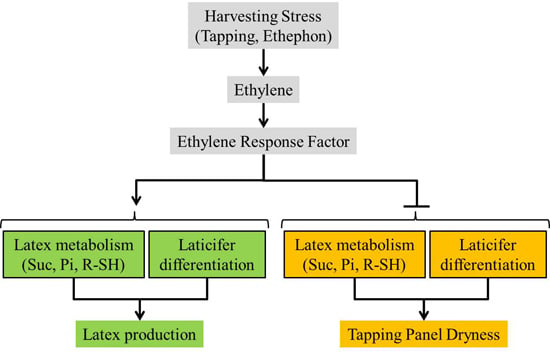
Involvement of Ethylene in the Latex Metabolism and Tapping Panel Dryness of Hevea brasiliensis
Abstract
:1. Introduction

2. Results
2.1. Metabolic Typology of Three Rubber Clones with Contrasting Latex Metabolism

2.2. Evolution of Latex Diagnosis Parameters in Response to Various Harvesting Systems for Three Rubber Clones with Contrasting Latex Metabolism

2.3. Occurrence of Tapping Panel Dryness in Three Rubber Clones with Contrasting Latex Metabolism

| Source | Degree of Freedom | Year | |||
|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | ||
| F | F | F | F | ||
| Clone | 2 | 11.244 * | 10.795 * | 3.498 * | 0.595 |
| Tapping | 2 | 0.87 | 0.798 | 1.899 | 7.535 * |
| Ethephon | 2 | 17.25 | 2.633 | 0.927 | 6.579 * |
| Clone * Tapping | 4 | 0.42 | 2.253 | 2.219 | 1.203 |
| Clone * Ethephon | 4 | 0.661 | 0.893 | 1.049 | 0.301 |
| Tapping * Ethephon | 4 | 0.665 | 1.254 | 0.216 | 0.518 |
| Clone * Tapping * Ethephon | 8 | 0.53 | 1.046 | 0.543 | 1.859 |
2.4. Production of Dry Rubber during the Occurrence of TPD

2.5. Evolution of Latex Diagnosis Parameters in the Latex of Healthy and TPD-Affected Trees

2.6. Anatomical Study of Rubber Tree Soft Bark


2.7. Fold Change in the Transcript Abundance of Genes Involved in the Ethylene Biosynthesis and Signalling Pathways and ROS-Scavenging System under Tapping Panel Dryness

3. Discussion
3.1. High Latex Metabolism Clones Are More Susceptible to TPD
3.2. Involvement of the Antioxidant System in the Onset of TPD
3.3. Involvement of Ethylene Biosynthesis and Signalling in the Onset of TPD
3.4. Identification of Expression Marker Genes during ROS-TPD
4. Experimental Section
4.1. Plant Material
4.2. Histo-Cytological Analysis
4.3. Measurement of the Dry Cut Length and Latex Diagnosis
4.4. Total RNA Isolation
4.5. Primer Design and Analysis of Transcript Abundance by Real-Time RT-PCR
4.6. Statistical Analysis for the Comparison of Relative Transcript Abundance and for the Analysis of Interactions between the Effect of Ethephon, TPD and a Combination of both in Mature Trees
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abraham, P.D. Fields trials with ethrel. Plant. Bull. 1970, 111, 306. [Google Scholar]
- De Faÿ, E.; Jacob, J.L. Anatomical organization of the laticiferous system in the bark. In Physiology of Rubber Tree Latex; d’Auzac, J., Jacob, J.L., Chrestin, H., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1989; pp. 4–14. [Google Scholar]
- D’Auzac, J.; Jacob, J.L. The composition of latex from Hevea brasiliensis as a laticiferous cytoplasm. In Physiology of Rubber Tree Latex; d’Auzac, J., Jacob, J.L., Chrestin, H., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1989; p. 58. [Google Scholar]
- Chrestin, H.; Gidrol, X.; Kush, A. Towards a latex molecular diagnostic of yield potential and the genetic engineering of the rubber tree. Euphytica 1997, 96, 77–82. [Google Scholar] [CrossRef]
- D’Auzac, J.; Jacob, J.L.; Prévôt, J.C.; Clément, A.; Gallois, R.; Crestin, H.; Lacote, R.; Pujade-Renaud, V.; Gohet, E. The regulation of cis-polyisoprene production (natural rubber) from Hevea brasiliensis. Recent Res. Dev. Plant Physiol. 1997, 1, 273–332. [Google Scholar]
