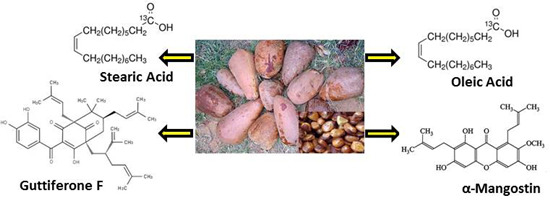
Allanblackia Oil: Phytochemistry and Use as a Functional Food
Abstract
:1. Introduction
2. Results and Discussion
2.1. Botanical Description and Geographic Distribution



| Species | A. floribunda | A. parviflora | A. stuhlmannii |
|---|---|---|---|
| Mature height/Trunk Diameter | 30 m/80 cm | 25 (–33) m/80 cm | 35 (–45) m/65 cm |
| Outer bark | Reddish-brown to blackish; scales small and irregular | Yellowish-brown or reddish-brown; scales small and irregular | Dark grey to black, smooth or rarely flaking with square scales |
| Inner bark | Granular, reddish or brown, exuding clear sap | Reddish-brown with sometimes pale yellow streaks; exuding colorless or pale yellow sap | Red to pale brown with white stripes, fibrous to granular, exuding a clear sap that turns yellowish upon oxidation |
| Leaves | Opposite, simple, entire, glabrous, estipulate; blade elliptical to ovate, rarely obovate; base rounded or cuneate; apex acuminate; 8–25 cm × 3–8 cm; petiole 1 cm | Opposite, simple, entire, glabrous, estipulate; blade elliptical to narrowly obovate; base cuneate; apex acuminate; 12–25 cm × 5–9 cm; petiole 1–1.5 cm. | Opposite, simple, entire, estipulate; blade oblong to elliptical-oblong; base cuneate, apex shortly acuminate; 5–20 cm × 1–7 cm; petiole 1–2 cm |
| Inflorescence | Terminal raceme or panicle with strongly reduced branches or flowers single or in pairs in leaf axils | Terminal raceme or panicle with strongly reduced branches or flowers single or in pairs in leaf axils | Flowers solitary in leaf axils or crowded at the end of branches |
| Flowers | Unisexual, regular, five-merous, pinkish or reddish (rarely white); pedicel 3–8 cm | Unisexual, regular, five-merous, pinkish or reddish, fragrant; pedicel 1–3 cm | Unisexual, regular, five-merous, cream to reddish, fragrant; pedicel (3.5–) 6.5–8 cm |
| Sepals | Orbicular, unequal, outer ones 5–8 mm in diameter, inner ones 12–15 mm in diameter, glabrous | Ovate or obovate, unequal, 6–18 mm × 4–15 mm, glabrous | Orbicular to ovate, unequal, outer ones 4–9 mm in diameter, inner ones ca. 2 cm in diameter, pale yellow |
| Petals | Obovate to orbicular, 20–25 mm long, glabrous | Obovate, ca. 20 mm long, glabrous | Orbicular to spathulate, 27–37 mm × 18–26 mm, glabrous |
| Stamens | Numerous, in 5 bundles opposite the petals, 10–15 mm long, anthers arranged on the internal face of the bundle; disk star-shaped with deeply folded glands | Numerous, in five bundles opposite the petals, obtriangular, ca. 18 mm long, anthers arranged on the internal face of the bundle; disk star-shaped with smooth or slightly folded glands | Numerous, in five bundles opposite the petals, ca. 2 cm long, inner surface angled, anthers arranged on the 2 faces of the bundles; disk star-shaped |
| Ovary | Superior, incompletely five-celled, stigma sessile, staminal bundles reduced to a few free 4–5 mm long staminodes, disk glands grooved | Superior, incompletely five-celled, stigma sessile | Superior, incompletely five-celled, stigma sessile; staminal bundles reduced to a few free, ca. 4 mm long staminodes |
| Fruit | Large ellipsoid berry 20–50 cm × 5–14 cm, with five longitudinal ridges, 40–80 seeded | Large ellipsoid berry 10–50 cm × ca. 15 cm, with five longitudinal ridges, brown warty appearance, 40–100 seeded | Large oblong to globose or cone-shaped berry 16–34 cm × 15–17 cm, 2.5–6 kg, red-brown, 60–140 seeded |
