Abstract
A series of 1,4-disubstituted-3,4-dihydroisoquinoline derivatives designed as tubulin polymerization inhibitors were synthesized. Their cytotoxic activities against the CEM leukemia cell line were evaluated. Most of them displayed moderate cytotoxic activities, and compounds 21 and 32 showed good activities with IC50 of 4.10 and 0.64 μM, respectively. The most potent compound 32 was further confirmed to be able to inhibit tubulin polymerization, and its hypothetical binding mode with tubulin was obtained by molecular docking.
1. Introduction
Microtubules, which are composed of the α/β heterodimeric tubulin proteins [1], have an important role in a variety of cellular process including mitosis and cell division [2,3]. The compounds, which can regulate the polymerization dynamics of the tubulin, may prove useful in the development of anticancer drugs [4,5]. They can be divided into the tubulin polymerization inhibitors such as colchicine, Combretastatin A-4 (CA-4) (Figure 1) and the vinca alkaloids, and microtubule stabilizing agents such as paclitaxel and epothilone [6].
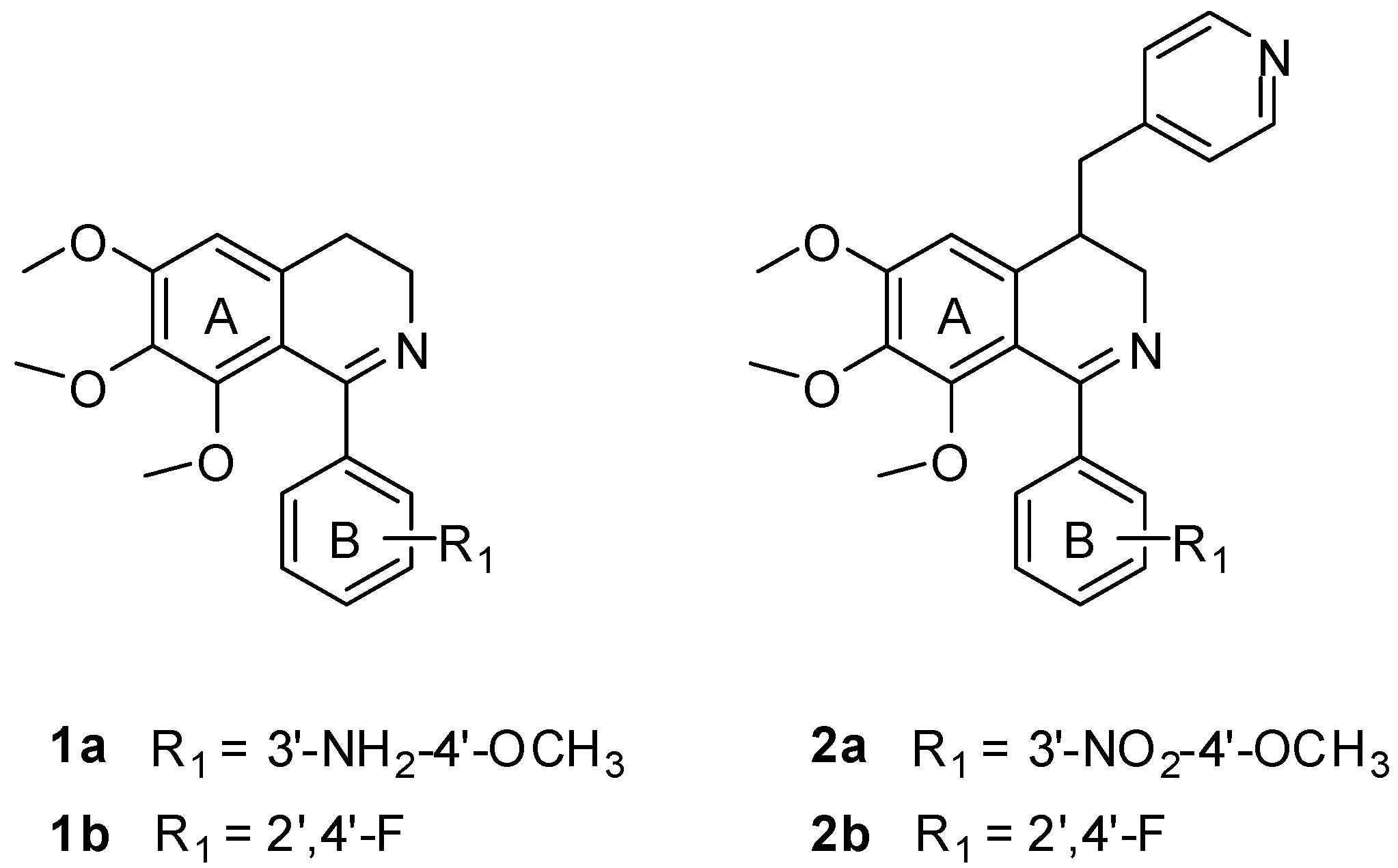
1-Phenyl-3,4-dihydroisoquinoline derivatives (e.g., compounds 1a and 1b) (Figure 2) designed as CA-4 like tubulin polymerization inhibitors were synthesized by our group previously, which showed moderate cytotoxic activities [7,8]. Their docking poses in the tubulin binding site were similar to colchicine. The two phenyl rings of these inhibitors interacted with the hydrophobic P1 and P2 pocket, and another region P3 in binding site would be the potential binding site for further modification. Based on this idea, a pyridinylmethyl sidechain was introduced at the C-4 position of the isoquinoline ring which might form interactions with the region proposed by our group. As an initial attempt, some 1,4-disubstituted-3,4-dihydroisoquinoline derivatives (e.g., compounds 2a and 2b, Figure 2) were synthesized and found to show moderate cytotoxic activities [8]. However, no data on their inhibition of tubulin polymerization were reported. In this paper, we aim at further elucidating the structure-activity relationship (SAR) of these compounds by the syntheses and evaluation of a series of new 1,4-disubstituted-3,4-dihydroisoquinoline derivatives against human CEM leukemia cell line. The best compound was further validated for its tubulin polymerization inhibitory activity for the first time. Molecular docking studies were also performed to explain the SAR.
Figure 1.
Structure of CA-4.
Figure 1.
Structure of CA-4.
