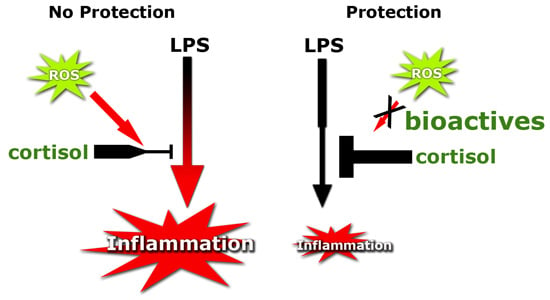
Food-Derived Bioactives Can Protect the Anti-Inflammatory Activity of Cortisol with Antioxidant-Dependent and -Independent Mechanisms
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Effect of Bioactives and Protection against GC Resistance
2.2. Antioxidant Effect of Flavonoid Metabolites and Protection against GC Resistance

| Compound | Abbr. | Antioxidant Effect (%) | Protection Cortisol Response (%) |
|---|---|---|---|
| Flavanol | |||
| (−)-epicatechin | EC | 86 ± 11 | 49 ± 44 |
| 3′-O-methyl(-)-epicatechin | 3′ME | 67 ± 13 | 36 ± 53 |
| 4′-O-methyl(-)-epicatechin | 4′ME | 56 ± 14 | 39 ± 31 |
| (−)-epicatechin-7-O-β-d-glucuronide | E7G | 69 ± 7 | 42 ± 46 |
| 4′-O-methyl(−)-epicatechin-7-O-β-d-glucuronide | 4′ME7G | 39 ± 21 | 35 ± 46 |
| (−)-epicatechin-4′-O-sulfate | E4′S | 27 ± 12 | 41 ± 12 |
| Flavanonol | |||
| (±)-Taxifolin | Tax | 78 ± 3 | 56 ± 11 |
| Flavone | |||
| Chrysin | Chr | 14 ± 2 | 97 ± 9 |
| Flavonol | |||
| 7-mono-O-(β-hydroxyethyl)-rutoside | MH | 88 ± 2 | 71 ± 16 |
| Quercetin | Q | 100 ± 2 | −30 ± 47 |
| 3-O-methyl-quercetin | Q3M | - | 57 ± 6 |
| Quercetin-3-O-glucuronide | Q3G | - | 55 ± 24 |
| Isoflavone | |||
| Genistein | Gen | 83 ± 3 | 92 ± 13 |
| Curcuminoid | |||
| Curcumin | Cur | −4 ± 6 | 100 ± 10 |
| Stilbenoid | |||
| Resveratrol | Res | 57 ± 3 | 99 ± 14 |
| Methylxanthine | |||
| Theophylline | Theo | 11 ± 4 | 69 ± 13 |


3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Intracellular Oxidative Stress
4.4. Determination of Inflammation and GC Resistance
4.5. Statistics
5. Conclusions
References
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [PubMed]
- Heiss, C.; Keen, C.L.; Kelm, M. Flavanols and cardiovascular disease prevention. Eur. Heart J. 2010, 31, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- Weseler, A.R.; Bast, A. Pleiotropic-acting nutrients require integrative investigational approaches: The example of flavonoids. J. Agric. Food Chem. 2012, 60, 8941–8946. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Serafini, M.; Colombi, B.; del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Duenas, M.; Gonzalez-Manzano, S.; Gonzalez-Paramas, A.; Santos-Buelga, C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J. Pharm. Biomed. Anal. 2010, 51, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Weseler, A.R.; Bast, A. Oxidative stress and vascular function: Implications for pharmacologic treatments. Curr. Hypertens. Rep. 2010, 12, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Sundar, I.K.; Yao, H.; Rahman, I. Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases. Antioxid. Redox Signal. 2013, 18, 1956–1971. [Google Scholar] [CrossRef] [PubMed]
- Van der Vliet, A.; Bast, A. Effect of oxidative stress on receptors and signal transmission. Chem. Biol. Interact. 1992, 85, 95–116. [Google Scholar] [CrossRef]
- Rickard, A.J.; Young, M.J. Corticosteroid receptors, macrophages and cardiovascular disease. J. Mol. Endocrinol. 2009, 42, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Adcock, I.M. Glucocorticoid resistance in inflammatory diseases. Lancet 2009, 373, 1905–1917. [Google Scholar] [CrossRef]
- Yang, N.; Ray, D.W.; Matthews, L.C. Current concepts in glucocorticoid resistance. Steroids 2012, 77, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Keenan, C.R.; Salem, S.; Fietz, E.R.; Gualano, R.C.; Stewart, A.G. Glucocorticoid-resistant asthma and novel anti-inflammatory drugs. Drug Discov. Today 2012, 17, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hanazawa, T.; Tomita, K.; Barnes, P.J.; Adcock, I.M. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: Role of tyrosine nitration. Biochem. Biophys. Res. Commun. 2004, 315, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ruijters, E.J.; Haenen, G.R.; Weseler, A.R.; Bast, A. The cocoa flavanol (−)-epicatechin protects the cortisol response. Pharmacol. Res. 2014, 79, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Ruijters, E.J.B.; Haenen, G.R.M.M.; Weseler, A.R.; Bast, A. The anti-inflammatory efficacy of dexamethasone is protected by (−)-epicatechin. PharmaNutrition 2014, 2, 47–52. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patan, F.; Llorach, R.; Garrido, I.; Gomez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Toro-Funes, N.; Cifuentes-Gomez, T.; Cortese-Krott, M.; Heiss, C.; Spencer, J.P. Uptake and metabolism of (−)-epicatechin in endothelial cells. Arch. Biochem. Biophys. 