Targeted Delivery Systems for Molecular Therapy in Skeletal Disorders
Abstract
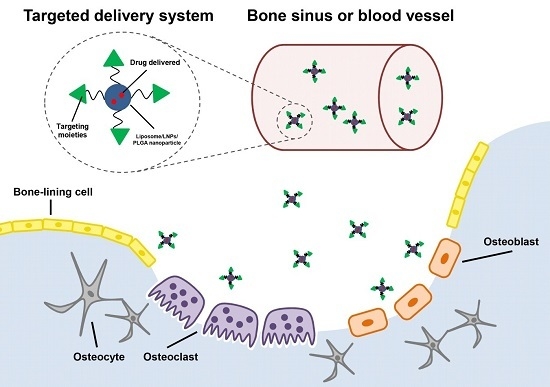
:1. Introduction
2. Targeting Moieties
2.1. Bone Tissue-Targeting Moiety
2.1.1. Synthetic Compounds
Bisphosphonates
Tetracycline
2.1.2. Biological Molecules
Cyclic Arginine-Glycine-Aspartic Acid-Tyrosine-Lysine Peptide (cRGDyk)
2.2. Cell-Specific Targeting Moiety
2.2.1. Osteoblast-Targeting Moiety
Tripeptide Aspartate-Serine-Serine (DSS)
Osteoblast-Targeting Aptamer
2.2.2. Osteoclast-Targeting Moiety
Acid Octapeptide with Aspartic Acid
2.2.3. Bone Marrow Mesenchymal Stem Cell-Specific Moieties
Bone Marrow Mesenchymal Stem Cell-Specific Aptamer
2.3. Potential Targeting Moieties
Pseudopurpurin
3. Nanoparticles
3.1. Liposomes
3.2. Lipid Nanoparticles
3.3. Poly(Lactic-co-Glycolic Acid) Nanoparticles
4. Challenges and Perspectives
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allgrove, J. Metabolic bone disease. Paediatr. Child Health 2010, 21, 187–193. [Google Scholar] [CrossRef]
- Itaka, K.; Ohba, S.; Miyata, K.; Kawaguchi, H.; Nakamura, K.; Takato, T.; Chung, U.I.; Kataoka, K. Bone regeneration by regulated in vivo gene transfer using biocompatible polyplex nanomicelles. Mol. Ther. 2007, 15, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Muro, S. Challenges in design and characterization of ligand-targeted drug delivery systems. J. Control. Release 2012, 164, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.Y.; Wu, C.T.; Chen, J.Z.; Xiao, Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int. J. Nanomed. 2013, 8, 2305–2317. [Google Scholar] [CrossRef] [PubMed]
- Sung-Wook Choi, J.-H.K. Design of surface-modified poly(d,l-lactide-co-glycolide) nanoparticles for targeted drug delivery to bone. J. Control. Release 2007, 122, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, I.; Thamake, S.L.R.; Gryczynski, Z.; Ranjan, A.P.; Vishwanatha, J.K. Alendronate coated poly-lactic-co-glycolic acid (PLGA) nanoparticles for active targeting of metastatic breast cancer. Biomaterials 2012, 33, 7164–7173. [Google Scholar]
- Miller, K.; Eldar-Boock, A.; Polyak, D.; Segal, E.; Benayoun, L.; Shaked, Y.; Satchi-Fainaro, R. Antiangiogenic antitumor activity of HPMA copolymer-paclitaxel alendronate conjugate on breast cancer bone metastasis mouse model. Mol. Pharm. 2011, 8, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Neale, J.R.; Richter, N.B.; Merten, K.E.; Taylor, K.G.; Singh, S.; Waite, L.C.; Emery, N.K.; Smith, N.B.; Cai, J.; Pierce, W.M., Jr. Bone selective effect of an estradiol conjugate with a novel tetracycline-derived bone-targeting agent. Bioorg. Med. Chem. Lett. 2008, 19, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.C.L.; Zhang, R.; Chen, Z.; Zhu, L. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J. Control. Release 2014, 28, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Guo, B.S.; Wu, H.; Tang, T.; Zhang, B.T.; Zheng, L.Z.; He, Y.X.; Yang, Z.J.; Pan, X.H.; Chow, H.; et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat. Med. 2012, 18, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Guo, B.; Wu, H.; Shao, N.; Li, D.; Liu, J.; Dang, L.; Wang, C.; Li, H.; Li, S.; et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel rna interference-based bone anabolic strategy. J. Nat. Med. 2015, 21, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dang, L.; Li, D.F.; Liang, C.; He, X.J.; Wu, H.; Qian, A.R.; Yang, Z.J.; Au, D.W.T.; Chiang, M.W.L.; et