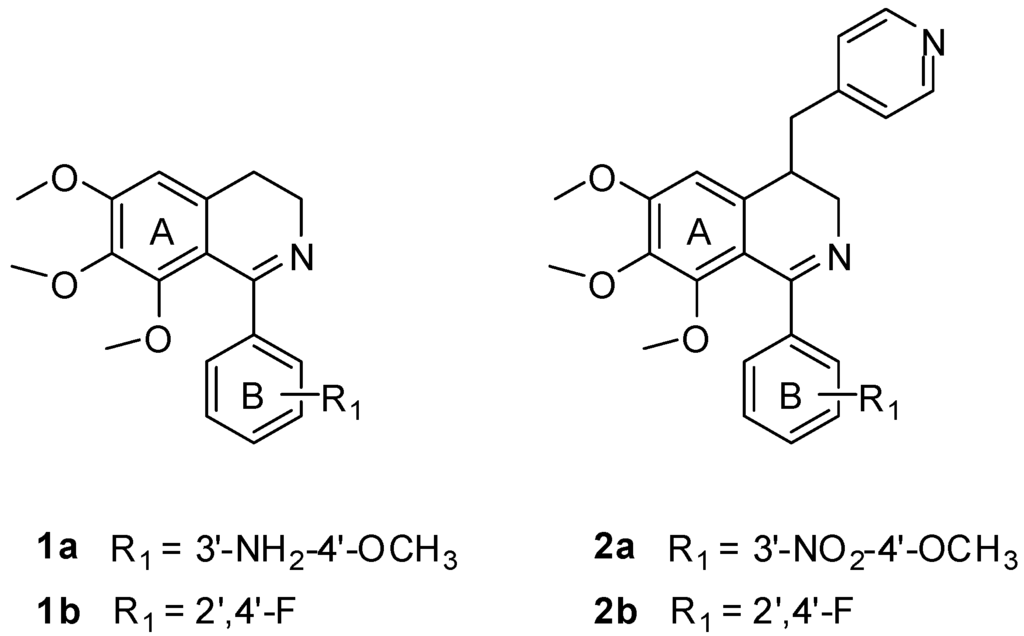
Figure 2.
Structure of compounds 1a, 1b, 2a and 2b.
Figure 2.
Structure of compounds 1a, 1b, 2a and 2b.
2. Results and Discussion
2.1. Chemistry
Thirteen new derivatives of 1,4-disubstituted-3,4-dihydroisoquinoline were synthesized. The general method used for the synthesis of the 3,4-dihydroisoquinoline derivatives was outlined in Scheme 1. The condensation of 3 with aromatic aldehyde gave 4a–d, which were subsequently treated with NaBH4 to afford amines 6a–d in good yield via independent two-step reduction reaction [9,10], then 6a–d was reacted with the substituted benzoic acids to give the substituted benzamides 7–19. The target compounds 20–32 were finally obtained by Bischler-Napieralski cyclization of the corresponding benzamides 7–19. The chemical structures of all the target compounds (Table 1) were confirmed by 1H NMR and MS (ESI) data.
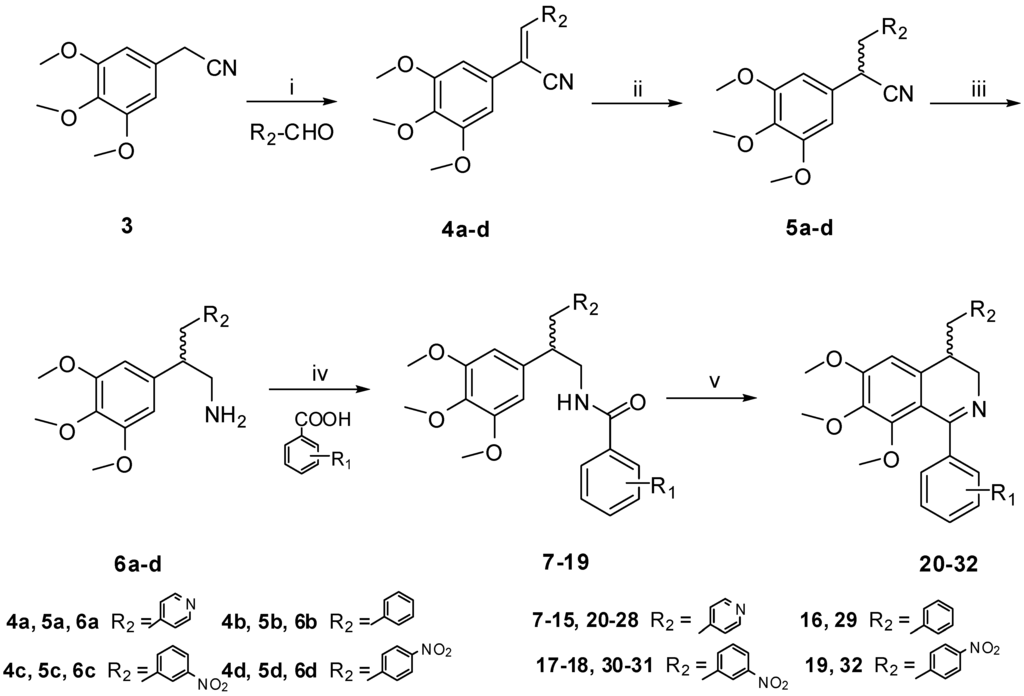
Scheme 1.
Synthetic route of the target compounds. Reagents and conditions: (i) NaOH, EtOH, 0 °C, 0.5 h, 85%; (ii) NaBH4, MeOH, 0 °C, 0.5 h, 86%; (iii) BF3, NaBH4, THF, 0 °C, 96%; (iv) EDC, DMAP, CH2Cl2, 25 °C, 59%–85%; (v) CH3CN, POCl3, reflux, 3 h, 63%–95%.
Scheme 1.
Synthetic route of the target compounds. Reagents and conditions: (i) NaOH, EtOH, 0 °C, 0.5 h, 85%; (ii) NaBH4, MeOH, 0 °C, 0.5 h, 86%; (iii) BF3, NaBH4, THF, 0 °C, 96%; (iv) EDC, DMAP, CH2Cl2, 25 °C, 59%–85%; (v) CH3CN, POCl3, reflux, 3 h, 63%–95%.
