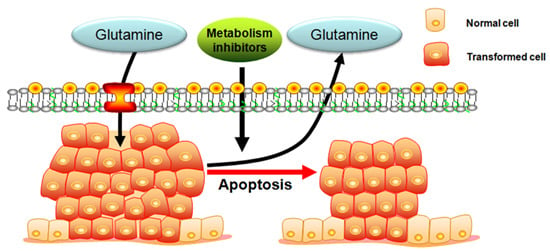
Targeting Glutamine Induces Apoptosis: A Cancer Therapy Approach
Abstract
:1. Introduction
2. Glutamine Metabolism
2.1. Glutamine Metabolism in Normal Tissue

2.2. Glutamine Metabolism in Cancers

2.3. Regulation of Glutamine Metabolism in Cancers
2.3.1. MYC in Glutamine Metabolism in Cancers
2.3.2. p53 in Glutamine Metabolism in Cancers
2.3.3. Ras in Glutamine Metabolism in Cancers
2.3.4. Hypoxia-Inducible Factor (HIF) in Glutamine Metabolism in Cancers
3. Targeting Glutamine Induces Apoptosis in the Cancer Therapy
3.1. Glutamine Deprivation

3.2. Restriction of Glutamine Uptake
| Compound | Target | References |
|---|---|---|
| BPTES | GLS1 | [84,85,86,87,88,89] |
| 968 | GAC | [85,90,91,92,93] |
| CB-839 | GLS1 | [15,94] |
| Ebselen | GLS1, GLS2 | [95] |
| Chelerythrine | GLS1, GLS2 | [95] |
| Apomorphine | GLS1, GLS2 | [95] |
| DON | Glutamine antagonist | [96,97,98,99] |
| Acivicin | γ-Glutamyl transpeptidase glutamine amidotransferase; Glutamine antagonist | [99,100,101,102] |
| BCH | Glutamine transporter (SLC7A5) | [79,80] |
| α-Methyl-dl-tryptophan | Glutamine transporter (SLC6A14) | [103,104] |
| Tamoxifen | Glutamine transporter (ASCT2) | [77] |
| Raloxifene | Glutamine transporter (ASCT2) | [77] |
| GPNA | Glutamine transporter (ASCT2) | [2,77] |
| EGCG | GDH | [105,106,107] |
| l-Asparaginase | Glutamine | [81,108,109] |
| Phenylacetate | Glutamine | [110,111] |
3.3. Inhibition of Glutaminase
3.3.1. Glutaminase (GLS)
3.3.2. Regulators of GLS
3.3.3. GLS1 Inhibitors in Cancer Therapy
BPTES (Bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3)
968 (5-(3-Bromo-4-(dimethylamino)phenyl)-2,2-dimethyl-2,3,5,6-tetrahydrobenzo[a]phenanthridin-4(1H)-one)
CB-839 (N-(5-(4-(6-((2-(3-(Trifluoromethoxy)phenyl)acetyl)amino)-3-pyridazinyl)butyl)-1,3,4-thiadiazol-2-yl)-2-pyridineacetamide)
3.3.4. GLS2 in Cancer Therapy
4. Conclusions
5. Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 17AGG | 17-Allylaminogeldanamycin |
| 968 | 5-(3-Bromo-4-(dimethylamino)phenyl)-2,2-dimethyl-2,3,5,6-tetrahydrobenzo[a]phenanthridin-4(1H)-one |
| α-KG | α-Ketoglutarate |
| ABT-199 | Venetoclax |
| ALL | Acute Leukemias |
| Akt | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| ATP | Adenosine-triphosphate |
| ASCT | System ASC amino acid transporters 1 and 2 |
| BAX | Bcl-2 associated X protein |
| BCH | 2-Aminobicyclo-(2,2,1)-heptane-2-carboxylic acid |
| BPTES | Bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 |
| CB-839 | N-(5-(4-(6-((2-(3-(Trifluoromethoxy)phenyl)acetyl)amino)-3-pyridazinyl)butyl)-1,3,4-thiadiazol-2-yl)-2-pyridineacetamide |
| CD95 | Cluster of differentiation 95 |
| Dbl | Diffuse B cell lymphoma |
| DON | 6-Diazo-5-oxo-l-norleucine |
| DR | Death receptor |
| DMAP1 | Dnmt1 associated protein |
| EAA | Essential amino acid |
| EGF | Epidermal Growth Factor |
| ErbB2 | Receptor tyrosine-protein kinase erbB-2 |
| GAC | Enlongated kidney glutaminase variant |
| GADD | Growth arrest and DNA damage-induced genes |
| GBM | Glioblastoma multiforme |
| GCL | Glutamate-cysteine ligase |
| GCS | Glutamylcysteine synthetase |
| GLS | Glutaminase |
| GLUD | Glutamate dehydrogenase |
| GLUT | Glucose transporters |
| GOT | Aspartate transaminase |
| GPNA | γ-l-Glutamylp-nitroanilide |
| GSH | Glutathione |
| HG | Hydroxyglutarate |
| HIF | Hypoxia-inducible factor |
| HSP | Heat shock protein |
| IDH | Isocitrate dehydrogenase |
| KGA | Kidney glutaminase |
| KGDH | Ketoglutarate dehydrogenase |
| LAT | l-Type amino acid transporters |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| LDHA | Lactate dehydrogenase |
| LGA | Liver glutaminase |
| LKB | Liver kinase B |
| MAPK | Mitogen-activated protein kinase |
| MDM2 | Mouse double minute 2 homolog |
| mTOR | Mammalian target of rapamycin |
| NAC | N-Acetylcysteine |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOXA | Phorbol-12-myristate-13-acetate-induced protein 1 |
| OAA | Oxaloacetic acid |
| PARP | Poly(ADP-ribose) polymerase |
| PC | Pyruvate carboxylase |
| PDK | Pyruvate dehydrogenase kinase |
| PDH | Pyruvate dehydrogenase |
| PI3K | Phosphatidylinositol-3 kinase |
| PP242 | 2-(4-Amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-3-yl)-1H-indol-5-ol |
| PTEN | Phosphatase and tensin homolog |
| PUMA | p53 upregulated modulator of apoptosis |
| ROS | Reactive oxygen species |
| SLC7A5 | Solute carrier family 7 member 5 |
| SMAC | Second mitochondria-derived activator of caspases |
| SN1 | System N amino acid transporter 1 |
| STAT | Signal transducer and activator of transcription 1 |
| TCA | Tricarboxylic acid cycle |
| TNF-α | Tumor necrosis factor-α |
References