- D’Auzac, J.; Bouteau, F.; Chrestin, H.; Clément, A.; Jacob, J.L.; Lacrotte, R.; Prévot, J.C.; Pujade-Renaud, V.; Rona, J.P. Stress ethylene in Hevea brasiliensis: Physiological, cellular and molecular aspects. In Cellular and Molecular Aspects of the Plant Hormone Ethylene; Pech, J., Latché, A., Balagué, C., Eds.; Springer Netherlands: Heidelberg, Germany, 1993; Volume 16, pp. 205–210. [Google Scholar]
- Chrestin, H. Biochemical aspects of bark dryness induced by overstimulation of rubber trees with ethrel. In Physiology of Rubber Tree Latex; d’Auzac, J., Jacob, J.L., Chrestin, H., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1989; pp. 431–439. [Google Scholar]
- Jacob, J.L.; Prévôt, J.C.; Lacrotte, R. Tapping panel dryness in Hevea brasiliensis. Plant. Rech. Dev. 1994, 2, 15–21. [Google Scholar]
- Gidrol, X.; Chrestin, H.; Tan, H.L.; Kush, A. Hevein, a lectin-like protein from Hevea brasiliensis (rubber tree) is involved in the coagulation of latex. J. Biol. Chem. 1994, 269, 9278–9283. [Google Scholar] [PubMed]
- Faridah, Y.S.; Arija, M.A.; Ghandimathi, H. Changes in some physiological latex paramers in relation to over exploitation and onset of induced tapping panel dryness. J. Nat. Rubber Res. 1996, 10, 182–186. [Google Scholar]
- Obouayeba, S.; Soumahin, E. Relationship between tapping intensity and tapping panel dryness susceptibility of some clones of Hevea brasiliensis in Southwestern Côte d’Ivoire. Agric. Biol. J. N. Am. 2011, 2, 1151–1159. [Google Scholar] [CrossRef]
- De Faÿ, E.; Jacob, J.L. Symptomatological, histological and cytological aspects. In Physiology of Rubber Tree Latex; d’Auzac, J., Jacob, J.L., Chrestin, H., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1989; pp. 407–428. [Google Scholar]
- Nicole, M.; Thouvenel, J.C.; Giannotti, J.; Chrestin, H.; Geiger, J.P.; Nandris, D.; Rio, B. The histology of Hevea brasiliensis phloem necrosis. Eur. J. For. Pathol. 1991, 21, 27–35. [Google Scholar] [CrossRef]
- Moraes, L.A.C.; Moreira, A.; de Figueiredo Moraes, V.H.; Tsai, S.M.; Cordeiro, E.R. Relationship between cyanogenesis and latex stability on tapping panel dryness in rubber trunk girth. J. Plant Interact. 2014, 9, 418–424. [Google Scholar] [CrossRef]
- De Faÿ, E.; Moraes, L.A.C.; Moraes, V.H.D.F. Cyanogenesis and the onset of tapping panel dryness in rubber tree. Pesqui. Agropecu. Bras. 2010, 45, 1372–1380. [Google Scholar]
- Jacob, J. Review on tapping panel dryness in rubber tree. In Workshop on the Tapping Panel Dryness in Rubber Tree; Rubber Research Insitute of India: Kottayam, India, 2005. [Google Scholar]
- Sobhana, P.; Thomas, M.; Jacob, J. Genetic distance between the root stock and scion of healthy and TPD affected trees of Hevea brasiliensis. Nat. Rubber Res. 2005, 18, 101–104. [Google Scholar]
- Jacob, J.L.; Prévôt, J.C.; Roussel, D.; Lacrotte, R.; Serres, E.; d’Auzac, J.; Eschbach, J.M.; Omont, H. Yield limiting factors, latex physiological parameters, latex diagnosis, and clonal typology. In Physiology of Rubber Tree Latex; d’Auzac, J, J., J.-L., Chrestin, H, Eds.; CRC Press, Inc.: Boca Raton, FL, 1989; pp. 345–382. [Google Scholar]
- Jacob, J.L.; Eschbach, J.M.; Prévot, J.L.; Roussel, D.; Lacrotte, R.; Chrestin, H.; Auzac, J.D. Physiological basis for latex diagnosis of the functioning of the laticiferous system in rubber trees. In Proceeding of International Rubber Conference, Kuala Lumpur, Malaysia, 20–25 October 1986.
