Expression of Human DNAJ (Heat Shock Protein-40) B3 in Humanized UDP-glucuronosyltransferase 1 Mice
Abstract
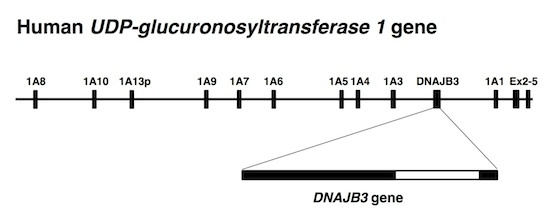
:1. Introduction

2. Results
2.1. Specificity of the Primers

2.2. Tissue-Specific Expression of Human DNAJ Family Homolog, Subfamily B, Member 3 (DNAJB3) in Humanized UDP-Glucuronosyltransferase 1 (hUGT1) Mice


2.3. Effects of a High-Fat Diet on the Expression of Human DNAJB3 in hUGT1 Mice

2.4. Effects of a UGT Inducer on the Expression of Human DNAJB3 in hUGT1 Mice

3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Animals and Treatments
4.3. Reverse-Transcription Polymerase Chain Reaction (RT-PCR) and Quantitative (Q)-RT-PCR
4.4. Western Blotting Analysis
4.5. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schlesinger, M.J. Heat shock proteins. J. Biol. Chem. 1990, 265, 12111–12114. [Google Scholar] [PubMed]
- Morimoto, R.I. Cells in stress: Transcriptional activation of heat shock genes. Science 1993, 259, 1409–1410. [Google Scholar] [CrossRef] [PubMed]
- Argon, Y.; Simen, B.B. GRP94, an ER chaperone with protein and peptide binding properties. Semin. Cell Dev. Biol. 1999, 10, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Q.; Zhao, X.; Kariya, Y.; Teshigawara, K.; Uchida, A. Inhibition of proliferation and induction of apoptosis by abrogation of heat-shock protein (HSP) 70 expression in tumor cells. Cancer Immunol. Immunother. 1995, 40, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Li, C.L.; Lang, Z.W.; Gao, G.F.; Tien, P. Significant correlation between expression level of HSP gp96 and progression of hepatitis B virus induced diseases. World J. Gastroenterol. 2004, 10, 1141–1145. [Google Scholar] [PubMed]
- Doiguchi, M.; Kaneko, T.; Urasoko, A.; Nishitani, H.; Iida, H. Identification of a heat-shock protein Hsp40, DjB1, as an acrosome- and a tail-associated component in rodent spermatozoa. Mol. Reprod. Dev. 2007, 74, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Michels, A.A.; Kanon, B.; Konings, A.W.; Ohtsuka, K.; Bensaude, O.; Kampinga, H.H. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. J. Biol. Chem. 1997, 272, 33283–33289. [Google Scholar] [CrossRef] [PubMed]
- Michels, A.A.; Kanon, B.; Bensaude, O.; Kampinga, H.H. Heat shock protein (HSP) 40 mutants inhibit Hsp70 in mammalian cells. J. Biol. Chem. 1999, 274, 36757–36763. [Google Scholar] [CrossRef] [PubMed]
- Szyperski, T.; Pellecchia, M.; Wall, D.; Georqopoulos, C.; Wüthrich, K. NMR structure determination of the Escherichia coli DnaJ molecular chaperone: Secondary structure and backbone fold of the N-terminal region (residues 2–108) containing the highly conserved J domain. Proc. Natl. Acad. Sci. USA 1994, 91, 11343–11347. [Google Scholar] [CrossRef] [PubMed]
- Corsi, A.K.; Schekman, R. The lumenal domain of Sec63p stimulates the ATPase activity of BiP and mediates BiP recruitment to the translocon in Saccharomyces cerevisiae. J. Cell Biol. 1997, 137, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.B.; Shao, Y.M.; Miao, S.; Wang, L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell. Mol. Life Sci. 2006, 63, 2560–2570. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.J.; Haqeman, J.; Carra, S.; Kampinqa, H.H. Structural and functional diversities between members of the human HSPB, HSPH, HSPA and DNAJ chaperone families. Biochemistry 2008, 47, 7001–7011. [Google Scholar] [CrossRef] [PubMed]
- Abubaker, J.; Tiss, A.; Abu-Farha, M.; Al-Ghimlas, F.; Al-Khairi, I.; Baturcam, E.; Cherian, P.; Elkum, N.; Hammad, M.; John, J.; Kavalakatt, S.; et al. DNAJB3/HSP-40 cochaperone is downregulated in obese humans and is restored by physical exercise. PLoS ONE 2013, 8, e69217. [Google Scholar] [CrossRef] [PubMed]