- Bergstrom, J.; Furst, P.; Noree, L.O.; Vinnars, E. Intracellular free amino acid concentration in human muscle tissue. J. Appl. Physiol. 1974, 36, 693–697. [Google Scholar]
- Mohamed, A.; Deng, X.; Khuri, F.R.; Owonikoko, T.K. Altered glutamine metabolism and therapeutic opportunities for lung cancer. Clin. Lung Cancer 2014, 15, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.C.; Bode, B.P. Stressing out over survival: Glutamine as an apoptotic modulator. J. Surg. Res. 2006, 131, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.G.; Kim, E.Y.; Kim, T.; Park, H.; Park, H.S.; Choi, E.J.; Kim, S. Glutamine-dependent antiapoptotic interaction of human glutaminyl-tRNA synthetase with apoptosis signal-regulating kinase 1. J. Biol. Chem. 2001, 276, 6030–6036. [Google Scholar] [CrossRef] [PubMed]
- Lora, J.; Alonso, F.J.; Segura, J.A.; Lobo, C.; Marquez, J.; Mates, J.M. Antisense glutaminase inhibition decreases glutathione antioxidant capacity and increases apoptosis in Ehrlich ascitic tumour cells. Eur. J. Biochem. 2004, 271, 4298–4306. [Google Scholar] [CrossRef]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Rajendram, D.R.; Preedy, V.R.; Patel, V.B. Glutamine in Clinical Nutrition; Springer: Berlin, Germany, 2014. [Google Scholar]
- Daye, D.; Wellen, K.E. Metabolic reprogramming in cancer: Unraveling the role of glutamine in tumorigenesis. Semin. Cell Dev. Biol. 2012, 23, 362–369. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2009, 29, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, A.V.; Waters, A.H.; Golubeva, A.V.; Dmitriev, R.I.; Papkovsky, D.B. Availability of the key metabolic substrates dictates the respiratory response of cancer cells to the mitochondrial uncoupling. Biochim. Biophys. Acta 2014, 1837, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Herrera, K.N.; Lee, J.; Haigis, M.C. Intersections between mitochondrial sirtuin signaling and tumor cell metabolism. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.I.; Demo, S.D.; Dennison, J.B.; Chen, L.; Chernov-Rogan, T.; Goyal, B.; Janes, J.R.; Laidig, G.J.; Lewis, E.R.; Li, J.; et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther. 2014, 13, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Qing, G.; Li, B.; Vu, A.; Skuli, N.; Walton, Z.E.; Liu, X.; Mayes, P.A.; Wise, D.R.; Thompson, C.B.; Maris, J.M. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell 2012, 22, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A.; Sánchez-Jiménez, F.; Márquez, J.; Quesada, A.R.; de Castro Núñez, I. Relevance of glutamine metabolism to tumor cell growth. Mol. Cell. Biochem. 1992, 113, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.C.; Bode, B.P. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime? Semin. Cancer Biol. 2005, 15, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S. Adaptation of plasma membrane amino acid transport mechanisms to physiological demands. Pflügers Arch. 2002, 444, 457–466. [Google Scholar] [PubMed]
- Dolińska, M.; Dybel, A.; Zabłocka, B.; Albrecht, J. Glutamine transport in C6 glioma cells shows ASCT2 system characteristics. Neurochem. Int. 2003, 43, 501–507. [Google Scholar] [CrossRef]
- Wasa, M.; Wang, H.-S.; Okada, A. Characterization of l-glutamine transport by a human neuroblastoma cell line. Am. J. Physiol. Cell Physiol. 2002, 282, C1246–C1253. [Google Scholar] [CrossRef] [PubMed]
- Kron, C.; Bode, B. Glutamine transporter expression profiling reveal major role for ASCT2 and LAT1 in primary and metastatic human hepatocellular carcinoma cells. FASEB J. 2015, 29. [Google Scholar] [CrossRef]
- Shimizu, K.; Kaira, K.; Tomizawa, Y.; Sunaga, N.; Kawashima, O.; Oriuchi, N.; Kana, Y.; Yamada, M.; Oyama, T.; Takeyoshi, I. P0143 ASC amino acid transporter 2 (ASCT2) as a novel prognostic marker in non-small-cell lung cancer. Eur. J. Cancer 2014, 50, e49. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Khelifa, S.; Feng, Y.; Lau, E.; Cardiff, R.; Kim, H.; Rimm, D.L.; Kluger, Y.; Ronai, Z.E. RNF5 mediates ER stress-induced degradation of SLC1A5 in breast cancer. Cancer Res. 2014, 74, 2440–2440. [Google Scholar] [CrossRef]
- Cheng, T.; Sudderth, J.; Yang, C.; Mullen, A.R.; Jin, E.S.; Matés, J.M.; DeBerardinis, R.J. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc. Natl. Acad. Sci. 2011, 108, 8674–8679. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V.; O’Donnell, K.A.; Zeller, K.I.; Nguyen, T.; Osthus, R.C.; Li, F. The c-Myc target gene network. Semin. Cancer Biol. 2006, 16, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Lutz, W.; Stöhr, M.; Schürmann, J.; Wenzel, A.; Löhr, A.; Schwab, M. Conditional expression of N-myc in human neuroblastoma cells increases expression of α-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene 1996, 13, 803–812. [Google Scholar] [PubMed]