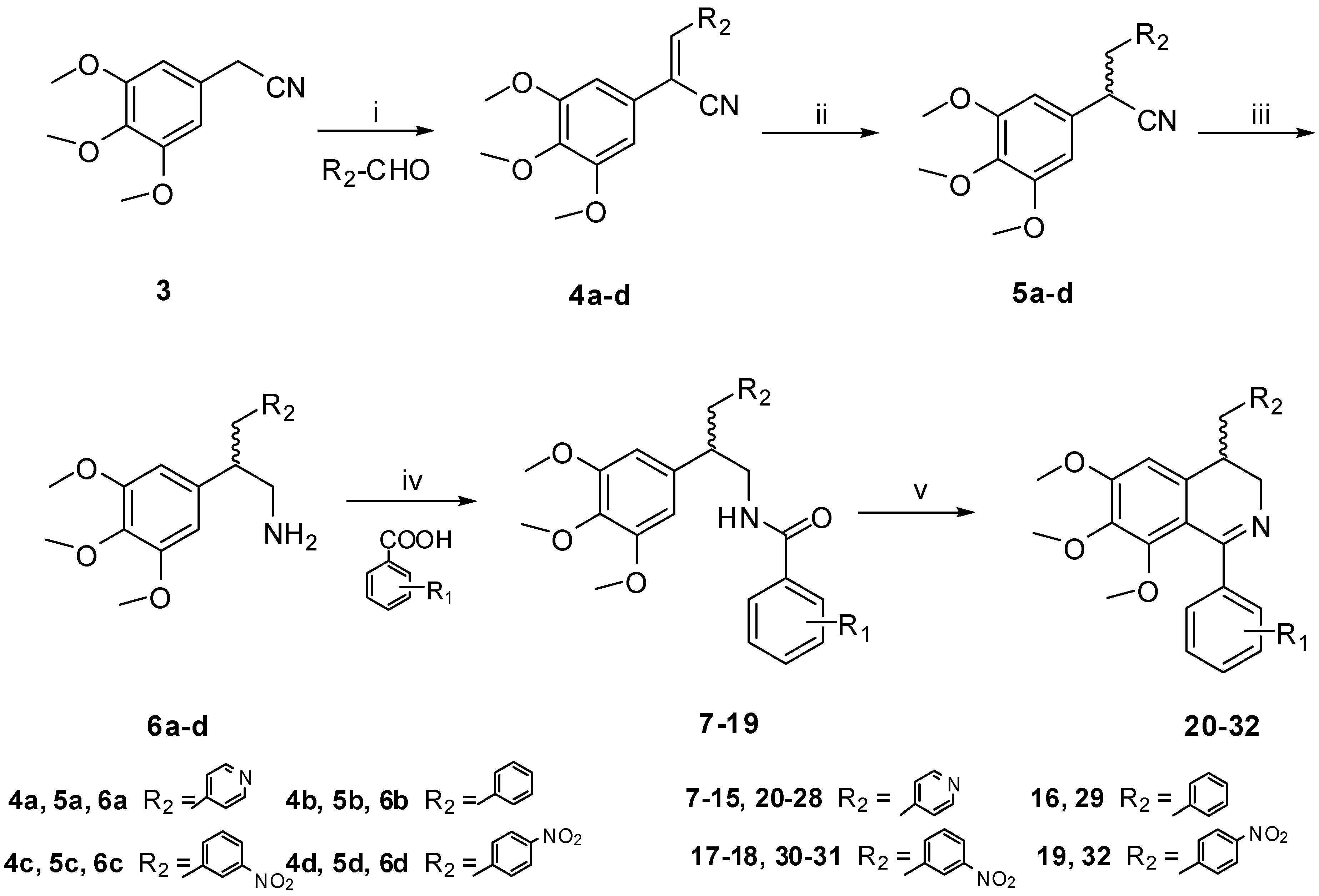

Table 1.
Cytotoxic activities of the target compounds.
| Compound | R1 | R2 | IC50 (μM) |
|---|---|---|---|
| CEM | |||
| 20 | 3',4'-OCH3 | 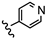 | 36.29 |
| 21 | 3'-NH2-4'-OCH3 | 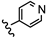 | 4.10 |
| 22 | 3'-NHCOCH3-4'-OCH3 | 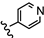 | 24.32 |
| 23 | 4'-OCH3 | 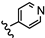 | 17.76 |
| 24 | 4'-OH | 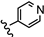 | 40.33 |
| 25 | 4'-OCOCH3 | 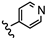 | >100 |
| 26 | 4'-F | 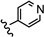 | 44.58 |
| 27 | 4'-CH3 | 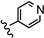 | >100 |
| 28 | 3'-CH3 | 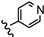 | 44.07 |
| 29 | 3'-NH2-4'-OCH3 | 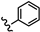 | 46.11 |
| 30 | 4'-OH | 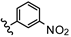 | 32.48 |
| 31 | 2',4'-F | 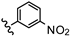 | 3.08 |
| 32 | 2',4'-F | 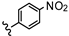 | 0.64 |
| 2a | 3'-NO2-4'-OCH3 | 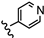 | 29.25 |
| 2b | 2',4'-F |  | 15.21 |
| Colchicine | / | / | 0.004 |
2.2. In Vitro Cytotoxic Activity
The cytotoxicities of the target compounds were evaluated against human CEM leukemia cell line by the MTT assay (Table 1). The cytotoxic activity of 21 (IC50 = 4.10 μM) was slightly higher than that of 1a (IC50 = 6.92 μM), which has the same substitutions in the B ring. This indicated that introducing a pyridin-4-ylmethyl moiety to the C-4 position of the isoquinoline ring of the lead compounds was in favor of activity. On the contrary, introducing a benzyl moiety to this position was detrimental to the activity, which was demonstrated by the reduced activity of compound 29 than 1a. However, further introduction of a nitro group to the 4-benzyl can significantly improve the cytotoxic activity. Compounds 31 (IC50 = 3.08 μM) and 32 (IC50 = 0.64 μM) which bear 3'-nitro and 4'-nitro at the benzyl group are 13-fold and 61-fold more potent than 1b (IC50 = 39.15 μM), which has the same substitution in the B ring. The results also confirmed that 4'-nitro had better activities than 3'-nitro. Compound 32 showed more than 20-fold improvement than previously reported structurally related compound 2b (IC50 = 15.21 μM) in which there was only a pyridinylmethyl sidechain at the C-4 position. Based on these results, it showed that introducing a polar sidechain to the C-4 position of the isoquinoline ring of the lead compounds was a practical strategy to increase their cytotoxic activities.
The substituents of the B ring are also very important to the activity. This was evidenced by compound 23, which has a 4'-OCH3 in the B ring. It showed more active than 24, 25, 26 and 27 which have 4'-OH, 4'-OCOCH3, 4'-F and 4'-CH3 substituted B-rings. An amino group at the 3'-position of the B-ring is necessary for high cytotoxic activity. The potent compound 21 bears 3'-NH2 group, while 3'-OCH3 and 3'-NHCOCH3 substituted compounds, such as 20 and 22, showed significantly lower activities. Compared with the reported compound 2a (IC50 = 29.25 μM), the cytotoxic activity of compound 21 was about 7-fold more potent against CEM leukemia cell line. Generally, the electron-donating groups in the B ring were more favorable to the cytotoxic activities, which is consistent with the SARs of the CA-4 derivatives [11].
2.3. In Vitro Tubulin Polymerization Inhibitory Activity
To test whether these 1,4-disubstituted-3,4-dihydroisoquinoline compounds could inhibit tubulin polymerization, the most potent compound 32 was subjected to evaluation by the tubulin assembly assay. Compound 32 showed inhibitory effect (52%) of microtubule assembly from pure tubulin at 40 μM level (Figure 3).