- Tukey, R.H.; Strassburg, C.P. Human UDP-glucuronosyltransferases: Metabolism, expression, and disease. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 581–616. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, R.; Nguyen, N.; Chen, S.; Tukey, R.H. Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus. Proc. Natl. Acad. Sci. USA 2010, 107, 5024–5029. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, R.; Chen, S.; Karin, M.; Tukey, R.H. Reduced expression of UGT1A1 in intestines of humanized UGT1 mice via inactivation of NF-κB leads to hyperbilirubinemia. Gastroenterology 2012, 142, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R.; Cobellis, G.; Scarpa, D.; Fienga, G.; Pierantoni, R.; Fasano, S. Detection of msj-1 gene expression in the frog, Rana esculenta testis, brain, and spinal cord. Mol. Reprod. Dev. 2004, 68, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Nguyen, N.; Peterkin, V.; Yang, Y.S.; Hotz, K.; La Placa, D.B.; Chen, S.; Tukey, R.H.; Stevens, J.C. A humanized UGT1 mouse model expressing the UGT1A1*28 allele for assessing drug clearance by UGT1A1-dependent glucuronidation. Drug. Metab. Dispos. 2010, 38, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Kutsuno, Y.; Sumida, K.; Itoh, T.; Tukey, R.H.; Fujiwara, R. Glucuronidation of drugs in humanized UDP-glucuronosyltransferase 1 mice: Similarity with glucuronidation in human liver microsomes. Pharmacol. Res. Perspect. 2013, 1, e00002. [Google Scholar] [CrossRef] [PubMed]
- Kutsuno, Y.; Itoh, T.; Tukey, R.H.; Fujiwara, R. Glucuronidation of drugs and drug-induced toxicity in humanized UDP-glucuronosyltransferase 1 mice. Drug. Metab. Dispos. 2014, 42, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Rui, G.; XiXia, L.; Huizhen, W. Stage-specific expression analysis of mouse testis-specific genes in spermatogenic cells. Chin. J. Zool. 2009, 44, 39–46. [Google Scholar]
- Yu, S.S.; Takenaka, O. Molecular cloning, structure, and testis-specific expression of MFSJ1, a member of the DNAJ protein family, in the Japanese monkey (Macaca fuscata). Biochem. Biophys. Res. Commun. 2003, 301, 443–449. [Google Scholar] [CrossRef]
- Berruti, G.; Martegani, E. The deubiquitinating enzyme mUBPy interacts with the sperm-specific molecular chaperone MSJ-1: The relation with the proteasome, acrosome, and centrosome in mouse male germ cells. Biol. Reprod. 2005, 72, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R.; Cobellis, G.; Berruti, G.; Junier, M.P.; Ceriani, M.; Boilée, S.; Pierantoni, R.; Fasano, S. Mouse sperm cell-specific DnaJ first homologue: An evolutionarily conserved protein for spermiogenesis. Biol. Reprod. 2002, 66, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Hahn, Y.; Lee, B. Identification of nine human-specific frameshift mutations by comparative analysis of the human and the chimpanzee genome sequences. Bioinformatics 2005, 21, i186–i194. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Puig, A.; Jimenez-Liñan, M.; Lowell, B.B.; Hamann, A.; Hu, E.; Spiegelman, B.; Flier, J.S.; Moller, D.E. Regulation of PPARγ gene expression by nutrition and obesity in rodents. J. Clin. Investig. 1996, 97, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D.B.; Klaassen, C.D. Induction of mouse UDP-glucuronosyltransferase mRNA expression in liver and intestine by activators of aryl-hydrocarbon receptor, constitutive androstane receptor, pregnane X receptor, peroxisome proliferator-activated receptor alpha, and nuclear factor erythroid 2-related factor 2. Drug Metab. Dispos. 2009, 37, 847–856. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsugi, R.; Itoh, T.; Fujiwara, R. Expression of Human DNAJ (Heat Shock Protein-40) B3 in Humanized UDP-glucuronosyltransferase 1 Mice. Int. J. Mol. Sci. 2015, 16, 14997-15008. https://doi.org/10.3390/ijms160714997
Mitsugi R, Itoh T, Fujiwara R. Expression of Human DNAJ (Heat Shock Protein-40) B3 in Humanized UDP-glucuronosyltransferase 1 Mice. International Journal of Molecular Sciences. 2015; 16(7):14997-15008. https://doi.org/10.3390/ijms160714997
Chicago/Turabian StyleMitsugi, Ryo, Tomoo Itoh, and Ryoichi Fujiwara. 2015. "Expression of Human DNAJ (Heat Shock Protein-40) B3 in Humanized UDP-glucuronosyltransferase 1 Mice" International Journal of Molecular Sciences 16, no. 7: 14997-15008. https://doi.org/10.3390/ijms160714997
APA StyleMitsugi, R., Itoh, T., & Fujiwara, R. (2015). Expression of Human DNAJ (Heat Shock Protein-40) B3 in Humanized UDP-glucuronosyltransferase 1 Mice. International Journal of Molecular Sciences, 16(7), 14997-15008. https://doi.org/10.3390/ijms160714997