- Vita, M.; Henriksson, M. The Myc oncoprotein as a therapeutic target for human cancer. Semin. Cancer Biol. 2006, 16, 318–330. [Google Scholar] [CrossRef]
- Rudolph, C.; Adam, G.; Simm, A. Determination of copy number of c-Myc protein per cell by quantitative Western blotting. Anal. Biochem. 1999, 269, 66–71. [Google Scholar] [PubMed]
- Wise, D.R.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.-Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Tchernyshyov, I.; Chang, T.-C.; Lee, Y.-S.; Kita, K.; Ochi, T.; Zeller, K.I.; de Marzo, A.M.; van Eyk, J.E.; Mendell, J.T. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009, 458, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Yuneva, M.O.; Fan, T.W.; Allen, T.D.; Higashi, R.M.; Ferraris, D.V.; Tsukamoto, T.; Matés, J.M.; Alonso, F.J.; Wang, C.; Seo, Y. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012, 15, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Veas-Perez de Tudela, M.; Delgado-Esteban, M.; Cuende, J.; Bolanos, J.P.; Almeida, A. Human neuroblastoma cells with MYCN amplification are selectively resistant to oxidative stress by transcriptionally up-regulating glutamate cysteine ligase. J. Neurochem. 2010, 113, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Westermark, U.K.; Wilhelm, M.; Frenzel, A.; Henriksson, M.A. The MYCN oncogene and differentiation in neuroblastoma. Semin. Cancer Biol. 2011, 21, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Felsher, D.W. MYC inactivation elicits oncogene addiction through both tumor cell-intrinsic and host-dependent mechanisms. Genes Cancer 2010, 1, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Kruspig, B.; Nilchian, A.; Bejarano, I.; Orrenius, S.; Zhivotovsky, B.; Gogvadze, V. Targeting mitochondria by α-tocopheryl succinate kills neuroblastoma cells irrespective of MycN oncogene expression. Cell. Mol. Life Sci. 2012, 69, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Takenobu, H.; Ohira, M.; Nakazawa, A.; Yoshida, S.; Akita, N.; Shimozato, O.; Iwama, A.; Nakagawara, A.; Kamijo, T. Novel 1p tumour suppressor Dnmt1-associated protein 1 regulates MYCN/ataxia telangiectasia mutated/p53 pathway. Eur. J. Cancer 2014, 50, 1555–1565. [Google Scholar] [CrossRef]
- Jackson, J.; Lozano, G. The mutant p53 mouse as a pre-clinical model. Oncogene 2013, 32, 4325–4330. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2002, 2, 103–112. [Google Scholar] [CrossRef]
- Nieminen, A.I.; Eskelinen, V.M.; Haikala, H.M.; Tervonen, T.A.; Yan, Y.; Partanen, J.I.; Klefstrom, J. Myc-induced AMPK-phospho p53 pathway activates Bak to sensitize mitochondrial apoptosis. Proc. Natl. Acad. Sci. USA 2013, 110, E1839–E1848. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.; Levine, A. p53 Research: The past thirty years and the next thirty years. Cold Spring Harb. Perspect. Biol. 2010, 2, a000893. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, C.; Wu, R.; Sun, Y.; Levine, A.; Feng, Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc. Natl. Acad. Sci. USA 2010, 107, 7455–7460. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Tanaka, T.; Poyurovsky, M.V.; Nagano, H.; Mayama, T.; Ohkubo, S.; Lokshin, M.; Hosokawa, H.; Nakayama, T.; Suzuki, Y. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc. Natl. Acad. Sci. USA 2010, 107, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.-J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.; Wang, Y.Z.; Gout, P.W. The xc- cystine/glutamate antiporter: A potential target for therapy of cancer and other diseases. J. Cell. Physiol. 2008, 215, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Puzio-Kuter, A.M. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 2010, 330, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, D.; Metallo, C.M.; Gameiro, P.A.; Hiller, K.; Danna, L.S.; Balestrieri, C.; Alberghina, L.; Stephanopoulos, G.; Chiaradonna, F.; et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol. Syst. Biol. 2011, 7, 523. [Google Scholar] [CrossRef] [PubMed]
- White, E. Exploiting the bad eating habits of Ras-driven cancers. Genes Dev. 2013, 27, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Lyssiotis, C.A.; Ying, H.; Wang, X.; Hua, S.; Ligorio, M.; Perera, R.M.; Ferrone, C.R.; Mullarky, E.; Shyh-Chang, N. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013, 496, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, D.; Soldati, C.; Vanoni, M.; Alberghina, L.; Chiaradonna, F. Glutamine deprivation induces abortive s-phase rescued by deoxyribonucleotides in k-ras transformed fibroblasts. PLoS ONE 2009, 4, e4715. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Russo, J. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim. Biophys. Acta 2012, 1826, 370–384. [Google Scholar] [PubMed]