al. A delivery system specifically approaching bone resorption surfaces to facilitate therapeutic modulation of micrornas in osteoclasts. Biomaterials 2015, 52, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; Pan, H.; Wang, D.; Bowman, B.M.; Kopeckova, P.; Kopecek, J. Feasibility of using a bone-targeted, macromolecular delivery system coupled with prostaglandin E1 to promote bone formation in aged, estrogen-deficient rats. Pharm. Res. 2008, 25, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Wei, L.; Miron, R.J.; Shi, B.; Bian, Z. Anabolic bone formation via a site-specific bone-targeting delivery system by interfering with semaphorin 4D expression. J. Bone Miner. Res. 2014, 30, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Sekido, T.; Sakura, N.; Higashi, Y.; Miya, K.; Nitta, Y.; Nomura, M.; Sawanishi, H.; Morito, K.; Masamune, Y.; Kasugai, S.; et al. Novel drug delivery system to bone using acidic oligopeptide: Pharmacokinetic characteristics and pharmacological potential. J. Drug Target. 2001, 9, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Richter, D. Vital staining of bones with madder. Biochem. J. 1937, 31, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Drake, M.T.; Mullan, R.J.; Mauck, K.F. Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: A systematic review and network meta-analysis. J. Clin. Endocrinol. Metab. 2012, 97, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Khademi, F.; Poursina, F.; Hosseini, E.; Akbari, M.; Safaei, H.G. Helicobacter pylori in Iran: A systematic review on the antibiotic resistance. Iran J. Basic Med. Sci. 2015, 18, 2–7. [Google Scholar] [PubMed]
- Russell, R.G.G. Bisphosphonates: Mode of action and pharmacology. Pediatrics 2007, 119, S150–S162. [Google Scholar] [CrossRef] [PubMed]
- Geralad, A.M.; Finerman, R.A.M. In vitro binding of tetracyclines to calcium. Nature 1963, 198, 486–487. [Google Scholar]
- Widler, L.; Jahnke, W.; Green, J.R. The chemistry of bisphosphonates: From antiscaling agents to clinical therapeutics. Anti-Cancer Agents Med. Chem. 2012, 12, 95–101. [Google Scholar] [CrossRef]
- Selander, K.S.; Mönkkönen, J.; Karhukorpi, E.K.; Harkonen, P.; Hannumiemi, R.; Vaananen, H.K. Characteristics of clodronate-induced apoptosis in osteoclasts and macrophages. Mol. Pharmacol. 1996, 50, 1127–1138. [Google Scholar] [PubMed]
- Cantrill, J.A.; Anderson, D.C. Treatment of paget’s disease of bone. Clin. Endocrinol. 1990, 32, 507–518. [Google Scholar] [CrossRef]
- Van Holten-Verzantvoort, A.T.; Bijvoet, O.L.; Cleton, F.J.; Hermans, J.; Kroon, H.M.; Harinck, H.I.; Vermey, P.; Elte, J.W.; Neijt, J.P.; Beex, L.V.; et al. Reduced morbidity from skeletal metastases in breast cancer patients during long-term bisphosphonate (APD)-treatment. Lancet 1987, 2, 983–985. [Google Scholar] [CrossRef]
- Storm, T.; Thamsborg, G.; Steiniche, T.; Genant, H.K.; Sørensen, O.H. Effect of intermittent cyclical etidronate therapy on bone mass and fracture rate in women with postmenopausal osteoporosis. N. Engl. J. Med. 1990, 322, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Heath, D. The treatment of hypercaalcaemia of maliganancy. Clin. Endocrinol. 1991, 34, 155–157. [Google Scholar] [CrossRef]
- Recklies, A.D.; Tiltman, K.J.; Stoker, T.A.; Poole, A.R. Secretion of proteinases from malignant and nonmalignant human breast tissue. Cancer Res. 1980, 40, 550–556. [Google Scholar] [PubMed]