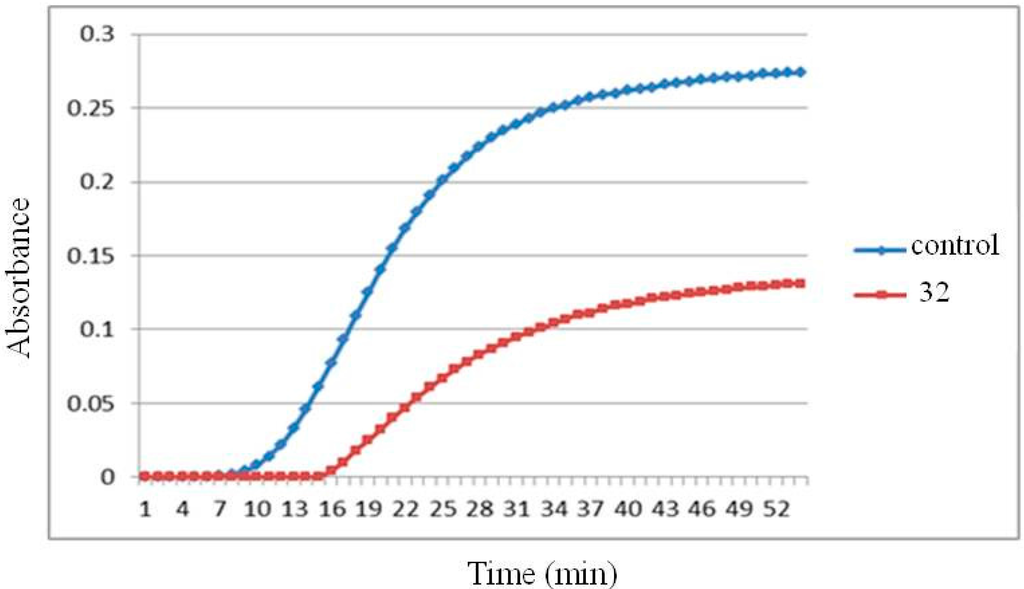
Figure 3.
Tubulin polymerization inhibition of compound 32 at 40 µM.
Figure 3.
Tubulin polymerization inhibition of compound 32 at 40 µM.
2.4. Molecular Docking Study
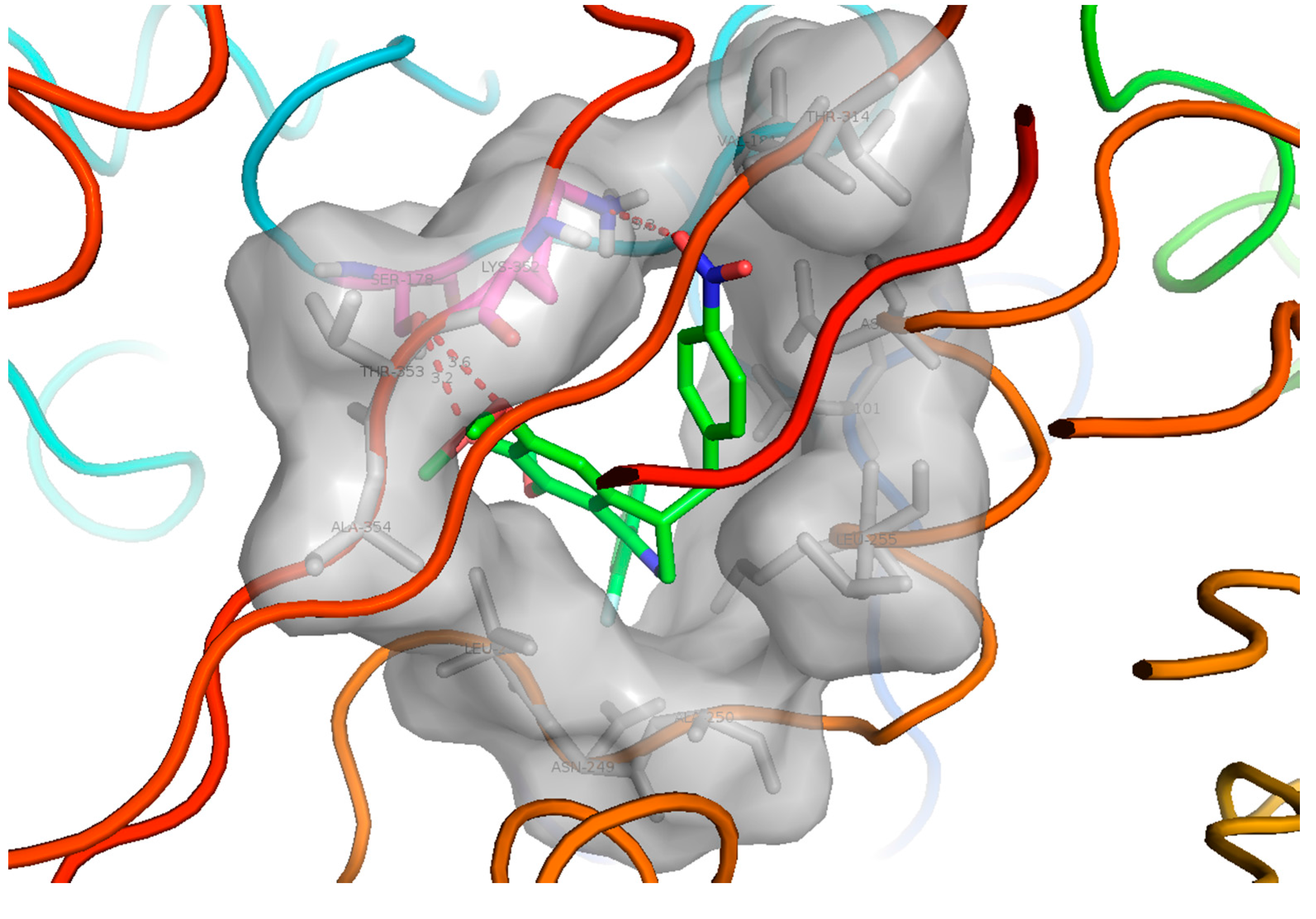
To further elucidate the binding mode with tubulin, molecular docking studies were performed on the most potent compound 32. The X-ray crystal structure of the DAMA-colchicine-tubulin complex (PDB entry: 1SA0) was used as the tubulin protein template. All the modeling studies were performed by using Discovery Studio 3.5 molecular simulation package. It is noted that the synthesized compounds were racemates. Therefore, the two isomers of compound 32 were both docked into the target. From the docking results, the S isomer of the compound 32 clearly had better intereactions with the target than the R isomer. The predictive docking mode of the S isomer of 32 to tubulin protein was found to be similar to that of colchicines, as shown in Figure 4. In its docking mode, the two phenyl rings of compound 32 interacted with the hydrophobic P1 and P2 pocket, which were the most important two pharmacophores of the tubulin polymerization inhibitors. The 4'-nitro benzyl moiety interacted with the region P3 as expected. The 6,7-di-methoxy group formed two hydrogen bonds with Ser178 of tubulin. It is also interesting to see an oxygen atom of the 4'-nitro group formed a hydrogen bond with the amino group at the flexible sidechain of Lys352, which exactly explain why the introduction of 4'-nitro benzyl to the C-4 position of the isoquinoline ring of the lead compounds is favorable to activity.