- Tupý, J. Sucrose supply and utilization for latex production. In Physiology of Rubber Tree Latex; d’Auzac, J., Jacob, J.L., Chrestin, H., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1989; pp. 179–218. [Google Scholar]
- Jacob, J.L.; Prévôt, J.C.; Lacrotte, R.; Clément, A.; Serres, E.; Gohet, E. Clonal typology of laticifer functioning in Hevea brasiliensis. Plant Rech. Dev. 1995, 2, 43–49. [Google Scholar]
- Yang, S.Q.; Fan, X.W. Physiological response of PR107 to intensive tapping with stimulation at early exploitation stage. Chin. J. Trop. Crop. Res. 1995, 16, 17–28. [Google Scholar]
- Eliathe, E.A.A.; Edmond, K.K.; Mathurin, O.K.; Justin, L.Y.; Pierre, N.A.S.; Kouadio, D.; Abdourahamane, S. Detection of Hevea brasilensis clones yield potential and susceptibility to tapping panel dryness in Côte d’Ivoire using the 32 and 35 KDa lutoidic proteins. Afr. J. Biotechnol. 2012, 11, 10200–10206. [Google Scholar]
- Venkatachalam, P.; Thulaseedharan, A.; Raghothama, K. Identification of expression profiles of tapping panel dryness (TPD) associated genes from the latex of rubber tree (Hevea brasiliensis Muell. Arg.). Planta 2007, 226, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Deng, Z.; Chen, C.; Xia, Z.; Wu, M.; He, P.; Chen, S. Identification and characterization of genes associated with tapping panel dryness from Hevea brasiliensis latex using suppression subtractive hybridization. BMC Plant Biol. 2010, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Deng, Z.; Qin, B.; Liu, X.; Men, Z. De novo assembly and characterization of bark transcriptome using Illumina sequencing and development of EST-SSR markers in rubber tree (Hevea brasiliensis Muell. Arg.). BMC Genom. 2012, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.-S.; Ghazali, A.-K.; Hoh, C.-C.; Mohd-Zainuddin, Z. RNA sequencing read depth requirement for optimal transcriptome coverage in Hevea brasiliensis. BMC Res. Notes 2014, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Gébelin, V.; Leclercq, J.; Kuswanhadi; Argout, X.; Chaidamsari, T.; Hu, S.; Tang, C.; Sarah, G.; Yang, M.; Montoro, P. The small RNA profile in latex from Hevea brasiliensis trees is affected by tapping panel dryness. Tree Physiol. 2013, 33, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Kuswanhadi; Leclercq, J.; Rio, M.; Tregear, J.; Ducamp-Collin, M.N.; Montoro, P. Isolation of three members of the multigene family encoding ACC oxidases in Hevea brasiliensis and Inverstigation of Their Responses to Ethylene Stimulation and Wounding. J. Rubber Res. 2010, 13, 185–205. [Google Scholar]
- Duan, C.; Argout, X.; Gébelin, V.; Summo, M.; Dufayard, J.-F.; Leclercq, J.; Hadi, K.; Piyatrakul, P.; Pirrello, J.; Rio, M.; et al. Identification of the Hevea brasiliensis AP2/ERF superfamily by RNA sequencing. BMC Genom. 2013, 14, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclercq, J.; Martin, F.; Sanier, C.; Clement-Vidal, A.; Fabre, D.; Oliver, G.; Lardet, L.; Ayar, A.; Peyramard, M.; Montoro, P. Over-expression of a cytosolic isoform of the HbCuZnSOD gene in Hevea brasiliensis changes its response to a water deficit. Plant Mol. Biol. 2012, 80, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Piyatrakul, P.; Yang, M.; Putranto, R.-A.; Pirrello, J.; Dessailly, F.; Hu, S.; Summo, M.; Theeravatanasuk, K.; Leclercq, J.; Montoro, P. Sequence and expression analyses of ethylene response factors highly expressed in latex cells from Hevea brasiliensis. PLoS ONE 2014, 9, e99367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacote, R.; Gabla, O.; Obouayeba, S.; Eschbach, J.M.; Rivano, F.; Dian, K.; Gohet, E. Long-term effect of ethylene stimulation on the yield of rubber trees is linked to latex cell biochemistry. Field Crop. Res. 2010, 115, 94–98. [Google Scholar] [CrossRef]
- Le Guen, V.; Garcia, D.; Doaré, F.; Mattos, C.R.R.; Condina, V.; Couturier, C.; Chambon, A.; Weber, C.; Espéout, S.; Seguin, M. A rubber tree’s durable resistance to Microcyclus ulei is conferred by a qualitative gene and a major quantitative resistance factor. Tree Genet. Genom. 2011, 7, 877–889. [Google Scholar] [CrossRef]
