liu, D.; et al. Repairing large porcine full-thickness defects of articular cartilage using autologous chondrocyte-engineered cartilage. Tissue Eng. 2002, 8, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Ishiguro, T.; Okumura, M.; Iwanaga, T.; Kadosawa, T.; Fujinaga, T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp. Hematol. 2004, 32, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Muraglia, A.; Corsi, A.; Bianco, P.; Marcacci, M.; Martin, I.; Boyde, A.; Ruspantini, I.; Chistolini, P.; Rocca, M.; et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J. Biomed. Mater. Res. 2000, 49, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Peruffo, A.; Cozzi, B. Bovine brain: An in vitro translational model in developmental neuroscience and neurodegenerative research. Front. Pediatr. 2014, 2, 74. [Google Scholar] [CrossRef] [PubMed]
- Emoto, N.; Gonzalez, A.M.; Walicke, P.A.; Wada, E.; Simmons, D.M.; Shimasaki, S.; Baird, A. Basic fibroblast growth factor (FGF) in the central nervous system: Identification of specific loci of basic FGF expression in the rat brain. Growth Factors 1989, 2, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Riese, D.J., II. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays 1998, 20, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Nowakowski, R.S.; Vaccarino, F.M. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev. Neurosci. 2004, 26, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, A.L.; Parati, E.A.; Gritti, A.; Poulin, P.; Ferrario, M.; Wanke, E.; Frolichsthal-Schoeller, P.; Cova, L.; Arcellana-Panlilio, M.; Colombo, A.; et al. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp. Neurol. 1999, 156, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Park, E.M.; Joh, T.H.; Volpe, B.T.; Chu, C.K.; Song, G.; Cho, S. A neuroprotective role of extracellular signal-regulated kinase in N-acetyl-O-methyldopamine-treated hippocampal neurons after exposure to in vitro and in vivo ischemia. Neuroscience 2004, 123, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Tropepe, V.; Sibilia, M.; Ciruna, B.G.; Rossant, J.; Wagner, E.F.; van der Kooy, D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev. Biol. 1999, 208, 166–188. [Google Scholar] [CrossRef] [PubMed]
- Sutterlin, P.; Williams, E.J.; Chambers, D.; Saraf, K.; von Schack, D.; Reisenberg, M.; Doherty, P.; Williams, G. The molecular basis of the cooperation between EGF, FGF and eCB receptors in the regulation of neural stem cell function. Mol. Cell. Neurosci. 2013, 52, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Supeno, N.E.; Pati, S.; Hadi, R.A.; Ghani, A.R.; Mustafa, Z.; Abdullah, J.M.; Idris, F.M.; Han, X.; Jaafar, H. IGF-1 acts as controlling switch for long-term proliferation and maintenance of EGF/FGF-responsive striatal neural stem cells. Int. J. Med. Sci. 2013, 10, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.F.; Impagnatiello, F.; Girelli, M.; Cova, L.; Grioni, E.; Onofri, M.; Cavallaro, M.; Etteri, S.; Vitello, F.; Giombini, S.; et al. Isolation and characterization of neural stem cells from the adult human olfactory bulb. Stem Cells 2000, 18, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.L.; Kempermann, G. One mouse, two cultures: Isolation and culture of adult neural stem cells from the two neurogenic zones of individual mice. J. Vis. Exp. 2014, 84, e51225. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Li, X.; Zheng, D.; Guan, W.; Ma, Y. Isolation of a Pluripotent Neural Stem Cell from the Embryonic Bovine Brain. Int. J. Mol. Sci. 2015, 16, 5990-5999. https://doi.org/10.3390/ijms16035990
Gao Y, Li X, Zheng D, Guan W, Ma Y. Isolation of a Pluripotent Neural Stem Cell from the Embryonic Bovine Brain. International Journal of Molecular Sciences. 2015; 16(3):5990-5999. https://doi.org/10.3390/ijms16035990
Chicago/Turabian StyleGao, Yuhua, Xiangchen Li, Dong Zheng, Weijun Guan, and Yuehui Ma. 2015. "Isolation of a Pluripotent Neural Stem Cell from the Embryonic Bovine Brain" International Journal of Molecular Sciences 16, no. 3: 5990-5999. https://doi.org/10.3390/ijms16035990
APA StyleGao, Y., Li, X., Zheng, D., Guan, W., & Ma, Y. (2015). Isolation of a Pluripotent Neural Stem Cell from the Embryonic Bovine Brain. International Journal of Molecular Sciences, 16(3), 5990-5999. https://doi.org/10.3390/ijms16035990