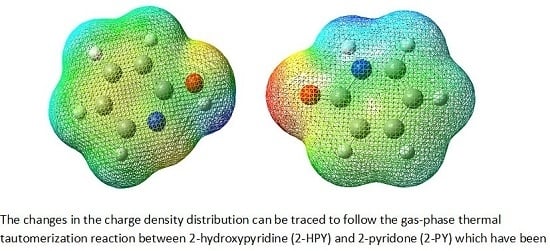
The Thermodynamic and Kinetic Properties of 2-Hydroxypyridine/2-Pyridone Tautomerization: A Theoretical and Computational Revisit
Abstract
Share and Cite
Hejazi, S.A.; Osman, O.I.; Alyoubi, A.O.; Aziz, S.G.; Hilal, R.H. The Thermodynamic and Kinetic Properties of 2-Hydroxypyridine/2-Pyridone Tautomerization: A Theoretical and Computational Revisit. Int. J. Mol. Sci. 2016, 17, 1893. https://doi.org/10.3390/ijms17111893
Hejazi SA, Osman OI, Alyoubi AO, Aziz SG, Hilal RH. The Thermodynamic and Kinetic Properties of 2-Hydroxypyridine/2-Pyridone Tautomerization: A Theoretical and Computational Revisit. International Journal of Molecular Sciences. 2016; 17(11):1893. https://doi.org/10.3390/ijms17111893
Chicago/Turabian StyleHejazi, Safiyah A., Osman I. Osman, Abdulrahman O. Alyoubi, Saadullah G. Aziz, and Rifaat H. Hilal. 2016. "The Thermodynamic and Kinetic Properties of 2-Hydroxypyridine/2-Pyridone Tautomerization: A Theoretical and Computational Revisit" International Journal of Molecular Sciences 17, no. 11: 1893. https://doi.org/10.3390/ijms17111893
APA StyleHejazi, S. A., Osman, O. I., Alyoubi, A. O., Aziz, S. G., & Hilal, R. H. (2016). The Thermodynamic and Kinetic Properties of 2-Hydroxypyridine/2-Pyridone Tautomerization: A Theoretical and Computational Revisit. International Journal of Molecular Sciences, 17(11), 1893. https://doi.org/10.3390/ijms17111893