- Orriss, I.R.; Key, M.L.; Colston, K.W.; Arnett, T.R. Inhibition of osteoblast function in vitro by aminobisphosphonates. J. Cell. Biochem. 2009, 106, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I.; Bivi, N.; Bellido, T. A bisphosphonate that does not affect osteoclasts prevents osteoblast and osteocyte apoptosis and the loss of bone strength induced by glucocorticoids in mice. Bone 2011, 49, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Favus, M.J. Diabetes and the risk of osteonecrosis of the jaw. J. Clin. Endocrinol. Metab. 2007, 92, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Burr, D.; Cauley, J.; Dempster, D.W.; Ebeling, P.R.; Felsenberg, D.; Gagel, R.F.; Gilsanz, V.; Guise, T.; Koka, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American society for bone and mineral reasearch. J. Bone Miner. Res. 2007, 22, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Mehrotra, B.; Rosenberg, T.J.; Engroff, S.L. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J. Oral Maxillofac. Surg. 2004, 62, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Goodger, N.M.; Pogrel, M.A. Osteonecrosis of the jaws associated with cancer chemotherapy. J. Oral Maxillofac. Surg. 2003, 61, 1104–1107. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef] [PubMed]
- Schlickewei, C.W.; Laaff, G.; Andresen, A.; Klatte, T.O.; Rueger, J.M.; Ruesing, J.; Epple, M.; Lehmann, M. Bone augmentation using a new injectable bone graft substitute by combining calcium phosphate and bisphosphonate as composite-an animal model. J. Orthop. Surg. Res. 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, C.; Verron, E.; Soueidan, A.; Josse, S.; Arshad, M.D.; Janvier, P.; Pilet, P.; Bouler, J.M.; Bujoli, B.; Guicheux, J. Controlled release of bisphosphonate from a calcium phosphate biomaterial inhibits osteoclastic resorption in vitro. J. Biomed. Mater. Res. 2008, 89, 46–56. [Google Scholar]
- Misra, D.N. Absorption and orientation of tetracycline on hydroxyapatite. Calcif. Tissue Int. 1991, 48, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Eden, H.S.; Chen, X.Y. Peptides in cancer nanomedicine: Drug carriers, targeting ligands and protease substrates. J. Control. Release 2012, 159, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Hynes., R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Duong, L.T.; Lakkakorpi, P.; Nakamura, I.; Rodan, G.A. Integrins and signaling in osteoclast function. Matrix Biol. 2000, 19, 97–105. [Google Scholar] [CrossRef]
- Wang, D.; Miller, S.C.; Shlyakhtenko, L.S.; Portillo, A.M.; Liu, X.M.; Papangkorn, K.; Kopekova, P.; Lyubchenko, Y.; Higuchi, W.I.; Kopecek, J. Osteotropic peptide that differentiates functional domains of the skeleton. Bioconj. Chem. 2007, 18, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Gold, L. The selex process: A surprising source of therapeutic and diagnostic compounds. Harvey Lect. 1995, 91, 47–57. [Google Scholar] [PubMed]
