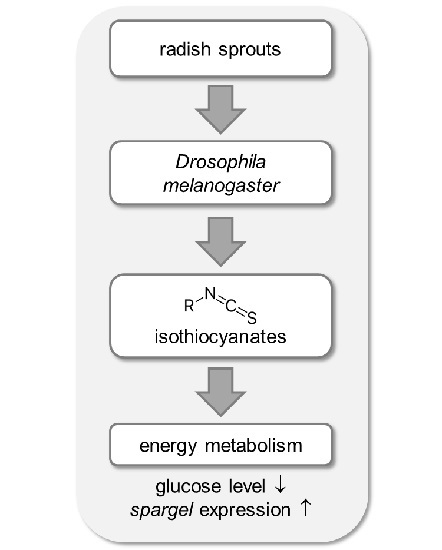
Metabolic Activity of Radish Sprouts Derived Isothiocyanates in Drosophila melanogaster
Abstract
:1. Introduction
2. Results and Discussion
2.1. Glucosinolate and Isothiocyanate Content in Radish Sprouts
2.2. Evaluation of Food Intake and Fitness in Drosophila melanogaster
2.3. Sulforaphene, Sulforaphane, Indole-3-Carbinole, and Sulforaphane-Cysteine Concentrations in Fly Homgenates
2.4. Inhibition of α-Amylase and α-Glucosidase in Vitro by Radish Sprouts
2.5. Energy Metabolism in Drosophila melanogaster
3. Materials and Methods
3.1. Radish Sprout Production
3.2. Analyses of GLS and ITC in Radish Sprouts and Drosophila Melanogaster by HPLC-DAD-ESI-MSn and UHPLC-QqQ-MS/MS
3.3. In Vitro α-Amylase and α-Glucosidase Assay of Radish Sprouts
3.4. Drosophila melanogaster Stocks and Treatment
3.5. Gustatory Assay
3.6. Negative Geotaxis Assay: Climbing Activity
3.7. Glucose Analysis
3.8. Real-Time PCR
3.9. Statistics
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| GLS | glucosinolates |
| ITC | isothiocyanates |
| PGC1α | PPARγ co-activator 1 α |
| PPARγ | peroxisome proliferator activated receptor γ |
| GSH | glutathione |
| GST | glutathione-S-transferase |
| NAC | N-acetyl-cysteine |
| cys | cysteine |
| cys–gly | cysteinylglycine |
| SFE | sulforaphene |
| SFN | sulforaphane |
| RPS | raphasatin |
| SFN–CYS | sulforaphane-cysteine |
| RpL32 | Drosophila melanogaster ribosomal protein L32 |
| Srl | spargel |
References
- Tzioumis, E.; Adair, L.S. Childhood dual burden of under- and overnutrition in low- and middle-income countries: A critical review. Food Nutr. Bull. 2014, 35, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Potential efficacy of broccoli sprouts as a unique supplement for management of type 2 diabetes and its complications. J. Med. Food 2013, 16, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ul-Haq, M.; Cavar, S.; Qayum, M.; Imran, I.; de Feo, V. Compositional studies: Antioxidant and antidiabetic activities of Capparis decidua (Forsk.) Edgew. Int. J. Mol. Sci. 2011, 12, 8846–8861. [Google Scholar] [CrossRef] [PubMed]
- Poornima, J.; Mirunalini, S. Regulation of carbohydrate metabolism by indole-3-carbinol and its metabolite 3,3′-diindolylmethane in high-fat diet-induced C57BL/6J mice. Mol. Cell. Biochem. 2014, 385, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Rajab, A.; Asghari, G.; Azizi, F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Diabetes Res. Clin. Pract. 2012, 96, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Garcia-Viguera, C.; Moreno, D.A. Biotic elicitors effectively increase the glucosinolates content in Brassicaceae sprouts. J. Agric. Food Chem. 2014, 62, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Orlandi, M.; Bartolini, G.; Barillari, J.; Iori, R.; Paolini, M.; Ferroni, F.; Grazia Fumo, M.; Pedulli, G.F.; Valgimigli, L. Cytotoxic and antioxidant activity of 4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L. (kaiware daikon) sprouts. J. Agric. Food Chem. 2008, 56, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, M.B.; Lim, S.B. Formation and stabilization of raphasatin and sulforaphene from radish roots by endogenous enzymolysis. Prev. Nutr. Food Sci. 2015, 20, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.P.; Wang, M.L.; Chan, M.H.; Chiu, Y.S.; Chen, Y.H. Antiobesity activities of indole-3-carbinol in high-fat-diet-induced obese mice. Nutrition 2011, 27, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Jeffery, E. Glucosinolate hydrolysis and bioavailability of resulting isothiocyanates: Focus on glucoraphanin. J. Funct. Foods 2014, 7, 67–76. [Google Scholar] [CrossRef]