- Lespinasse, D.; Grivet, L.; Troispoux, V.; Rodier-Goud, M.; Pinard, F.; Seguin, M. Identification of QTLs involved in the resistance to South American leaf blight (Microcyclus ulei) in the rubber tree. Theor. Appl. Genet. 2000, 100, 975–984. [Google Scholar] [CrossRef]
- Jacob, J.L.; d’Auzac, J.; Prévôt, J.C. The composition of natural latex from Hevea brasiliensis. Clin. Rev. Allegy 1993, 11, 325–337. [Google Scholar]
- Sewelam, N.; Kazan, K.; Thomas-Hall, S.R.; Kidd, B.N.; Manners, J.M.; Schenk, P.M. Ethylene response factor 6 is a regulator of reactive oxygen species signaling in arabidopsis. PLoS ONE 2013, 8, e70289. [Google Scholar] [CrossRef] [PubMed]
- Baxter-Burrell, A.; Yang, Z.; Springer, P.S.; Bailey-Serres, J. RopGAP4-Dependent rop GTPase rheostat control of arabidopsis oxygen deprivation tolerance. Science 2002, 296, 2026–2028. [Google Scholar] [CrossRef] [PubMed]
- Coupé, M.; Chrestin, H. Physico-chemical and bio-chemical mechanisms of the hormonal (ethylene) stimulation: Early biochemical events induced in Hevea latex, by hormonal bark stimulation. In Physiology of Rubber Tree Latex; d’Auzac, J., Jacob, J.L., Chrestin, H., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1989; pp. 295–319. [Google Scholar]
- Pramoolkit, P.; Lertpanyasampatha, M.; Viboonjun, U.; Kongsawadworakul, P.; Chrestin, H.; Narangajavana, J. Involvement of ethylene-responsive microRNAs and their targets in increased latex yield in the rubber tree in response to ethylene treatment. Plant Physiol. Biochem. 2014, 84, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Woeste, K.E.; Theologis, A.; Kieber, J.J. Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc. Natl. Acad. Sci. USA 1998, 95, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Woeste, K.E.; Ye, C.; Kieber, J.J. Two arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of l-aminocyclopropane-l-carboxylic acid synthase. Plant Physiol. 1999, 119, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.C.; Yoshida, H.; Lurin, C.; Ecker, J.R. Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature 2004, 428, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.B.; Cancel, J.D. A recessive mutation in the RUB1-conjugating enzyme, RCE1, reveals a requirement for RUB modification for control of ethylene biosynthesis and proper induction of basic chitinase and PDF1.2 in Arabidopsis. Plant J. 2004, 38, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Bostick, M.; Lochhead, S.R.; Honda, A.; Palmer, S.; Callis, J. Related to Ubiquitin 1 and 2 are redundant and essential and regulate vegetative growth, auxin signaling, and ethylene production in Arabidopsis. Plant Cell 2004, 16, 2418–2432. [Google Scholar] [CrossRef] [PubMed]
- Ziliotto, F.; Begheldo, M.; Rasori, A.; Bonghi, C.; Tonutti, P. Transcriptome profiling of ripening nectarine (Prunus persica L. Batsch) fruit treated with 1-MCP. J. Exp. Bot. 2008, 59, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Kida, Y.; Arai, T.; Kishi, Y.; Manago, Y.; Murai, M.; Matsushita, Y. Overexpression of tobacco ethylene response factor NtERF3 gene and its homologues from tobacco and rice induces hypersensitive response-like cell death in tobacco. J. Gen. Plant Pathol. 2011, 78, 8–17. [Google Scholar] [CrossRef]
- Ogata, T.; Kida, Y.; Tochigi, M.; Matsushita, Y. Analysis of the cell death-inducing ability of the ethylene response factors in group VIII of the AP2/ERF family. Plant Sci. 2013, 209, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Kosmacz, M.; Weits, D.A.; Giuntoli, B.; Giorgi, F.M.; Voesenek, L.A.; Perata, P.; van Dongen, J.T. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 2011, 479, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.