| Seeds | Ovoid, 2.5–3 cm × 1.5–2 cm, enclosed in a pinkish aril; embryo small, embedded in oily endosperm | Ovoid, ca. 3 cm × 2 cm, enclosed in a pinkish aril | 4-Angular, ca. 4 cm × 2–3 cm, one angle with a small fleshy aril; embryo small, embedded in oily endosperm |
| Germination | Seedling with hypogeal germination | Seedling with hypogeal germination; epicotyl 4–5 cm long | Seedling with hypogeal germination |
2.2. Traditional Allanblackia Seed Oil Use
2.3. Cultivation and Wildcrafting of Allanblackia Seeds
2.4. General Characteristics of the Seed Oil
2.4.1. Allanblackia floribunda Seed Oil
2.4.2. Allanblackia parviflora Seed Oil
2.4.3. Allanblackia stuhlmannii Seed Oil
2.4.4. Other Allanblackia Seed Oils
2.5. Phytochemistry and Medicinal Use of Allanblackia Seeds
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gunstone, F. Production and trade of vegetable oils. In Vegetable Oils in Food Technology: Composition, Properties and Uses, 2nd ed.; Gunstone, F., Ed.; Blackwell Publishing Ltd.: Chichester, UK, 2011; pp. 1–24. [Google Scholar]
- Da Silva, F.J.; de Lima, A.G.B.; da Costa, Y.J.R.; Rilho, C.R.B.; Grilo, M.B. Duel-fuel (natural gas/biodiesel) engines: Fundamentals, performance and environmental impact. In Alternative Energies: Updates on Progress; Ferreira, G., Ed.; Springer-Verlag: Berlin, Germany, 2013; pp. 47–68. [Google Scholar]
- Betoret, E.; Betoret, N.; Vidal, D.; Fito, P. Functional foods development: Trends and technologies. Trends Food Sci. Technol. 2011, 22, 498–508. [Google Scholar] [CrossRef]
- WHO. Diet, nutrition and the prevention of chronic diseases. Available online: http://www.fao.org/docrep/005/AC911E/AC911E00.HTM#Contents (accessed on 29 June 2015).
- Stevens, P. Clusiaceae-Guttiferae. In The Families and Genera of Vascular Plants IX. Flowering Plants Eudicots; Kubitzki, K., Ed.; Springer Verlag: Berlin, Germany, 2007; pp. 48–66. [Google Scholar]
- Sweeney, P.W. Phylogeny and floral diversity in the genus Garcinia (Clusiaceae) and relatives. Int. J. Plant Sci. 2008, 169, 1288–1303. [Google Scholar] [CrossRef]
- Ruhfel, B.R.; Bittrich, V.; Bove, C.P.; Gustafsson, M.H.G.; Philbrick, C.T.; Rutishauser, R.; Xi, Z.; Davis, C.C. Phylogeny of the clusioid clade (Malpighiales): Evidence from the plastid and mitochondrial genomes. Am. J. Bot. 2011, 98, 306–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, E.M.; Kamal-Eldin, A. Cosmetic and pharmaceutical properties of fats and oils. In Processing and Nutrition of Fats and Oils; Erickson, D., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Bamps, P.; Robson, N.; Verdcourt, B. Flora of Tropical East Africa: Guttiferae; Crown Agents: London, UK, 1978. [Google Scholar]
- Orwa, C.; Munjuga, M. Allanblackia floribunda Oliv. In PROTA 14: Vegetable oils/Oléagineux; van der Vossen, H.A.M., Mkamilo, G.S., Eds.; PROTA: Wageningen, The Netherlands, 2007. [Google Scholar]
- Orwa, C.; Oyen, L.P.A. Allanblackia parviflora A.Chev. In PROTA 14: Vegetable oils/Oléagineux; van der Vossen, H.A.M., Mkamilo, G.S., Eds.; PROTA: Wageningen, The Netherlands, 2007. [Google Scholar]
- Munjuga, M.; Mwaura, L.; Schmidt, L.H. Allanblackia stuhlmannii Engl. Seed Leaflet. Available online: http://www.prota4u.org (accessed on 20 June 2015).