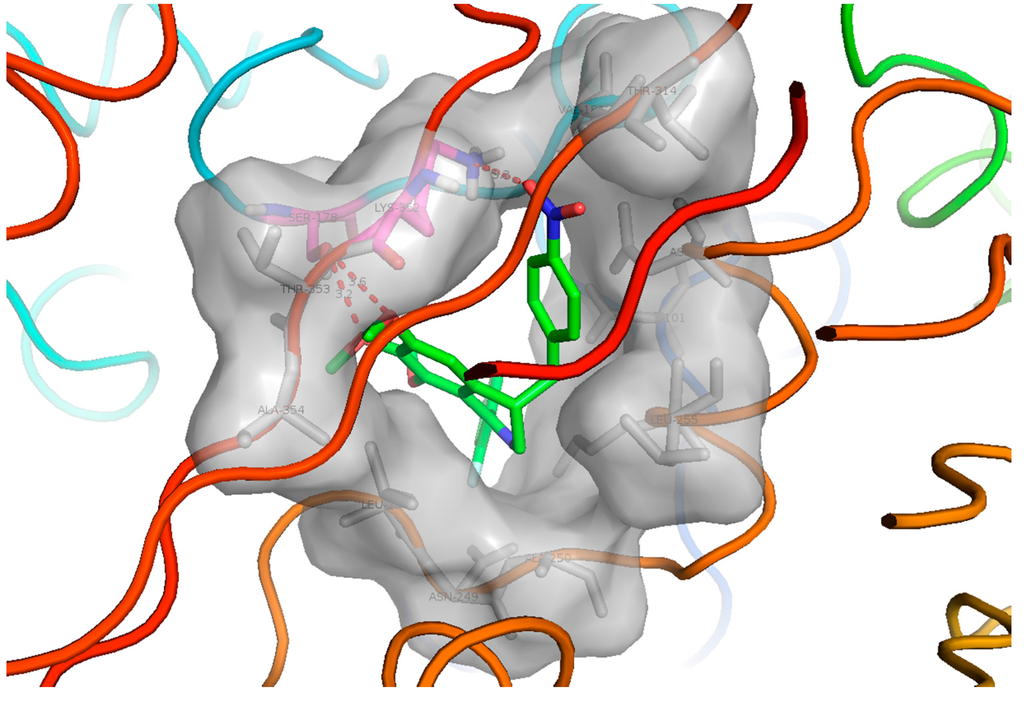
Figure 4.
The hypothetical binding mode of the S isomer of compound 32 to tubulin protein. P1 and P2 are the two hydrophobic pockets, and P3 is a polar region in the interface between α/β-tubulin. The figure was generated using PyMol (http://pymol.souceforge.net/).
Figure 4.
The hypothetical binding mode of the S isomer of compound 32 to tubulin protein. P1 and P2 are the two hydrophobic pockets, and P3 is a polar region in the interface between α/β-tubulin. The figure was generated using PyMol (http://pymol.souceforge.net/).
3. Experimental Section
3.1. Chemistry
The melting point was determined on a XT4A microscope melting-point apparatus (Keyi Electron Optical Instrument Factory, Beijing, China) without correction.1H NMR and 13C NMR spectra were recorded on BRUKER AVANCE 300 and 600 spectrometers (Bruker Company, Rheinstetten, Germany), with TMS as an internal standard and CDCl3 as the solvent. ESI mass spectra were performed on an API-3000 LC-MS spectrometer (Applied Biosystems, Toronto, ON, Canada). Flash column chromatography was performed with silica gel 300–400 mesh (Qingdao Haiyang Chemical, Qingdao, China). All solvents and reagents were purchased from commercial suppliers and, when necessary, were purified and dried by standard protocols. Organic solutions were dried over anhydrous sodium sulfate. The purity of the final compounds was assessed with an Agilent 1200 HPLC (Agilent Technologies, Santa Clara, CA, USA), and the results were greater than 95%.
(Z)-3-(Pyridin-4-yl)-2-(3,4,5-trimethoxyphenyl)acrylonitrile (4a)
A mixture of 2-(3,4,5-trimethoxyphenyl)acetonitrile 3 (0.52 g, 2.5 mmol) and isonicotinaldehyde (0.54 g, 5.0 mmol) was dissolved in EtOH (10 mL) at 0 °C, and then NaOH (0.10 g, 2.5 mmol) was added to the solution mixture. After stirring for 30 min, the solution was filtrated and the residue was collected under suction filtration and dried to afford compound 4a (0.63 g, 85.04%) as a yellow green crystals. mp 134–135 °C. 1H NMR (300 MHz, CDCl3): δ 3.91 (s, 3H), 3.43 (s, 3H), 3.95 (s, 6H), 6.90 (s, 2H), 7.40 (s, 1H), 7.71 (dd, 2H, J = 1.5, 4.8 Hz), 8.75 (dd, 2H, J = 1.5, 4.5 Hz).
The synthetic methods for the intermediates 4b–d were similar to the synthesis of intermadiate 4a.
3-(Pyridin-4-yl)-2-(3,4,5-trimethoxyphenyl)propanenitrile (5a)
A mixture of 4a (0.62 g, 2.1 mmol), NaBH4 (0.32 g, 8.5 mmol) and 20 mL MeOH was heated under 50 °C for 0.5 h. The mixture was evaporated and the residue diluted with 25 mL EtOAc. The organic layer was dried and filtered and the solvent removed by evaporation. After the solution was cooled and stayed overnight to give the compound 5a (2.18 g, 86.21%) as a white crystals. mp 129–130 °C. 1H NMR (300 MHz, CDCl3): δ 3.10–3.24 (m, 2H), 3.82 (s, 6H), 3.85 (s, 3H), 3.99 (t, 1H), 6.40 (s, 2H), 7.09 (d, 2H, J = 4.5 Hz), 8.56 (d, 2H, J = 4.5 Hz).
The synthetic methods for the intermediates 5b–d were similar to the synthesis of intermadiate 5a.
3-(Pyridin-4-yl)-2-(3,4,5-trimethoxyphenyl)propan-1-amine (6a)
BF3·O(C2H5)2 (7.5 mmol) was slowly added to a stirred solution of 5a (0.76 g, 2.5 mmol) and NaBH4 (10 mmol) in THF (10 mL) at 0 °C. The solution was refluxed for 1 h, poured into water, and extracted with EtOAc (15 mL × 3). The combined extracts were dried over anhydrous Na2SO4 and filtered. The solvents were removed by evaporation to afford 6a (0.72 g, 96.61%) as a yellow oil. 1H NMR (300 MHz, CDCl3): δ 2.96–2.79 (m, 5H), 3.81 (s, 6H), 3.83 (s, 3H), 6.32 (s, 2H), 6.98 (dd, 2H, J = 1.5, 4.5 Hz), 8.43 (dd, 2H, J = 1.5, 4.5 Hz).
The synthetic methods for the intermediates 6b–d were similar to the synthesis of intermadiate 6a.