- MacKenzie, E.D.; Selak, M.A.; Tennant, D.A.; Payne, L.J.; Crosby, S.; Frederiksen, C.M.; Watson, D.G.; Gottlieb, E. Cell-permeating α-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol. Cell. Biol. 2007, 27, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Lane, A.N.; Hamaker, M.; Bose, S.; Gouw, A.; Barbi, J.; Tsukamoto, T.; Rojas, C.J.; Slusher, B.S.; Zhang, H. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012, 15, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Butler, E.; Tan, M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013, 4, e532. [Google Scholar] [CrossRef] [PubMed]
- Cassago, A.; Ferreira, A.P.; Ferreira, I.M.; Fornezari, C.; Gomes, E.R.; Greene, K.S.; Pereira, H.M.; Garratt, R.C.; Dias, S.M.; Ambrosio, A.L. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc. Natl. Acad. Sci. USA 2012, 109, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.C.; Denko, N.C. Hypoxic regulation of glutamine metabolism through HIF 1 and SIAH2 supports lipid synthesis that is necessary fot tumor growth. Cell Metab. 2014, 19, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Metallo, C.M.; Gameiro, P.A.; Bell, E.L.; Mattaini, K.R.; Yang, J.; Hiller, K.; Jewell, C.M.; Johnson, Z.R.; Irvine, D.J.; Guarente, L.; et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2012, 481, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Vincent, E.E.; Griss, T.; Samborska, B.; Izreig, S.; Svensson, R.U.; Mamer, O.A.; Avizonis, D.; Shackelford, D.B.; Shaw, R.J.; et al. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1α. Proc. Natl. Acad. Sci. USA 2014, 111, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Pathways from glutamine to apoptosis. Front. Biosci. 2006, 11, 3164–3180. [Google Scholar] [CrossRef] [PubMed]
- Petronini, P.G.; Urbani, S.; Alfieri, R.; Borghetti, A.F.; Guidotti, G.G. Cell susceptibility to apoptosis by glutamine deprivation and rescue: Survival and apoptotic death in cultured lymphoma-leukemia cell lines. J. Cell. Physiol. 1996, 169, 175–185. [Google Scholar] [CrossRef]
- Oehler, R.; Pusch, E.; Dungel, P.; Zellner, M.; Eliasen, M.M.; Brabec, M.; Roth, E. Glutamine depletion impairs cellular stress response in human leucocytes. Br. J. Nutr. 2002, 87, S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Jäättelä, M.; Wissing, D.; Kokholm, K.; Kallunki, T.; Egeblad, M. Hsp70 exerts its anti-apoptotic function downstream of caspase 3-like proteases. EMBO J. 1998, 17, 6124–6134. [Google Scholar] [CrossRef] [PubMed]
- Paquette, J.C.; Guérin, P.J.; Gauthier, E.R. Rapid induction of the intrinsic apoptotic pathway by l-glutamine starvation. J. Cell. Physiol. 2005, 202, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.C.; Perez, J.C.; Suetterlin, J.E.; Chaudhry, S.B.; Bode, B.P. Inducible antisense RNA targeting amino acid transporter ATB0/ASCT2 elicits apoptosis in human hepatoma cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G467–G478. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.M.; Sontheimer, H. Glutamate transporters in the biology of malignant gliomas. Cell. Mol. Life Sci. 2014, 71, 1839–1854. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.L.; Carretero, J.; Obrador, E.; Gambini, J.; Asensi, M.; Rodilla, V.; Estrela, J.M. Tumor cytotoxicity by endothelial cells impairment of the mitochondrial system for glutathione uptake in mouse b16 melanoma cells that survive after in vitrointeraction with the hepatic sinusoidal endothelium. J. Biol. Chem. 2003, 278, 13888–13897. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.A.; Wang, W.-I.; Rosales, K.R.; Welliver, M.X.; Pan, M.; Kong, M. The B55α subunit of PP2A drives a p53-dependent metabolic adaptation to glutamine deprivation. Mol. Cell 2013, 50, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999, 27, 922–935. [Google Scholar] [CrossRef]
- Cardaci, S.; Rizza, S.; Filomeni, G.; Bernardini, R.; Bertocchi, F.; Mattei, M.; Paci, M.; Rotilio, G.; Ciriolo, M.R. Glutamine deprivation enhances antitumor activity of 3-bromopyruvate through the stabilization of monocarboxylate transporter-1. Cancer Res. 2012, 72, 4526–4536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Cai, M.-Y.; Ma, K.; Yang, J.; Zhou, J.; Fu, W.; Wei, F.-Z.; Wang, L.; Xie, D. XBP-1u suppresses autophagy by promoting the degradation of FoxO1 in cancer cells. Cell Res. 2013, 23, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Abcouwer, S.F.; Schwarz, C.; Meguid, R.A. Glutamine deprivation induces the expression of GADD45 and GADD153 primarily by mRNA stabilization. J. Biol. Chem. 1999, 274, 28645–28651. [Google Scholar] [CrossRef] [PubMed]