- Pakenham, T. The Scramble for Africa; Weidenfeld & Nicolson: London, UK, 1991; pp. 1876–1912. [Google Scholar]
- Pye-Smith, C. Seeds of Hope: A Public-Private Partnership to Domesticate a Native Tree, Allanblackia, Is Transforming Lives in Rural Africa; World Agroforestry Centre: Nairobi, Kenya, 2009. [Google Scholar]
- Ruffo, C.K.; Birnie, A.; Tengnäs, B. Edible wild plants of Tanzania; Regional Land Management Unit/SIDA: Nairobi, Kenya, 2002. [Google Scholar]
- Adubofuor, J.; Sefah, W.; Oldham, J.H. Nutrient composition of Allanblackia paviflora seed kernels and oil compared with some plant fats and oils and application of the oil in soap preparation. J. Cereals Oil Seeds 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Hutchinson, J.; Dalziel, J.M.; Keay, R.W.J. Flora of West Tropical Africa; Crown Agents: London, UK, 1954. [Google Scholar]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Agroforestree Database: A Tree Reference and Selection Guide Version 4.0, 2009. Available online: http://www.worldagroforestry.org (accessed on 20 June 2015).
- Buss, C.; Tissari, J. Allanblackia—An ingredient for poverty reduction? Rural 21 2010, 3, 37–39. [Google Scholar]
- Bürkle, E.; Palenberg, M. Allanblackia supply chain as a strategic alliance with Unilever R & D Netherlands. Available online: http://www.entwicklung.at/uploads/media/7_Allanblackia_Supply_chain_as_a_Strategic_Alliance_with_Unilever_R_D_Netherlands_Tanzania.pdf (accessed on 13 June 2015).
- Silou, T. Oils and Fats for the Future. A Case Study: Safou (Dacryodes edulis) from the Congo Basin Countries in Africa; Nova Science Publishers: Hauppauge, NY, USA, 2012. [Google Scholar]
- Loumouamou, B.W.; Binaki, A.F.; Silou, T. Oleaginous character and profiles in fatty acids and in triacylglycerols of the seeds of Allanblackia floribunda Oliv. of Congo. Adv. J. Food Sci. Technol. 2014, 6, 308–315. [Google Scholar]
- Shrestha, R.B.; Akangaamkum, A.D.; Agyare, E.; Torpey, K.; Fordjor, M.M.; Hinneh, K.O. Relative attractiveness of Allanblackia cultivation in Ghana: Farmer’s perceptions and willingness. Available online: http://www.snvworld.org/download/publications/2._relative_attractiveness_of_allanblackia_cultivation_in_ghana_farmers_perceptions_and_willingness.pdf (accessed on 10 September 2015).
- Shrestha, R.B.; Akangaamkum, A.D. Novella Partnership, a Partnership for Poverty Reduction through Sustainable Enterprise Development Based on Allanblackia; SNV Ghana: Accra, Ghana, 2008. [Google Scholar]
- Tetens, I. Opinion of the scientific panel on dietetic products, nutrition and allergies on a request from the commission related to the safety of Allanblackia seed oil for use in yellow fat and cream based spreads: Request N° EFSA-Q-2007-059. Eur. Food Saf. Auth. 2007, 580, 1–10. [Google Scholar]
- Li, X.; Lao, Y.; Zhang, H.; Wang, X.; Tan, H.; Lin, Z.; Xu, H. The natural compound guttiferone F sensitizes prostate cancer to starvation induced apoptosis via calcium and JNK elevation. BMC Cancer 2015, 15, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.W.; Blunt, J.W.; Boswell, J.L.; Cardellina, J.H.; Boyd, M.R. Guttiferone F, the first prenylated benzophenone from Allanblackia stuhlmannii. J. Nat. Prod. 1999, 62, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Ofori, D.A.; Kehlenbeck, K.; Munjuga, M.; Asaah, E.; Kattah, C.; Rutatina, F.; Jamnadass, R. Allanblackia species: A model for the domestication of high potential tree crops in Africa. World Agrofor. Cent. 2011. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Minor Oil Crops. Part II–Non-Edible Oils. Available online: http://www.fao.org/docrep/x5043e/x5043E0d.htm#Allanblackia (accessed on 8 July 2015).