3,4-Dimethoxy-N-(3-(pyridin-4-yl)-2-(3,4,5-trimethoxyphenyl)propyl)benzamide (7)
A mixture of 3,4-dimethoxybenzoic acid (2.1 mmol), EDC·HCl (0.54 g, 2.8 mmol), DMAP (0.03 g, 0.28 mmol) and CH2Cl2 (20 mL), was stirred at room temperature for 10 min, Then 6a (0.73 g, 2.4 mmol) dissolved in CH2Cl2 (10 mL) was added through a dropping funnel for about 5 min. After stirring for about 30 min, the mixture was washed by water (30 mL × 2) and saturated aqueous Na2CO3 (30 mL × 2). The organic layer was dried over anhydrous MgSO4 and filtered. Then the filtrate was concentrated by evaporation and 7 (0.59 g, 52.68%) was obtained as white powder. 1H NMR (300 MHz, CDCl3): δ 2.92–3.04 (m, 2H), 3.08–3.21 (m, 1H), 3.38–3.44 (m, 1H), 3.78 (s, 6H), 3.83 (s, 3H), 3.90 (s, 6H), 3.95–4.02 (m, 1H), 5.93–6.01 (m, 1H), 6.35 (s, 2H), 6.81–6.98 (m, 2H), 7.01–7.05 (m, 2H), 7.31 (s, 1H), 8.45 (dd, 2H, J = 1.5, 4.2 Hz).
The synthetic methods for the intermediates 8–19 were similar to the synthesis of intermadiate 7.
1-(3,4-Dimethoxyphenyl)-6,7,8-trimethoxy-4-(pyridin-4-ylmethyl)-3,4-dihydroisoquinoline (20)
A mixture of 7 (0.70 g, 1.5 mmol), POCl3 (0.82 mL, 9 mmol) and CH3CN (15 mL) was stirred and heated under reflux for 4 h, then the solvents were removed by evaporation, and the residue was dissolved in EtOAc (30 mL). Then the solution was neutralied to pH = 7 with saturated aqueous Na2CO3 and washed by water (30 mL × 3). The organic layer was dried over anhydrous MgSO4 and filtered. Then the filtrate was concentrated under reduced pressure, The residue after evaporation was purified by flash chromatography on silica gel (eluent: CH2Cl2/MeOH = 100:1 v/v) and crystallized from EtOAc to give 20 (0.13 g, 19.3%) as white powder. mp 121–122 °C. 1H NMR (300 MHz, CDCl3): δ 2.77–2.80 (m, 3H), 3.43 (s, 3H), 3.47 (dd, 1H, J = 4.8, 14.7 Hz), 3.76 (s, 3H), 3.80 (s, 3H), 3.93 (s, 3H), 3.94 (s, 3H), 4.04 (dd, 1H, J = 3.0, 14.4 Hz), 6.20 (s, 1H), 6.88 (d, 1H, J = 8.4 Hz), 7.02 (dd, 1H, J = 1.8, 5.1 Hz), 7.07 (dd, 2H, J = 1.5, 4.5 Hz), 7.17 (d, 1H, J = 1.8 Hz), 8.51 (dd, 2H, J = 1.5, 4.5 Hz). ESI-MS (m/z): 449.3 [M + 1]+.
The synthetic methods for the compounds 21–32 were similar to the synthesis of compound 20.
2-Methoxy-5-(6,7,8-trimethoxy-4-(pyridin-4-ylmethyl)-3,4-dihydroisoquinolin-1-yl)aniline (21)
Yellow crystal. mp 109–110 °C. 1H NMR (300 MHz, CDCl3): δ 2.74–2.84 (m, 3H), 3.40–3.46 (m, 4H), 3.75 (s, 3H), 3.79 (s, 3H), 3.82 (s, 2H, N–H), 3.89 (s, 3H), 4.03 (dd, 1H, J = 2.4, 14.7 Hz), 6.18 (s, 1H), 6.79 (d, 1H, J = 8.1 Hz), 6.86 (dd, 1H, J = 1.8, 8.1 Hz), 6.97 (d, 1H, J = 1.8 Hz), 7.06 (dd, 2H, J = 1.5, 4.5 Hz), 8.50 (dd, 2H, J = 1.5, 4.5 Hz). ESI-MS (m/z): 434.3 [M + 1]+.
N-(2-Methoxy-5-(6,7,8-trimethoxy-4-(pyridin-4-ylmethyl)-3,4-dihydroisoquinolin-1-yl)phenyl)acetamide (22)
White powder, mp 173–176 °C. 1H NMR (300 MHz, CDCl3): δ 2.18 (s, 3H), 2.75–2.86 (m, 3H), 3.44 (s, 3H), 3.45 (dd, 1H, J = 4.2, 15.0 Hz), 3.76 (s, 3H), 3.80 (s, 3H), 3.94 (s, 3H), 4.04 (dd, 1H, J = 2.4, 15.0 Hz), 6.20 (s, 1H), 6.93 (d, 1H, J = 8.4 Hz), 7.08 (dd, 2H, J = 1.2, 4.5 Hz), 7.32 (d, 1H, J = 9.0 Hz), 7.77 (s, 1H), 8.48 (s, 1H), 8.49 (dd, 2H, J = 1.5, 4.5 Hz). ESI-MS (m/z): 476.3 [M + 1]+.
6,7,8-Trimethoxy-1-(4-methoxyphenyl)-4-(pyridin-4-ylmethyl)-3,4-dihydroisoquinoline (23)
White powder, mp 118–119 °C. 1H NMR (300 MHz, CDCl3): δ 2.75–2.87 (m, 3H), 3.40 (s, 3H), 3.47 (dd, 1H, J = 4.5, 14.7 Hz), 3.75 (s, 3H), 3.80 (s, 3H), 3.86 (s, 3H), 4.05 (dd, 1H, J = 2.7, 15.0 Hz), 6.19 (s, 1H), 6.90–6.95 (m, 2H), 7.06 (dd, 2H, J = 1.5, 4.2 Hz), 7.45–7.50 (m, 2H), 8.50 (dd, 2H, J = 1.5, 4.2 Hz). ESI-MS (m/z): 419.3 [M + 1]+.
4-(6,7,8-Trimethoxy-4-(pyridin-4-ylmethyl)-3,4-dihydroisoquinolin-1-yl)phenol (24)
White crystal, mp 140–141 °C. 1H NMR (300 MHz, CDCl3): δ 3.75–3.90 (m, 3H), 3.38 (s, 3H), 3.36 (dd, 1H, J = 4.8, 15.0 Hz), 3.77 (s, 6H), 4.01 (dd, 1H, J = 2.4, 14.7 Hz), 6.24 (s, 1H), 6.65 (d, 2H, J = 8.7 Hz), 7.06 (dd, 2H, J = 1.2, 4.5 Hz), 7.30 (d, 2H, J = 8.4 Hz), 8.46 (dd, 2H, J = 1.2, 4.5 Hz). ESI-MS (m/z): 405.2 [M + 1]+.
4-(6,7,8-Trimethoxy-4-(pyridin-4-ylmethyl)-3,4-dihydroisoquinolin-1-yl)phenyl acetate (25)
White powder, mp 119–120 °C. 1H NMR (300 MHz, CDCl3): δ 2.32 (s,3H), 2.75–2.89 (m, 3H), 3.40 (s, 3H), 3.48 (dd, 1H, J = 4.5, 15.0 Hz), 3.76 (s, 3H), 3.79 (s, 3H), 4.07 (dd, 1H, J = 2.7, 15.0 Hz), 6.21 (s, 1H), 7.07 (dd, 2H, J = 1.5, 4.5 Hz), 7.11–7.16 (m, 2H), 7.49–7.53 (m, 2H), 8.51 (dd, 2H, J = 1.8, 4.5 Hz). ESI-MS (m/z): 447.2 [M + 1]+.