- Desideri, E.; Ciriolo, M.R. Glutamine addiction of cancer cells. In Glutamine in Clinical Nutrition; Springer: Berlin, Germany, 2015; pp. 99–111. [Google Scholar]
- Sidoryk, M.; Matyja, E.; Dybel, A.; Zielinska, M.; Bogucki, J.; Jaskolski, D.J.; Liberski, P.P.; Kowalczyk, P.; Albrecht, J. Increased expression of a glutamine transporter SNAT3 is a marker of malignant gliomas. Neuroreport 2004, 15, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.K.; Kaufmann, Y.; Luo, S.; Klimberg, V.S. Tamoxifen and raloxifene suppress the proliferation of estrogen receptor-negative cells through inhibition of glutamine uptake. Cancer Chemother. Pharmacol. 2011, 67, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hardie, R.A.; Hoy, A.J.; van Geldermalsen, M.; Gao, D.; Fazli, L.; Sadowski, M.C.; Balaban, S.; Schreuder, M.; Nagarajah, R.; et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 2015, 236, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Kaira, K.; Oriuchi, N.; Shimizu, K.; Tominaga, H.; Yanagitani, N.; Sunaga, N.; Ishizuka, T.; Nagamori, S.; Promchan, K. Inhibition of l-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010, 30, 4819–4828. [Google Scholar] [PubMed]
- Janpipatkul, K.; Suksen, K.; Borwornpinyo, S.; Jearawiriyapaisarn, N.; Hongeng, S.; Piyachaturawat, P.; Chairoungdua, A. Downregulation of LAT1 expression suppresses cholangiocarcinoma cell invasion and migration. Cell Signal. 2014, 26, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, L.A.; Holton, T.; Yuneva, M.; Louie, R.J.; Padro, M.; Daemen, A.; Hu, M.; Chan, D.A.; Ethier, S.P.; van’t Veer, L.J.; et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 2013, 24, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Goossens, V.; Grooten, J.; Fiers, W. The oxidative metabolism of glutamine A modulator of reactive oxygen intermediate-mediated cytotoxicity of tumor necrosis factor in L929 fibrosarcoma cells. J. Biol. Chem. 1996, 271, 192–196. [Google Scholar] [PubMed]
- Liu, S.-L.; Shi, D.-Y.; Shen, Z.-H.; Wu, Y.-D. Effects of glutamine on tumor growth and apoptosis of hepatoma cells. Acta Pharmacol. Sin. 2000, 21, 668–672. [Google Scholar] [PubMed]
- Hartwick, E.W.; Curthoys, N.P. BPTES inhibition of hGA124–551, a truncated form of human kidney-type glutaminase. J. Enzym. Inhib. Med. Chem. 2012, 27, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Katt, W.P.; Cerione, R.A. Glutaminase regulation in cancer cells: A druggable chain of events. Drug Discov. Today 2014, 19, 450–457. [Google Scholar] [CrossRef] [PubMed]
- DeLaBarre, B.; Gross, S.; Fang, C.; Gao, Y.; Jha, A.; Jiang, F.; Song, J.J.; Wei, W.; Hurov, J.B. Full-length human glutaminase in complex with an allosteric inhibitor. Biochemistry 2011, 50, 10764–10770. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, K.; Pan, C.Q.; Karlberg, T.; Balaji, G.; Uttamchandani, M.; Suresh, V.; Schüler, H.; Low, B.C.; Sivaraman, J. Structural basis for the allosteric inhibitory mechanism of human kidney-type glutaminase (KGA) and its regulation by Raf-Mek-Erk signaling in cancer cell metabolism. Proc. Natl. Acad. Sci. USA 2012, 109, 7705–7710. [Google Scholar] [CrossRef] [PubMed]
- Kvamme, E.; Nissen-Meyer, L.S.H.; Roberg, B.Å.; Torgner, I.A. Novel form of phosphate activated glutaminase in cultured astrocytes and human neuroblastoma cells, PAG in brain pathology and localization in the mitochondria. Neurochem. Res. 2008, 33, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, K.; Chong, Q.Y.; Low, B.C.; Sivaraman, J. Structural basis for the active site inhibition mechanism of human kidney-type glutaminase (KGA). Sci. Rep. 2014, 4, 3827. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-B.; Erickson, J.W.; Fuji, R.; Ramachandran, S.; Gao, P.; Dinavahi, R.; Wilson, K.F.; Ambrosio, A.L.; Dias, S.M.; Dang, C.V. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 2010, 18, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.W.; Cerione, R.A. Glutaminase: A hot spot for regulation of cancer cell metabolism? Oncotarget 2010, 1, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Stalnecker, C.; Erickson, J.; Ramachandran, S.; DeBerardinis, R.; Cerione, R. Abstract C42: Mitochondrial glutaminase regulation and small molecule inhibition of glutamine metabolism. Cancer Res. 2013, 73, C42. [Google Scholar] [CrossRef]