- Famulok, M.; Mayer, G.; Blind, M. Nucleic acid aptamers-from selection in vitro to applications in vivo. Acc. Chem. Res. 2000, 33, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, D.K.; Hagerman, E.; Eckert, R.; He, J.; Choi, H.; Cao, N.; le, K.; Hedger, J.; Qi, F.X.; Anderson, M.; et al. Specific binding and mineralization of calcified surfaces by small peptides. Calcif. Tissue Int. 2010, 86, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.H.; Zhang, T.; Luo, H.; Yen, T.M.; Chen, P.W.; Han, Y.; Lo, Y.H. Nucleic acid aptamers: An emerging tool for biotechnology and biomedical sensing. Sensors 2015, 15, 16281–16313. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Pathol. Mech. Dis. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Sobacchi, C.; Schulz, A.; Coxon, F.P.; Villa, A.; Helfrich, M.H. Osteopetrosis: Genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 2013, 9, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Larson, B.L.; Vuoristo, J.T.; Cui, J.G.; Prockop, D.J. Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCS). J. Bone Miner. Res. 2004, 19, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Cheng, P.; Liang, M.K. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Investig. 2015, 125, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Li, X.B.; Han, T.S.; Li, P.; Wang, J.; Liu, G.; Wang, Z.; Ge, C.; Gao, S. Dietary pseudopurpurin improves bone geometry architecture and metabolism in red-bone guishan goats. PLoS ONE 2012, 7, e37469. [Google Scholar] [CrossRef] [PubMed]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D.; Needham, D. The “stealth” liposome: A prototypical biomaterial. Chem. Rev. 1995, 95, 2601–2628. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, X.H.; Carbone, E.J.; Nelson, C.; Kan, H.M.; Lo, K.W.H. Poly aspartic acid peptide-linked PLGA based nanoscale particles: Potential for bone-targeting drug delivery applications. Int. J. Pharm. 2014, 475, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G.; Neerunjun, D.E. Control of the rate of hepatic uptake and catabolism of liposome-entrapped proteins injected into rats. Eur. J. Biochem. 1974, 47, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Stamp, D. The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem. Biophys. Res. Commun. 1975, 63, 651–658. [Google Scholar] [CrossRef]
- Awasthi, V.D.; Garcia, D.; Goins, B.A.; Philips, W.T. Circulation and biodistribution profiles of long-circulating peg-liposomes of various sizes in rabbits. Int. J. Pharm. 2003, 253, 121–132. [Google Scholar] [CrossRef]
- Ferreira Ddos, S.; Boratto, F.A.; Cardoso, V.N.; Serakides, R.; Fernandes, S.O.; Ferreira, L.A.; Oliveira, M.C. Alendronate-coated long-circulating liposomes containing 99mtechnetium-ceftizoxime used to identify osteomyelitis. Int. J. Nanomed. 2015, 10, 2441–2450. [Google Scholar]
- Karita, M.; Tsuchiya, H.; Kawahara, M.; Kasaoka, S.; Tomita, K. The antitumor effect of liposome-encapsulated cisplatin on rat osteosarcoma and its enhancement by caffeine. Anticancer Res. 2008, 28, 1449–1457. [Google Scholar] [PubMed]
- Janknegt, R.; de Marie, S.; Bakker-Woudenberg, I.A.; Crommelin, D.J. Liposomal and lipid formulations of amphotericin B. Clinical pharmacokinetics. Clin. Pharmacokinet. 1992, 23, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Hamidi, M.; Azadi, A.; Rafiei, P. Pharmacokinetic consequences of pegylation. Drug Deliv. 2006, 13, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Sou, K.; Goins, B.; Takeoka, S.J.; Tsuchida, E.; Phillips, W.T. Selective uptake of surface-modified phospholipid vesicles by bone marrow macrophages in vivo. Biomaterials 2007, 28, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.L.; Ray, S.; Thakur, R.S. A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Rodríguez, A.; Delgado, D.; Solinís, M.A.; Gascón, A.R. Lipid nanoparticles as vehicles for macromolecules: Nucleic acids and peptides. Recent Pat. Drug Deliv. Formul. 