2014, 559, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Wilms, L.C.; Swennen, E.L.; Kleinjans, J.C.; Bast, A.; Haenen, G.R. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition 2008, 24, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Hrelia, S. Quercetin reduces inflammatory responses in LPS-stimulated cardiomyoblasts. Oxid. Med. Cell. Longev. 2012, 2012, 837104. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Li, H.; Schins, R.P.; Duffin, R.; Heemskerk, J.W.; Bast, A.; Haenen, G.R. The quercetin paradox. Toxicol. Appl. Pharmacol. 2007, 222, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, K.J.; Vrolijk, M.F.; Bouwman, F.G.; van der Vijgh, W.J.; Bast, A.; Haenen, G.R. The minor structural difference between the antioxidants quercetin and 4′O-methylquercetin has a major impact on their selective thiol toxicity. Int. J. Mol. Sci. 2014, 15, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Meja, K.K.; Rajendrasozhan, S.; Adenuga, D.; Biswas, S.K.; Sundar, I.K.; Spooner, G.; Marwick, J.A.; Chakravarty, P.; Fletcher, D.; Whittaker, P.; et al. Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. Am. J. Respir. Cell Mol. Biol. 2008, 39, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Cosio, B.G.; Tsaprouni, L.; Ito, K.; Jazrawi, E.; Adcock, I.M.; Barnes, P.J. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J. Exp. Med. 2004, 200, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.L.; Milara, J.; Lluch, J.; de Diego, A.; Sanz, C.; Cortijo, J. Phosphodiesterase-4 inhibition improves corticosteroid insensitivity in pulmonary endothelial cells under oxidative stress. Allergy 2013, 68, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.R. Flavonoids attenuate cardiovascular disease, inhibit phosphodiesterase, and modulate lipid homeostasis in adipose tissue and liver. Exp. Biol. Med. 2006, 231, 1287–1299. [Google Scholar]
- Ejiofor, S.; Turner, A.M. Pharmacotherapies for COPD. Clin. Med. Insights Circ. Respir. Pulm. Med. 2013, 7, 17–34. [Google Scholar] [PubMed]
- Bougarne, N.; Paumelle, R.; Caron, S.; Hennuyer, N.; Mansouri, R.; Gervois, P.; Staels, B.; Haegeman, G.; de Bosscher, K. PPARα blocks glucocorticoid receptor α-mediated transactivation but cooperates with the activated glucocorticoid receptor α for transrepression on NF-κB. Proc. Natl. Acad. Sci. USA 2009, 106, 7397–7402. [Google Scholar] [CrossRef] [PubMed]
- Neher, M.D.; Weckbach, S.; Huber-Lang, M.S.; Stahel, P.F. New insights into the role of peroxisome proliferator-activated receptors in regulating the inflammatory response after tissue injury. PPAR Res. 2012, 2012, 728461. [Google Scholar] [CrossRef] [PubMed]
- Matin, A.; Gavande, N.; Kim, M.S.; Yang, N.X.; Salam, N.K.; Hanrahan, J.R.; Roubin, R.H.; Hibbs, D.E. 7-Hydroxy-benzopyran-4-one derivatives: A novel pharmacophore of peroxisome proliferator-activated receptor α and -γ (PPARα and γ) dual agonists. J. Med. Chem. 2009, 52, 6835–6850. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruijters, E.J.B.; Haenen, G.R.M.M.; Willemsen, M.; Weseler, A.R.; Bast, A. Food-Derived Bioactives Can Protect the Anti-Inflammatory Activity of Cortisol with Antioxidant-Dependent and -Independent Mechanisms. Int. J. Mol. Sci. 2016, 17, 239. https://doi.org/10.3390/ijms17020239
Ruijters EJB, Haenen GRMM, Willemsen M, Weseler AR, Bast A. Food-Derived Bioactives Can Protect the Anti-Inflammatory Activity of Cortisol with Antioxidant-Dependent and -Independent Mechanisms. International Journal of Molecular Sciences. 2016; 17(2):239. https://doi.org/10.3390/ijms17020239
Chicago/Turabian StyleRuijters, Erik J. B., Guido R. M. M. Haenen, Mathijs Willemsen, Antje R. Weseler, and Aalt Bast. 2016. "Food-Derived Bioactives Can Protect the Anti-Inflammatory Activity of Cortisol with Antioxidant-Dependent and -Independent Mechanisms" International Journal of Molecular Sciences 17, no. 2: 239. https://doi.org/10.3390/ijms17020239
APA StyleRuijters, E. J. B., Haenen, G. R. M. M., Willemsen, M., Weseler, A. R., & Bast, A. (2016). Food-Derived Bioactives Can Protect the Anti-Inflammatory Activity of Cortisol with Antioxidant-Dependent and -Independent Mechanisms. International Journal of Molecular Sciences, 17(2), 239. https://doi.org/10.3390/ijms17020239