- Wilson, K.F.; Erickson, J.W.; Antonyak, M.A.; Cerione, R.A. Rho GTPases and their roles in cancer metabolism. Trends Mol. Med. 2013, 19, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Matre, P.; Shariati, M.; Velez, J.; Qi, Y.; Konoplev, S.; Su, X.; DiNardo, C.D.; Daver, N.; Majeti, R.; Andreeff, M. Efficacy of novel glutaminase inhibitor CB-839 in acute myeloid leukemia. Blood 2014, 124, 3763–3763. [Google Scholar]
- Thomas, A.G.; Rojas, C.; Tanega, C.; Shen, M.; Simeonov, A.; Boxer, M.B.; Auld, D.S.; Ferraris, D.V.; Tsukamoto, T.; Slusher, B.S. Kinetic characterization of ebselen, chelerythrine and apomorphine as glutaminase inhibitors. Biochem. Biophys. Res. Commun. 2013, 438, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Crosby, H.; Ihnat, M.; Miller, K. Evaluating the toxicity of the analgesic glutaminase inhibitor 6-Diazo-5-oxo-l-norleucine in vitro and on rat dermal skin fibroblasts. MOJ Toxicol. 2015. [Google Scholar] [CrossRef]
- Yoshioka, K.; Takehara, H.; Okada, A.; Komi, N. Glutamine antagonist with diet deficient in glutamine and aspartate reduce tumor growth. Tokushima J. Exp. Med. 1992, 39, 69–76. [Google Scholar] [PubMed]
- Shapiro, R.A.; Clark, V.M.; Curthoys, N.P. Inactivation of rat renal phosphate-dependent glutaminase with 6-diazo-5-oxo-l-norleucine. Evidence for interaction at the glutamine binding site. J. Biol. Chem. 1979, 254, 2835–2838. [Google Scholar] [PubMed]
- Rosenfeld, H.; Roberts, J. Enhancement of antitumor activity of glutamine antagonists 6-diazo-5-oxo-l-norleucine and acivicin in cell culture by glutaminase-asparaginase. Cancer Res. 1981, 41, 1324–1328. [Google Scholar] [PubMed]
- Maeda, K.; Nakajima, Y.; Motoyama, T.; Kitou, Y.; Kosaki, T.; Saito, T.; Nishiuchi, T.; Kanamaru, K.; Osada, H.; Kobayashi, T.; et al. Effects of acivicin on growth, mycotoxin production and virulence of phytopathogenic fungi. Lett. Appl. Microbiol. 2014, 59, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; Meck, R.; Yunis, A. The inhibition of γ-glutamyl transpeptidase from human pancreatic carcinoma cells by (α S,5S)-α-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid (AT-125; NSC-163501). Res. Commun. Chem. Pathol. Pharmacol. 1980, 27, 175–182. [Google Scholar] [PubMed]
- Hidalgo, M.; Rodriguez, G.; Kuhn, J.G.; Brown, T.; Weiss, G.; MacGovren, J.P.; von Hoff, D.D.; Rowinsky, E.K. A Phase I and pharmacological study of the glutamine antagonist acivicin with the amino acid solution aminosyn in patients with advanced solid malignancies. Clin. Cancer Res. 1998, 4, 2763–2770. [Google Scholar] [PubMed]
- Karunakaran, S.; Ramachandran, S.; Coothankandaswamy, V.; Elangovan, S.; Babu, E.; Periyasamy-Thandavan, S.; Gurav, A.; Gnanaprakasam, J.P.; Singh, N.; Schoenlein, P.V.; et al. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J. Biol. Chem. 2011, 286, 31830–31838. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.B.; Zhao, Y.; Muñoz-Pinedo, C.; Lu, J.; Tan, M. Stalling the engine of resistance: Targeting cancer metabolism to overcome therapeutic resistance. Cancer Res. 2013, 73, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Whitelaw, B.S.; Robinson, M.B. Inhibitors of glutamate dehydrogenase block sodium-dependent glutamate uptake in rat brain membranes. Front. Endocrinol. 2013, 4, 123. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ko, B.; Hensley, C.T.; Jiang, L.; Wasti, A.T.; Kim, J.; Sudderth, J.; Calvaruso, M.A.; Lumata, L.; Mitsche, M.; et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol. Cell 2014, 56, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, M.; Chen, P.; Narayan, S.; Matschinsky, F.M.; Bennett, M.J.; Stanley, C.A.; Smith, T.J. Green tea polyphenols control dysregulated glutamate dehydrogenase in transgenic mice by hijacking the ADP activation site. J. Biol. Chem. 2011, 286, 34164–34174. [Google Scholar] [CrossRef] [PubMed]
- Willems, L.; Jacque, N.; Jacquel, A.; Neveux, N.; Maciel, T.T.; Lambert, M.; Schmitt, A.; Poulain, L.; Green, A.S.; Uzunov, M.; et al. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood 2013, 122, 3521–3532. [Google Scholar] [CrossRef] [PubMed]
- Avramis, V.I.; Martin-Aragon, S.; Avramis, E.V.; Asselin, B.L. Pharmacoanalytical assays of Erwinia asparaginase (erwinase) and pharmacokinetic results in high-risk acute lymphoblastic leukemia (HR ALL) patients: Simulations of erwinase population PK-PD models. Anticancer Res. 2007, 27, 2561–2572. [Google Scholar] [PubMed]