- Amanor, K.S. Allanblackia standard setting and sustainable supply chain management. Socioeconomic conditions of Allanblackia production and perceptions of farmers and collectors. Available online: http://worldagroforestry.org/projects/allanblackia/workshop/2006/supporting_material/Allanblackia/Socioeconomic%20issues%20Ghana.pdf (accessed on 19 June 2015).
- Andersson, M. Allanblackia nuts in tropical Africa: A new source for food, oil and ecosystem services. In Bundling Agricultural Products with Ecosystem Services: Incentives for Ecoagriculture Landscapes; Andersson, M.S., Scherr, S.J., Shames, S., Eds.; Ecoagriculturepartners: Washington, DC, USA, 2009. [Google Scholar]
- Jamnadass, J.; Dawson, I.K.; Anegbeh, P.; Asaah, E.; Atangana, A.; Cordeiro, N.J.; Hendrickx, H.; Henneh, S.; Kadu, C.A.; Kattah, C.; et al. Allanblackia, a new tree crop in Africa for the global food industry: Market development, smallholder cultivation and biodiversity management. For. Trees Livelihoods 2010, 19, 251–268. [Google Scholar] [CrossRef]
- Russell, J.R.; Kadu, C.A.C.; Jamnadass, R.; Booth, A.; Cordeiro, N.J.; Woodhead, M.; Dawson, I.K. AFLP and SSR diversity in the African fruit tree Allanblackia: Implications for management of a genus newly subject to domestication for the edible oil industry. Tree Genet. Genomes 2009, 5, 517–527. [Google Scholar] [CrossRef]
- Alvum-Toll, K. Allanblackia stuhlmannii—A tree under current domestication: What are the soil requirements? Available online: http://stud.epsilon.slu.se/5966/1/alvum-toll_k_130821.pdf (accessed on 1 July 2015).
- Dougherty, R.; Allman, M.; Iacono, J. Effects of diets containing high or low amounts of stearic acid on plasma lipoprotein fractions and fecal fatty acid excretion of men. Am. J. Clin. Nutr. 1995, 61, 1120–1128. [Google Scholar] [PubMed]
- Tholstrup, T.; Marckmann, P.; Jespersen, J.; Sandström, B. Fat high in stearic acid favorably affects blood lipids and factor VII coagulant activity in comparison with fats high in palmitic acid or high myristic and lauric acids. Am. J. Clin. Nutr. 1994, 59, 371–377. [Google Scholar] [PubMed]
- Denke, M.; Grundy, S. Effects of fats high in stearic acid on lipid and lipoprotein concentrations in men. Am. J. Clin. Nutr. 1991, 54, 1036–1040. [Google Scholar] [PubMed]
- Nestel, P.J.; Pomeroy, S.; Kay, S.; Sasahara, T.; Yamashita, T. Effect of a stearic acid–rich, structured triacylglycerol on plasma lipid concentrations. Am. J. Clin. Nutr. 1998, 68, 1196–1201. [Google Scholar] [PubMed]
- Meara, M.L.; Zaky, Y.A.H. Fatty acids and glycerides of the seed fats of Allanblackia floribunda and Allanblackia parviflora. J. Soc. Chem. Ind. 1940, 59, 25–26. [Google Scholar]
- Noumi, G.B.; Njine, C.B.; Pengou, M.S.; Ngameni, E. Physicochemical analysis of the Cameroonian Allanblackia floribunda Oliver seeds oil extract. Riv. Ital. Sostanze Grasse 2011, 88, 38–45. [Google Scholar]
- Dike, M.C.; Asuquo, M.E. Proximate, phytochemical and mineral compositions of seeds of Allanblackia floribunda, Garcinia kola and Poga oleosa from Nigerian rainforest. Afr. J. Biotechnol. 2012, 11, 11096–11098. [Google Scholar]