1-(4-Fluorophenyl)-6,7,8-trimethoxy-4-(pyridin-4-ylmethyl)-3,4-dihydroisoquinoline (26)
White powder, mp 91–92 °C. 1H NMR (300 MHz, CDCl3): δ 2.75–2.90 (m, 3H), 3.39 (s, 3H), 3.48 (dd, 1H, J = 4.8, 15.0 Hz), 3.76 (s, 3H), 3.80 (s, 3H), 4.06 (dd, 1H, J = 3.0, 15.0 Hz), 6.20 (s, 1H), 7.05–7.12 (m, 4H), 7.46–7.53 (m, 2H), 8.51 (dd, 2H, J = 1.5, 4.5 Hz). ESI-MS (m/z): 407.2 [M + 1]+.
6,7,8-Trimethoxy-4-(pyridin-4-ylmethyl)-1-p-tolyl-3,4-dihydroisoquinoline (27)
Yellow crystal, mp 142–143 °C. 1H NMR (300 MHz, CDCl3): δ 2.40 (s, 3H), 2.74–2.89 (m, 3H), 3.38 (s, 3H), 3.47 (dd, 1H, J = 4.5, 14.7 Hz), 3.75 (s, 3H), 3.79 (s, 3H), 4.05 (dd, 1H, J = 2.7, 15.0 Hz), 6.19 (s, 1H), 7.06 (dd, 2H, J = 1.5, 4.5 Hz), 7.19–7.22 (m, 2H), 7.39–7.42 (m, 2H), 8.50 (dd, 2H, J = 1.5, 4.5 Hz). ESI-MS (m/z): 403.2 [M + 1]+.
6,7,8-Trimethoxy-4-(pyridin-4-ylmethyl)-1-m-tolyl-3,4-dihydroisoquinoline (28)
White powder, mp 138–139 °C. 1H NMR (300 MHz, CDCl3): δ 2.40 (s, 3H), 2.76–2.86 (m, 3H), 3.39 (s, 3H), 3.47 (dd, 1H, J = 4.5, 15.0 Hz), 3.76 (s, 3H), 3.78 (s, 3H), 4.07 (dd, 1H, J = 2.4, 14.7 Hz), 6.19 (s, 1H), 7.07 (dd, 2H, J = 1.5, 4.5 Hz), 7.19–7.31 (m, 3H), 7.33 (s, 1H), 8.50 (dd, 2H, J = 1.5, 4.5 Hz). ESI-MS (m/z): 403.3 [M + 1]+.
5-(4-Benzyl-6,7,8-trimethoxy-3,4-dihydroisoquinolin-1-yl)-2-methoxyaniline (29)
White powder, mp 96–97 °C. 1H NMR (300 MHz, CDCl3): δ 2.73–2.84 (m, 3H,), 3.44 (dd, 1H, J = 4.5, 14.4 Hz), 3.45 (s, 3H), 3.73 (s, 3H), 3.76–3.85 (m, 2H, N–H), 3.79 (s, 3H), 3.89 (s, 3H), 4.03 (dd, 1H, J = 2.4, 14.7 Hz), 6.18 (s, 1H), 6.79 (d, 1H, J = 8.4 Hz), 6.87 (dd, 1H, J = 2.1, 8.1 Hz), 6.99 (d, 1H, J = 2.1 Hz), 7.10–7.15 (m, 2H), 7.17–7.22 (m, 1H), 7.24–7.30 (m, 2H). ESI-MS (m/z): 433.3 [M + 1]+.
4-(6,7,8-Trimethoxy-4-(3-nitrobenzyl)-3,4-dihydroisoquinolin-1-yl)phenol (30)
Yellow powder, mp 201–203 °C. 1H NMR (300 MHz, CDCl3): δ 2.84–2.91 (m, 3H), 3.38 (s, 3H), 3.42–3.46 (m, 1H), 3.78 (s, 3H), 3.81 (s, 3H), 3.99 (d, 1H, J = 14.5 Hz), 6.32 (s, 1H), 6.60 (d, 2H, J = 8.5 Hz), 7.31 (d, 2H, J = 8.5 Hz), 7.36–7.41 (m, 2H), 8.05–8.06 (m, 2H). IR (KBr, cm−1) ν: 2940.81, 2839.79, 1593.73, 1523.77, 1351.70, 1317.11, 1247.45, 1127.89, 837.23. 13C NMR (500 MHz, CDCl3): δ 37.36, 39.66, 49.27, 56.07, 60.95, 61.47, 106.05, 115.18, 121.42, 123.78, 128.69, 129.28, 131.91, 136.03, 139.03, 141.47, 141.61, 148.34, 152.98, 155.99, 158.36, 166.59. ESI-MS (m/z): 449.2 [M + 1]+.
1-(2,4-Difluorophenyl)-6,7,8-trimethoxy-4-(3-nitrobenzyl)-3,4-dihydroisoquinoline (31)
White powder, mp 112–113 °C. 1H NMR (300 MHz, CDCl3): δ 2.85–2.96 (m, 3H), 3.43 (s, 3H), 3.52 (dd, 1H, J = 4.2, 15.0 Hz), 3.73 (s, 3H), 3.78 (s, 3H), 4.14 (d, 1H, J = 14.4 Hz), 6.14 (s, 1H), 6.80–6.87 (m, 1H), 6.94–7.00 (m, 1H), 7.35–7.45 (m, 2H), 7.53 (d, 1H, J = 7.2 Hz), 8.02–8.10 (m, 2H). ESI-MS (m/z): 469.2 [M + 1]+.
1-(2,4-Difluorophenyl)-6,7,8-trimethoxy-4-(4-nitrobenzyl)-3,4-dihydroisoquinoline (32)
Yellow crystal, mp 215–216 °C. 1H NMR (300 MHz, CDCl3): δ 2.85–2.96 (m, 3H), 3.43 (s, 3H), 3.52 (dd, 1H, J = 4.2, 15.3 Hz), 3.71 (s, 3H), 3.78 (s, 3H), 4.09–4.16 (m, 1H), 6.10 (s, 1H), 6.80–6.87 (m, 1H), 6.94–7.00 (m, 1H), 7.24–7.27 (m, 2H), 7.53 (dd, 1H, J = 8.1, 14.7 Hz), 8.14 (d, 2H, J = 8.7 Hz). ESI-MS (m/z): 469.2 [M + 1]+.
3.2. In Vitro Cytotoxic Activity
Cell culture. Cytotoxic effects were examined in the CEM human leukemia cell lines. Cancer cells were all cryopreservated and passaged by Pharmacological Laboratory of Shanghai Institute of pharmaceutical industry. The culture medium was DMEM with 10% fetal bovine serum (FBS) and double antibody. Culture was maintained at 37 °C with 5% CO2 in a humidified atmosphere.