2011, 5, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Howard, K.A.; Paludan, S.R.; Behlke, M.A.; Besenbacher, F.; Deleuran, B.; Kjems, J. Chitosan/siRNA nanoparticle-mediated TNF-α knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Mol. Ther. 2009, 17, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Horisawa, E.; Kubota, K.; Tuboi, I.; Sato, K.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Size-dependency of dl-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm. Res. 2002, 19, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mima, Y.; Hashimoto, Y.; Shimizu, T.; Kiwada, H.; Ishida, T. Anti-PEG IGM is a major contributor to the accelerated blood clearance of polyethylene glycol-conjugated protein. Mol. Pharm. 2015, 12, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.; Keller, S.; Manganiello, M.J.; Cheng, C.; Lee, C.C.; Opara, C.; Convertine, A.; Stayton, P.S. Ph-responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano 2013, 7, 3912–3925. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.M.; Carlini, A.S.; Chien, M.P.; Sonnenberg, S.; Luo, C.; Braden, R.L.; Osborn, K.G.; Li, Y.; Gianneschi, N.C.; Christman, K.L. Enzyme-responsive nanoparticles for targeted accumulation and prolonged retention in heart tissue after myocardial infarction. Adv. Mater. 2015, 27, 5547–5552. [Google Scholar] [CrossRef] [PubMed]

| Targeting Moieties | Targeted Tissues or Cells | Drugs Delivered | References |
|---|---|---|---|
| Bisphosphonate | Skeletal tissues | Curcumin, bortezomib and paclitaxel | [6,7] |
| Tetracycline derivate | Skeletal tissues | Estradiol | [8] |
| cRDGyk | Integrin-rich tumor cells | Cisplatin | [9] |
| (DSS)6 | Bone formation surfaces | CKIP-1siRNA | [10] |
| CH6 | Osteoblasts | CKIP-1siRNA | [11] |
| d-Asp8 | Bone resorption surfaces | microRNA modulator, PGE1 and sema4D siRNA | [12,13,14] |
| l-Asp6 | Bone resorption surfaces | Estradiol-17β | [15] |
| BMSCs-specific aptamer | BMSCs | Antagomir-188 | [16] |
| Nanoparticles | Targeting Moieties | References |
|---|---|---|
| Liposome | cRGDyk, (DSS)6 and d-Asp8 | [9,10,12] |
| LNPs | CH6 | [11] |
| PLGA nanoparticle | Bisphosphonate, poly-Asp | [6,54] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, L.; Liu, J.; Li, F.; Wang, L.; Li, D.; Guo, B.; He, X.; Jiang, F.; Liang, C.; Liu, B.; et al. Targeted Delivery Systems for Molecular Therapy in Skeletal Disorders. Int. J. Mol. Sci. 2016, 17, 428. https://doi.org/10.3390/ijms17030428
Dang L, Liu J, Li F, Wang L, Li D, Guo B, He X, Jiang F, Liang C, Liu B, et al. Targeted Delivery Systems for Molecular Therapy in Skeletal Disorders. International Journal of Molecular Sciences. 2016; 17(3):428. https://doi.org/10.3390/ijms17030428
Chicago/Turabian StyleDang, Lei, Jin Liu, Fangfei Li, Luyao Wang, Defang Li, Baosheng Guo, Xiaojuan He, Feng Jiang, Chao Liang, Biao Liu, and et al. 2016. "Targeted Delivery Systems for Molecular Therapy in Skeletal Disorders" International Journal of Molecular Sciences 17, no. 3: 428. https://doi.org/10.3390/ijms17030428
APA StyleDang, L., Liu, J., Li, F., Wang, L., Li, D., Guo, B., He, X., Jiang, F., Liang, C., Liu, B., Badshah, S. A., He, B., Lu, J., Lu, C., Lu, A., & Zhang, G. (2016). Targeted Delivery Systems for Molecular Therapy in Skeletal Disorders. International Journal of Molecular Sciences, 17(3), 428. https://doi.org/10.3390/ijms17030428