- Atangana, A.R.; van der Vlis, E.; Khasa, D.P.; van Houten, D.; Beaulieu, J.; Hendrickx, H. Tree-to-tree variation in stearic and oleic acid content in seed fat from Allanblackia floribunda from wild stands: Potential for tree breeding. Food Chem. 2011, 126, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Wilfred, S.; Adubofuor, J.; Oldham, J.H. Optimum conditions for expression of oil from Allanblackia floribunda seeds and assessing the quality and stability of pressed and solvent extracted oil. Afr. J. Food Sci. 2010, 4, 563–570. [Google Scholar]
- Alenyorege, E.A.; Hussein, Y.A.; Adongo, T.A. Extraction yield, efficiency and loss of the traditional hot water flotation (HWF) method of oil extraction from the seeds of Allanblackia floribunda. Int. J. Sci. Technol. Res. 2015, 4, 92–95. [Google Scholar]
- Heise, R. Über das Vorkommen des Oleodistearins in dem Fette der Samen von Theobroma-Cacao. Arbeiten aus dem Kaiserlichen Gesundheitsamte 1896, 12, 540. (In German) [Google Scholar]
- Mwaura, L.; Munjuga, M. Allanblackia stuhlmannii (Engl.) Engl. In PROTA 14: Vegetable Oils/Oléagineux; van der Vossen, H.A.M., Mkamilo, G.S., Eds.; PROTA: Wageningen, The Netherlands, 2007. [Google Scholar]
- Pengou, M.; Noumi, G.B.; Ngameni, E. Fatty acid composition and some physicochemical properties of oils from Allanblackia gabonensis and A. stanerana kernels. J. Am. Oil Chem. Soc. 2013, 90, 27–32. [Google Scholar] [CrossRef]
- Bilanda, D.C.; Dimo, T.; Dzeufiet Djomeni, P.D.; Bella, N.M.; Aboubaka, O.B.; Nguelefack, T.B.; Tan, P.V.; Kamtchouing, P. Antihypertensive and antioxidant effects of A. floribunda Oliv. (Clusiaceae) aqueous extract in alcohol- and sucrose-induced hypertensive rats. J. Ethnopharmacol. 2010, 128, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Meschak, C. Indigenous knowledge of Allanblackia stuhlmannii in the East Usambara Mountains, Tanzania. Available online: http://worldagroforestry.org/projects/allanblackia/Report/Tech/Ik_Tz.pdf (accessed on 28 June 2015).
- Epifano, F.; Fiorito, S.; Genovese, S. Phytochemistry and pharmacognosy of the genus Psorospermum. Phytochem. Rev. 2013, 12, 673–684. [Google Scholar] [CrossRef]
- Azebaze, A.G.B.; Menasria, F.; Noumi, L.G.; Nguemfo, E.L.; Tchamfo, M.F.; Nkengfack, A.E.; Kolb, J.P.; Meyer, M. Xanthones from the seeds of Allanblackia monticola and their apoptotic and antiproliferative activities. Planta Med. 2009, 75, 243–248. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crockett, S.L. Allanblackia Oil: Phytochemistry and Use as a Functional Food. Int. J. Mol. Sci. 2015, 16, 22333-22349. https://doi.org/10.3390/ijms160922333
Crockett SL. Allanblackia Oil: Phytochemistry and Use as a Functional Food. International Journal of Molecular Sciences. 2015; 16(9):22333-22349. https://doi.org/10.3390/ijms160922333
Chicago/Turabian StyleCrockett, Sara L. 2015. "Allanblackia Oil: Phytochemistry and Use as a Functional Food" International Journal of Molecular Sciences 16, no. 9: 22333-22349. https://doi.org/10.3390/ijms160922333
APA StyleCrockett, S. L. (2015). Allanblackia Oil: Phytochemistry and Use as a Functional Food. International Journal of Molecular Sciences, 16(9), 22333-22349. https://doi.org/10.3390/ijms160922333