- Jover-Cobos, M.; Noiret, L.; Lee, K.; Sharma, V.; Habtesion, A.; Romero-Gomez, M.; Davies, N.; Jalan, R. Ornithine phenylacetate targets alterations in the expression and activity of glutamine synthase and glutaminase to reduce ammonia levels in bile duct ligated rats. J. Hepatol. 2014, 60, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Ytrebo, L.M.; Kristiansen, R.G.; Maehre, H.; Fuskevag, O.M.; Kalstad, T.; Revhaug, A.; Cobos, M.J.; Jalan, R.; Rose, C.F. L-ornithine phenylacetate attenuates increased arterial and extracellular brain ammonia and prevents intracranial hypertension in pigs with acute liver failure. Hepatology 2009, 50, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.G.; O’Driscoll, C.M.; Bressler, J.; Kaufmann, W.; Rojas, C.J.; Slusher, B.S. Small molecule glutaminase inhibitors block glutamate release from stimulated microglia. Biochem. Biophys. Res. Commun. 2014, 443, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Segura, J.A.; Martín-Rufián, M.; Campos-Sandoval, J.A.; Alonso, F.J.; Márquez, J. Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer. Curr. Mol. Med. 2013, 13, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Elgadi, K.M.; Meguid, R.A.; Qian, M.; Souba, W.W.; Abcouwer, S.F. Cloning and analysis of unique human glutaminase isoforms generated by tissue-specific alternative splicing. Physiol. Genom. 1999, 1, 51–62. [Google Scholar]
- Shukla, K.; Ferraris, D.V.; Thomas, A.G.; Stathis, M.; Duvall, B.; Delahanty, G.; Alt, J.; Rais, R.; Rojas, C.; Gao, P. Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl) ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors. J. Med. Chem. 2012, 55, 10551–10563. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Lin, M.; Zhu, W.; Liang, Y.; Hong, X.; Zhao, Y.; Young, K.H.; Hu, W.; Feng, Z. Glutaminase 2 negatively regulates the PI3K/AKT signaling and shows tumor suppression activity in human hepatocellular carcinoma. Oncotarget 2014, 5, 2635–2647. [Google Scholar] [CrossRef] [PubMed]
- Campos-Sandoval, J.; Alonso, F.; Segura, J.; Manzanares, E.; Perez-Gomez, C.; Ruiz-Sánchez, P.; González, M.; Márquez, J.; Matés, J. Co-expression of glutaminase K and L isoenzymes in human tumour cells. Biochem. J. 2005, 386, 535–542. [Google Scholar]
- Lobo, C.; Ruiz-Bellido, M.; Aledo, J.; Marquez, J.; Núñez, D.C.I.; Alonso, F. Inhibition of glutaminase expression by antisense mRNA decreases growth and tumourigenicity of tumour cells. Biochem. J. 2000, 348, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, M.J.; Bennett, B.D.; Joshi, A.D.; Gao, P.; Thomas, A.G.; Ferraris, D.V.; Tsukamoto, T.; Rojas, C.J.; Slusher, B.S.; Rabinowitz, J.D. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010, 70, 8981–8987. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Stine, Z.E.; Xia, J.; Lu, Y.; O’Connor, R.S.; Altman, B.J.; Hsieh, A.L.; Gouw, A.M.; Thomas, A.G.; Gao, P.; et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J. Clin. Investig. 2015, 125, 2293–306. [Google Scholar] [CrossRef] [PubMed]
- Campos-Sandoval, J.A.; de la Oliva, A.R.L.; Lobo, C.; Segura, J.A.; Matés, J.M.; Alonso, F.J.; Márquez, J. Expression of functional human glutaminase in baculovirus system: Affinity purification, kinetic and molecular characterization. Int. J. Biochem. Cell Biol. 2007, 39, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Engler, J.; Gottesman, J.; Harkins, J.; Urazaev, A.; Lieberman, E.; Grossfeld, R. Properties of glutaminase of crayfish CNS: Implications for axon-glia signaling. Neuroscience 2002, 114, 699–705. [Google Scholar] [CrossRef]
- Velletri, T.; Romeo, F.; Tucci, P.; Peschiaroli, A.; Annicchiarico-Petruzzelli, M.; Niklison-Chirou, M.V.; Amelio, I.; Knight, R.A.; Mak, T.W.; Melino, G. GLS2 is transcriptionally regulated by p73 and contributes to neuronal differentiation. Cell Cycle 2013, 12, 3564–3573. [Google Scholar] [CrossRef] [PubMed]
- Qie, S.; Chu, C.; Li, W.; Wang, C.; Sang, N. ErbB2 activation upregulates glutaminase 1 expression which promotes breast cancer cell proliferation. J. Cell. Biochem. 2014, 115, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.R.; Lane, A.N.; Robertson, B.; Kemp, S.; Liu, Y.; Hill, B.G.; Dean, D.C.; Clem, B.F. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene 2014, 33, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, Y.; Tian, C.; Taylor, L.; Curthoys, N.; Wang, Y.; Vernon, H.; Zheng, J. Interferon-α regulates glutaminase 1 promoter through STAT1 phosphorylation: Relevance to HIV-1 associated neurocognitive disorders. PLoS ONE 2012, 7, e32995. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Xiao, C.; Finley, L.W.; Lahusen, T.; Souza, A.L.; Pierce, K.; Li, Y.-H.; Wang, X.; Laurent, G.; German, N.J. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 2013, 23, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Jun, S.A.; Tsukamoto, T.; Fathi, A.T.; Minden, M.D.; Dang, C.V. Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations. Exp. Hematol. 2014, 42, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Le, A.; Vander Jagt, D.L.; Tsukamoto, T.; Martinez, G.V.; Dang, C.V.; Gillies, R.J. Evaluation of LDH-A and glutaminase inhibition in vivo by hyperpolarized 13C-pyruvate magnetic resonance spectroscopy of tumors. Cancer Res. 2013, 73, 4190–4195. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Csibi, A.; Yang, S.; Hoffman, G.R.; Li, C.; Zhang, E.; Yu, J.J.; Blenis, J. Synthetic lethality of combined glutaminase and Hsp90 inhibition in mTORC1-driven tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, E21–E29. [Google Scholar] [CrossRef] [PubMed]