- Cartea, E.; Velasco, P. Glucosinolates in brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Wagner, A.E.; Terschluesen, A.M.; Rimbach, G. Health promoting effects of brassica-derived phytochemicals: From chemopreventive and anti-inflammatory activities to epigenetic regulation. Oxidative Med. Cell. Longev. 2013, 2013, 964539. [Google Scholar] [CrossRef] [PubMed]
- Scholl, C.; Eshelman, B.D.; Barnes, D.M.; Hanlon, P.R. Raphasatin is a more potent inducer of the detoxification enzymes than its degradation products. J. Food Sci. 2011, 76, C504–C511. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.H.; Chapple, I.L.; Milward, M.; Grant, M.M.; Hill, E.; Brown, J.; Griffiths, H.R. Sulforaphane restores cellular glutathione levels and reduces chronic periodontitis neutrophil hyperactivity in vitro. PLoS ONE 2013, 8, e66407. [Google Scholar]
- Steele, M.L.; Fuller, S.; Patel, M.; Kersaitis, C.; Ooi, L.; Munch, G. Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol. 2013, 1, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Pandey, K.B.; Abidi, A.B.; Rizvi, S.I. Markers of oxidative stress during diabetes mellitus. J. Biomark. 2013, 2013, 378790. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of maqui (Aristotelia chilensis) and murta (Ugni molinae Turcz.): Sources of antioxidant compounds and α-glucosidase/α-amylase inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Okulicz, M.; Hertig, I.; Chichlowska, J. Effects of indole-3-carbinol on metabolic parameters and on lipogenesis and lipolysis in adipocytes. Czech J. Anim. Sci. 2009, 54, 182–189. [Google Scholar]
- Kassi, E.; Papavassiliou, A.G. Could glucose be a proaging factor? J. Cell. Mol. Med. 2008, 12, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Leontieva, O.V.; Demidenko, Z.N.; Blagosklonny, M.V. Rapamycin reverses insulin resistance (IR) in high-glucose medium without causing IR in normoglycemic medium. Cell Death Dis. 2014, 5, e1214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schulz, T.J.; Zarse, K.; Voigt, A.; Urban, N.; Birringer, M.; Ristow, M. Glucose restriction extends caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007, 6, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Long, F.; Jhala, U.S.; Hedrick, S.; Quinn, R.; Bauer, A.; Rudolph, D.; Schutz, G.; Yoon, C.; Puigserver, P.; et al. Creb regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 2001, 413, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.C.; Puigserver, P.; Chen, G.; Donovan, J.; Wu, Z.; Rhee, J.; Adelmant, G.; Stafford, J.; Kahn, C.R.; Granner, D.K.; et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 2001, 413, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Rera, M.; Bahadorani, S.; Cho, J.; Koehler, C.L.; Ulgherait, M.; Hur, J.H.; Ansari, W.S.; Lo, T., Jr.; Jones, D.L.; Walker, D.W. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011, 14, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and Nrf1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Poulsen, P.; Carlsson, E.; Ridderstrale, M.; Almgren, P.; Wojtaszewski, J.; Beck-Nielsen, H.; Groop, L.; Vaag, A. Multiple environmental and genetic factors influence skeletal muscle PGC-1α and PGC-1β gene expression in twins. J. Clin. Investig. 2004, 114, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.O.; Bonetto, J.H.; Baregzay, B.; de Castro, A.L.; Puukila, S.; Forsyth, H.; Schenkel, P.C.; Llesuy, S.F.; Brum, I.S.; Araujo, A.S.; et al. Modulation of apoptosis by sulforaphane is associated with PGC-1α stimulation and decreased oxidative stress in cardiac myoblasts. Mol. Cell. Biochem. 