-P.; Loveridge, C.W. HvDRF1 is involved in abscisic acid-mediated gene regulation in barley and produces two forms of AP2 transcriptional activators, interacting preferably with a CT-rich element. Plant J. 2004, 37, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Egawa, C.; Kobayashi, F.; Ishibashi, M.; Nakamura, T.; Nakamura, C.; Takumi, S. Differential regulation of transcript accumulation and alternative splicing of a DREB2 homolog under abiotic stress conditions in common wheat. Genes Genet. Syst. 2006, 81, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Kakimoto, M.; Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Tran, L.S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J. 2007, 50, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Moffat, C.S.; Ingle, R.A.; Wathugala, D.L.; Saunders, N.J.; Knight, H.; Knight, M.R. ERF5 and ERF6 play redundant roles as positive regulators of JA/Et-Mediated defense against Botrytis cinerea in Arabidopsis. PLoS ONE 2012, 7, e35995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mase, K.; Ishihama, N.; Mori, H.; Takahashi, H.; Kaminaka, H.; Kodama, M.; Yoshioka, H. Ethylene-responsive AP2/ERF transcription factor MACD1 participates in phytotoxin-triggered programmed cell death. Mol. Plant Microbe Interact. 2013, 26, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Michaux-FerriAre, N.; Teisson, C.; Lardet, L.; Carron, M.P. Effects of carbohydrate addition on the induction of somatic embryogenesis in Hevea brasiliensis. Plant Cell Tissue Organ Cult. 1999, 59, 103–112. [Google Scholar] [CrossRef]
- Lillie, R.D.; Ashburn, L.L. Supersaturated solutions of fat stains in dilute isopropanol for demonstration of acute fatty degeneration not shown by Herxheimer’s technique. Arch. Path. 1943, 36, 432. [Google Scholar]
- Dische, Z.M. Methods in Carbohydrate Chemisrty; Whistler, R.L., Wolfrom, M.L., Eds.; Academia Press: New York, NY, USA, 1962; Volume 1, pp. 475–514. [Google Scholar]
- Taussky, H.H.; Shorr, E. A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 1953, 202, 675–685. [Google Scholar] [PubMed]
- McMullen, A.I. Thiols of low molecular weight in Hevea brasiliensis latex. Biochim. Biophys. Acta 1960, 41, 152. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning, A Laboratory Manual, 3rd ed.; Cold Spring Harbor Press: New York, NY, USA, 1989. [Google Scholar]
- Duan, C.; Rio, M.; Leclercq, J.; Bonnot, F.; Oliver, G.; Montoro, P. Gene expression pattern in response to wounding, methyl jasmonate and ethylene in the bark of Hevea brasiliensis. Tree Physiol. 2010, 30, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.K.; Czechowski, T.; Scheible, W.R. Eleven golden rules of quantitative RT-PCR. Plant Cell 2008, 20, 1736–1737. [Google Scholar] [CrossRef] [PubMed]
- Putranto, R.A.; Sanier, C.; Leclercq, J.; Duan, C.; Rio, M.; Jourdan, C.; Thaler, P.; Sabau, X.; Argout, X.; Montoro, P. Differential gene expression in different types of Hevea brasiliensis roots. Plant Sci. 2012, 183, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Piyatrakul, P.; Putranto, R.A.; Martin, F.; Rio, M.; Dessailly, F.; Leclercq, J.; Dufayard, J.-F.O.; Lardet, L.; Montoro, P. Some ethylene biosynthesis and AP2/ERF genes reveal a specific pattern of expression during somatic embryogenesis in Hevea brasiliensis. BMC Plant Biol. 2012, 12, 244. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putranto, R.-A.; Herlinawati, E.; Rio, M.; Leclercq, J.; Piyatrakul, P.; Gohet, E.; Sanier, C.; Oktavia, F.; Pirrello, J.; Kuswanhadi; et al. Involvement of Ethylene in the Latex Metabolism and Tapping Panel Dryness of Hevea brasiliensis. Int. J. Mol. Sci. 2015, 16, 17885-17908. https://doi.org/10.3390/ijms160817885
Putranto R-A, Herlinawati E, Rio M, Leclercq J, Piyatrakul P, Gohet E, Sanier C, Oktavia F, Pirrello J, Kuswanhadi, et al. Involvement of Ethylene in the Latex Metabolism and Tapping Panel Dryness of Hevea brasiliensis. International Journal of Molecular Sciences. 2015; 16(8):17885-17908. https://doi.org/10.3390/ijms160817885
Chicago/Turabian StylePutranto, Riza-Arief, Eva Herlinawati, Maryannick Rio, Julie Leclercq, Piyanuch Piyatrakul, Eric Gohet, Christine Sanier, Fetrina Oktavia, Julien Pirrello, Kuswanhadi, and et al. 2015. "Involvement of Ethylene in the Latex Metabolism and Tapping Panel Dryness of Hevea brasiliensis" International Journal of Molecular Sciences 16, no. 8: 17885-17908. https://doi.org/10.3390/ijms160817885
APA StylePutranto, R.-A., Herlinawati, E., Rio, M., Leclercq, J., Piyatrakul, P., Gohet, E., Sanier, C., Oktavia, F., Pirrello, J., Kuswanhadi, & Montoro, P. (2015). Involvement of Ethylene in the Latex Metabolism and Tapping Panel Dryness of Hevea brasiliensis. International Journal of Molecular Sciences, 16(8), 17885-17908. https://doi.org/10.3390/ijms160817885