- Katt, W.P.; Antonyak, M.A.; Cerione, R.A. Simultaneously targeting tissue transglutaminase and kidney type glutaminase sensitizes cancer cells to acid toxicity and offers new opportunities for therapeutic intervention. Mol. Pharm. 2014, 12, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Simpson, N.E.; Tryndyak, V.P.; Pogribna, M.; Beland, F.A.; Pogribny, I.P. Modifying metabolically sensitive histone marks by inhibiting glutamine metabolism affects gene expression and alters cancer cell phenotype. Epigenetics 2012, 7, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sasayama, T.; Irino, Y.; Takata, K.; Nagashima, H.; Masui, K.; Gini, B.; Yang, H.; Mischel, P.S.; Kohmura, E. NT-39glutaminase-mediated metabolic pathway involves glioblastoma resistance to mtor-targeted therapies. Neuro-Oncology 2014, 16, v167. [Google Scholar] [CrossRef]
- Tanaka, K.; Sasayama, T.; Irino, Y.; Takata, K.; Nagashima, H.; Satoh, N.; Kyotani, K.; Mizowaki, T.; Imahori, T.; Ejima, Y. Compensatory glutamine metabolism promotes glioblastoma resistance to mTOR inhibitor treatment. J. Clin. Investig. 2015, 125, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Terunuma, A.; Putluri, N.; Mishra, P.; Mathé, E.A.; Dorsey, T.H.; Yi, M.; Wallace, T.A.; Issaq, H.J.; Zhou, M.; Killian, J.K. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J. Clin. Investig. 2014, 124, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Santana, S.M.; Antonyak, M.A.; Cerione, R.A.; Kirby, B.J. Cancerous epithelial cell lines shed extracellular vesicles with a bimodal size distribution that is sensitive to glutamine inhibition. Phys. Biol. 2014, 11, 065001. [Google Scholar] [CrossRef] [PubMed]
- Parlati, F.; Demo, S.; Gross, M.; Janes, J.; Lewis, E.; MacKinnon, A.; Rodriguez, M.; Shwonek, P.J.; Wang, T.; Yang, J. CB-839, a novel potent and selective glutaminase inhibitor, has broad antiproliferative activity in cell lines derived from both solid tumors and hematological malignancies. Cancer Res. 2014, 74, 1416–1416. [Google Scholar] [CrossRef]
- Das, D.S.; Ravillah, D.; Ray, A.; Song, Y.; Munshi, N.C.; Richardson, P.G.; Chauhan, D.; Anderson, K.C. Anti-myeloma activity of a novel glutaminase inhibitor CB-839. Blood 2014, 124, 3439–3439. [Google Scholar]
- Jacque, N.; Ronchetti, A.M.; Larrue, C.; Meunier, G.; Birsen, R.; Willems, L.; Saland, E.; Decroocq, J.; Maciel, T.T.; Lambert, M. Targeting glutaminolysis has anti-leukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood 2015, 126, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Xie, G.; Liu, C.; Zhou, J.; Chen, J.; Yu, S.; Li, J.; Pang, X.; Shi, H.; Liang, H. Knock-down of glutaminase 2 expression decreases glutathione, NADH, and sensitizes cervical cancer to ionizing radiation. Biochim. Biophys. Acta 2013, 1833, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Z.; Yang, C.W.; Chang, H.Y.; Hsu, H.Y.; Chen, I.S.; Chang, H.S.; Lee, C.H.; Lee, J.C.; Kumar, C.R.; Qiu, Y.Q.; et al. Discovery of selective inhibitors of Glutaminase-2, which inhibit mTORC1, activate autophagy and inhibit proliferation in cancer cells. Oncotarget 2014, 5, 6087–6101. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V.; Le, A.; Gao, P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin. Cancer Res. 2009, 15, 6479–6483. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Cui, H. Targeting Glutamine Induces Apoptosis: A Cancer Therapy Approach. Int. J. Mol. Sci. 2015, 16, 22830-22855. https://doi.org/10.3390/ijms160922830
Chen L, Cui H. Targeting Glutamine Induces Apoptosis: A Cancer Therapy Approach. International Journal of Molecular Sciences. 2015; 16(9):22830-22855. https://doi.org/10.3390/ijms160922830
Chicago/Turabian StyleChen, Lian, and Hengmin Cui. 2015. "Targeting Glutamine Induces Apoptosis: A Cancer Therapy Approach" International Journal of Molecular Sciences 16, no. 9: 22830-22855. https://doi.org/10.3390/ijms160922830
APA StyleChen, L., & Cui, H. (2015). Targeting Glutamine Induces Apoptosis: A Cancer Therapy Approach. International Journal of Molecular Sciences, 16(9), 22830-22855. https://doi.org/10.3390/ijms160922830