2015, 401, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.M.; Jeffery, E.H. Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr. Cancer 2011, 63, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Perles, R.; Medina, S.; Moreno, D.A.; Garcia-Viguera, C.; Ferreres, F.; Gil-Izquierdo, A. A new ultra-rapid UHPLC/MS/MS method for assessing glucoraphanin and sulforaphane bioavailability in human urine. Food Chem. 2014, 143, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.A.T.; Wang, J.; Tang, J.Y.; Lee, Y.Z.; Ng, K. Evaluation of α-glucosidase inhibition potential of some flavonoids from epimedium brevicornum. LWT Food Sci. Technol. 2013, 53, 492–498. [Google Scholar] [CrossRef]
- Wagner, A.E.; Piegholdt, S.; Rabe, D.; Baenas, N.; Schloesser, A.; Eggersdorfer, M.; Stocker, A.; Rimbach, G. Epigallocatechin gallate affects glucose metabolism and increases fitness and lifespan in Drosophila melanogaster. Oncotarget 2015, 6, 30568–30578. [Google Scholar] [PubMed]
- Linford, N.J.; Bilgir, C.; Ro, J.; Pletcher, S.D. Measurement of lifespan in Drosophila melanogaster. J. Vis. Exp. 2013, 7, 50068. [Google Scholar]
- Piegholdt, S.; Rimbach, G.; Wagner, A.E. The phytoestrogen prunetin affects body composition and improves fitness and lifespan in male Drosophila melanogaster. FASEB J. 2016, 30, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Tinkerhess, M.J.; Healy, L.; Morgan, M.; Sujkowski, A.; Matthys, E.; Zheng, L.; Wessells, R.J. The Drosophila PGC-1α homolog spargel modulates the physiological effects of endurance exercise. PLoS ONE 2012, 7, e31633. [Google Scholar] [CrossRef] [PubMed]




| Glucosinolate Content in Radish Sprouts (mg/100 g F.W.) | |
|---|---|
| Glucoraphenin | 202 ± 18.3 |
| 4-Hydroxyglucobrassicin | 19.9 ± 1.32 |
| Glucoerucin | 8.74 ± 1.85 |
| Glucoraphasatin | 250 ± 23.5 |
| Glucobrassicin | 6.48 ± 0.36 |
| 4-Methioxyglucobrassicin | 19.5 ± 0.72 |
| Neoglucobrassicin | 6.51 ± 0.31 |
| Aliphatic GLS | 461 ± 42.3 |
| Indole GLS | 52.4 ± 1.97 |
| Total | 514 ± 44.0 |
| Isothiocyanate Content in Radish Sprouts (mg/100 g F.W.) | |
| Sulforaphene | 9.93 ± 0.01 |
| Sulforaphane | 0.97 ± 0.02 |
| Indole-3-carbinol | 1.00 ± 0.09 |
| Total | 11.9 ± 0.11 |
| Inhibitory Activity of Radish Sprouts | |
|---|---|
| α-Glucosidase IC50 | 60.8 ± 1.16 (mg/mL) |
| α -Amylase IC50 | 33.8 ± 4.00 (mg/mL) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baenas, N.; Piegholdt, S.; Schloesser, A.; Moreno, D.A.; García-Viguera, C.; Rimbach, G.; Wagner, A.E. Metabolic Activity of Radish Sprouts Derived Isothiocyanates in Drosophila melanogaster. Int. J. Mol. Sci. 2016, 17, 251. https://doi.org/10.3390/ijms17020251
Baenas N, Piegholdt S, Schloesser A, Moreno DA, García-Viguera C, Rimbach G, Wagner AE. Metabolic Activity of Radish Sprouts Derived Isothiocyanates in Drosophila melanogaster. International Journal of Molecular Sciences. 2016; 17(2):251. https://doi.org/10.3390/ijms17020251
Chicago/Turabian StyleBaenas, Nieves, Stefanie Piegholdt, Anke Schloesser, Diego A. Moreno, Cristina García-Viguera, Gerald Rimbach, and Anika E. Wagner. 2016. "Metabolic Activity of Radish Sprouts Derived Isothiocyanates in Drosophila melanogaster" International Journal of Molecular Sciences 17, no. 2: 251. https://doi.org/10.3390/ijms17020251
APA StyleBaenas, N., Piegholdt, S., Schloesser, A., Moreno, D. A., García-Viguera, C., Rimbach, G., & Wagner, A. E. (2016). Metabolic Activity of Radish Sprouts Derived Isothiocyanates in Drosophila melanogaster. International Journal of Molecular Sciences, 17(2), 251. https://doi.org/10.3390/ijms17020251