In vitro cytototoxic assay. The cytotoxicity was determined using MTT dye reduction assay. All tested compounds were dissolved in DMSO (Merck, Darmstadt, Germany) and was diluted with PBS. For each well of a 96-well microplate, 100 μL of cell dilution was seeded, allowed to attach overnight, and then exposed to varying concentrations (10−4–10−9 M) of compounds for 72 h maintained at 37 °C in 5% CO2. The plated was incubated for 4h after 20 μL of MTT (5 mg/mL) was added to each well. The resultant tetrazolium salt was dissolved in 100 μL dimethylsulfoxide and read at 570 nm using a microplate reader (Varioskan Flash; Thermo Fisher Scientific, Waltham, MA, USA).
Compounds were tested in triplicate in at least three independent assays. The IC50 values were determined from the linear portion of the curves by a nonlinear regression analysis. Average values were reported.
3.3. In Vitro Tubulin Polymerization Inhibitory Activity
Bovine brain tubulin was purchased from Cytoskeleton Inc. (Denver, CO, USA). Tubulin (>99% pure, 2 mg/mL) in 100 μL of general tubulin buffer (80 mM Na-Pipes, pH 6.9, 1 mM EGTA, 1 mM MgCl2, 1 mM GTP, and 5% glycerol) at 0 °C was placed in a pre-warmed 96-well plates at 37 °C in the presence of tested compounds at varying concentrations. The reaction was started by warming the samples at 37 °C. The mass of polymer formed was monitored by turbidimetry at 340 nm every 1 min for 45 min with a BioTek’s Synergy 4 multifunction microplate reader (Winooski, VT, USA) [12,13,14].
4. Conclusions
In summary, thirteen 1,4-disubstituted-3,4-dihydroisoquinoline derivatives were synthesized and evaluated for their anti-proliferative activities against human CEM leukemia cell line with the aim of elucidating the SARs of the lead compounds discovered earlier. Most of them displayed moderate cytotoxic activities, and compound 21 and 32 showed good activities. The SARs of the synthesized compounds were investigated. It was found that the electron-donating substituents in the B ring were more favorable to the cytotoxic activities. The most potent compound 32 was confirmed to be able to inhibit tubulin polymerization and its hypothetical binding mode to tubulin was proposed by molecular docking. Some compounds had more potent cytotoxic activities than the structurally related compounds reported in our paper. Further optimization of these compounds is ongoing in our laboratory.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (Grant No. 21172260, 30901859, 21102174), Shanghai Natural Science Foundation (Grant No. 09ZR1438800) and “Chen Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (2012).
Author Contributions
Ideas and experiment design: Ling Zhang, Canhui Zheng, Ju Zhu; Computational development: Ling Zhang, Jingjing Huang, Canhui Zheng, Yunlong Song; Chemistry and Biology: Ling Zhang, Jingjing Huang, Jia Liu, Wenwen Zhu, Yunlong Song, Youjun Zhou, Jiaguo Lv; Analysis and data interpretation: Ling Zhang, Jingjing Huang, Canhui Zheng; Writing and review of the manuscript: All the authors; Study supervision: Canhui Zheng, Ju Zhu.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Honore, S.; Pasquier, E.; Braguer, D. Understanding microtubule dynamics for improved cancer therapy. Cell. Mol. Life Sci. 2005, 62, 3039–3056. [Google Scholar] [CrossRef] [PubMed]
- Downing, K.H.; Nogales, E. Tublin structure: Insights into microtubule properties and functions. Curr. Opin. Struct. Biol. 1998, 8, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Sorger, P.K.; Dobles, M.; Tournebize, R.; Hyman, A.A. Coupling cell division and cell death to microtuble dynamics. Curr. Opin. Cell Biol. 1997, 9, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubles as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Liu, J.; Liu, N.; Shi, D.; Zhou, X.T.; Lv, J.G.; Zhu, J.; Zheng, C.H.; Zhou, Y.J. Imidazolone-amide bridges and their effects on tubulin polymerization in cis-locked vinylogous combretastatin-A4 analogues: Synthesis and biological evaluation. Bioorg. Med. Chem. 2011, 19, 3579–3584. [Google Scholar] [CrossRef] [PubMed]
- Altmann, K.H. Microtubule-stabilizing agents: A growing class of important anticancer drugs. Curr. Opin. Chem. Biol. 2001, 5, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.H.; Chen, J.; Liu, J.; Zhou, X.T.; Liu, N.; Shi, D.; Huang, J.J.; Lv, J.G.; Zhu, J.; Zhou, Y.J. Synthesis and biological evaluation of 1-phenyl-1,2,3,4-dihydroisoquinoline compounds as tubulin polymerization Inhibitors. Arch. Pharm. Chem. Life Sci. 2012, 345, 454–462. [Google Scholar] [CrossRef]
- Zhu, W.W.; Song, Y.L.; Zheng, C.H.; Lv, J.G.; Chen, J.; Huang, J.J.; Zhou, Y.J.; Zhu, J. Synthesis, crystal structure and antitumor activity of 6,7,8-trimethoxy-1-(4-methoxy-3-nitrophenyl)-4-(pyridin-4-ylmethyl)-3,4-dihydroisoquinoline. Chin. J. Struct. Chem. 2011, 30, 717–723. [Google Scholar]
- Jetter, M.C.; Youngman, M.A.; McNally, J.J.; McDonnell, M.E.; Zhang, S.P.; Dubin, A.E.; Nasser, N.; Codd, E.E.; Flores, C.M.; Dax, S.L. Heteroaryl β-tetralin ureas as novel antagonists of human TRPV1. Bioorg. Med. Chem. Lett. 2007, 17, 6160–6163. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.M.; Brown, H.C. Selective reductions. XII. Explorations in some representative applications of aluminum hydride for selective reductions. J. Am. Chem. Soc. 1968, 90, 2927–2938. [Google Scholar] [CrossRef]
- Shan, Y.; Zhang, J.; Liu, Z.; Wang, M.; Dong, Y. Developments of combretastatin A-4 derivatives as anticancer agents. Curr. Med. Chem. 2011, 18, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Bal, C.; Barbier, P.; Douillard, S.; McLeer-Florin, A.; Bourgarel-Rey, V.; Pierson, J.T.; Fedorov, A.Y.; Finet, J.P.; Boutonnat, J.; et al. Synthesis and biological evaluation of 4-arylcoumarin analogues of combretastatins. J. Med. Chem. 2003, 46, 5437–5444. [Google Scholar] [CrossRef] [PubMed]
- Hamel, E. Evaluation of antimiotic agents by quantitative comparisons of their effects on the polymerization of purified tubulin. Cell Biochem. Biophys. 2003, 38, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Peng, Y.Y.; Wang, X.I.; Keenan, S.M.; Arora, S.; Welsh, W.J. Highly potent triazole-based tubulin polymerization inhibitors. J. Med. Chem. 2007, 50, 749–754. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
