The Effects of Different Purifying Methods on the Chemical Properties, in Vitro Anti-Tumor and Immunomodulatory Activities of Abrus cantoniensis Polysaccharide Fractions
Abstract
:1. Introduction
2. Results
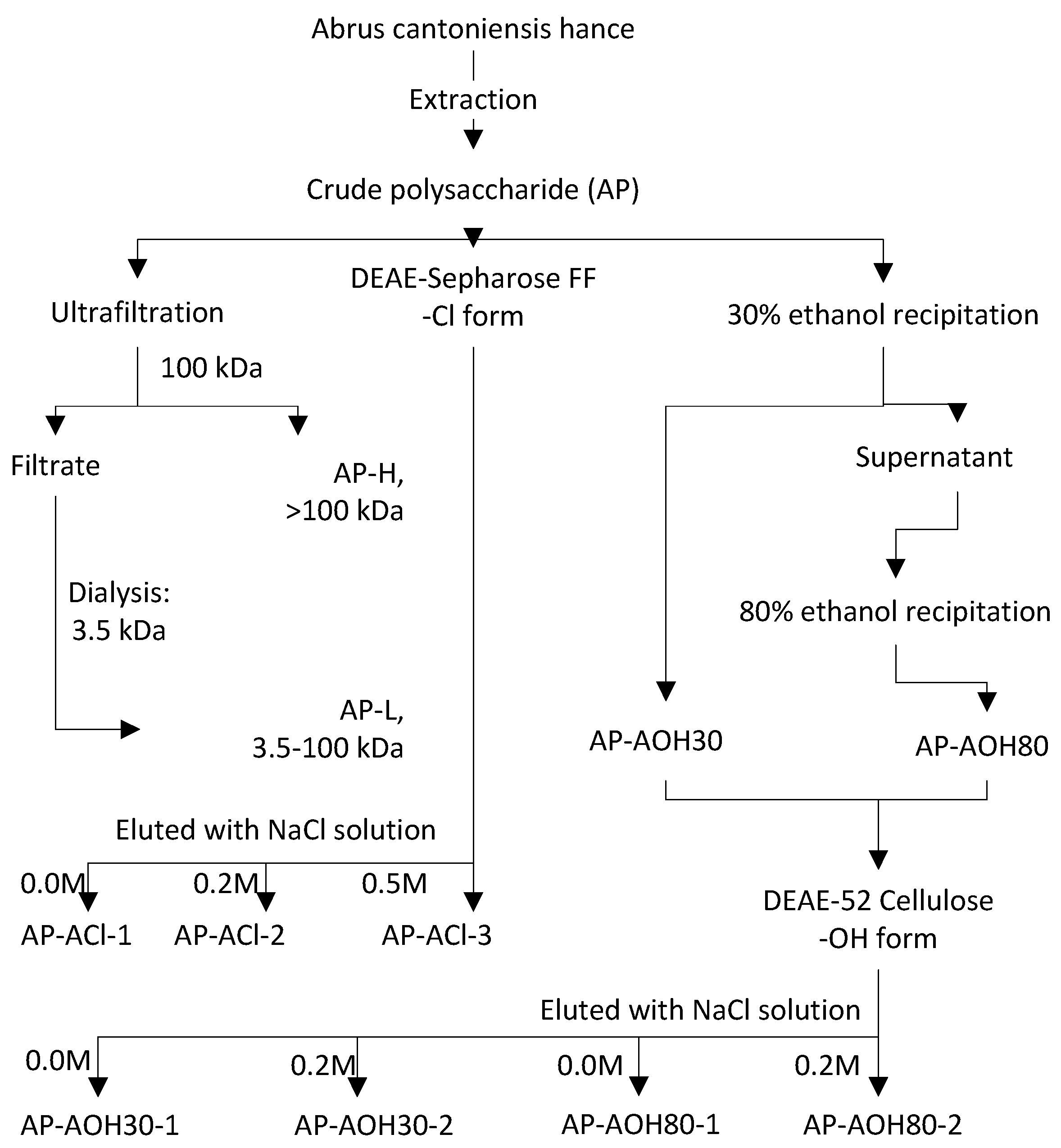
2.1. Isolation of Abrus Polysaccharides Fractions (APF)
2.2. Characterization Comparison for APF
2.2.1. Chemical Properties of APF
2.2.2. The Distribution of Molecular Weight
2.2.3. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
2.2.4. Monosaccharide Composition of APF
2.3. In Vitro Anti-Tumor Effects of APF
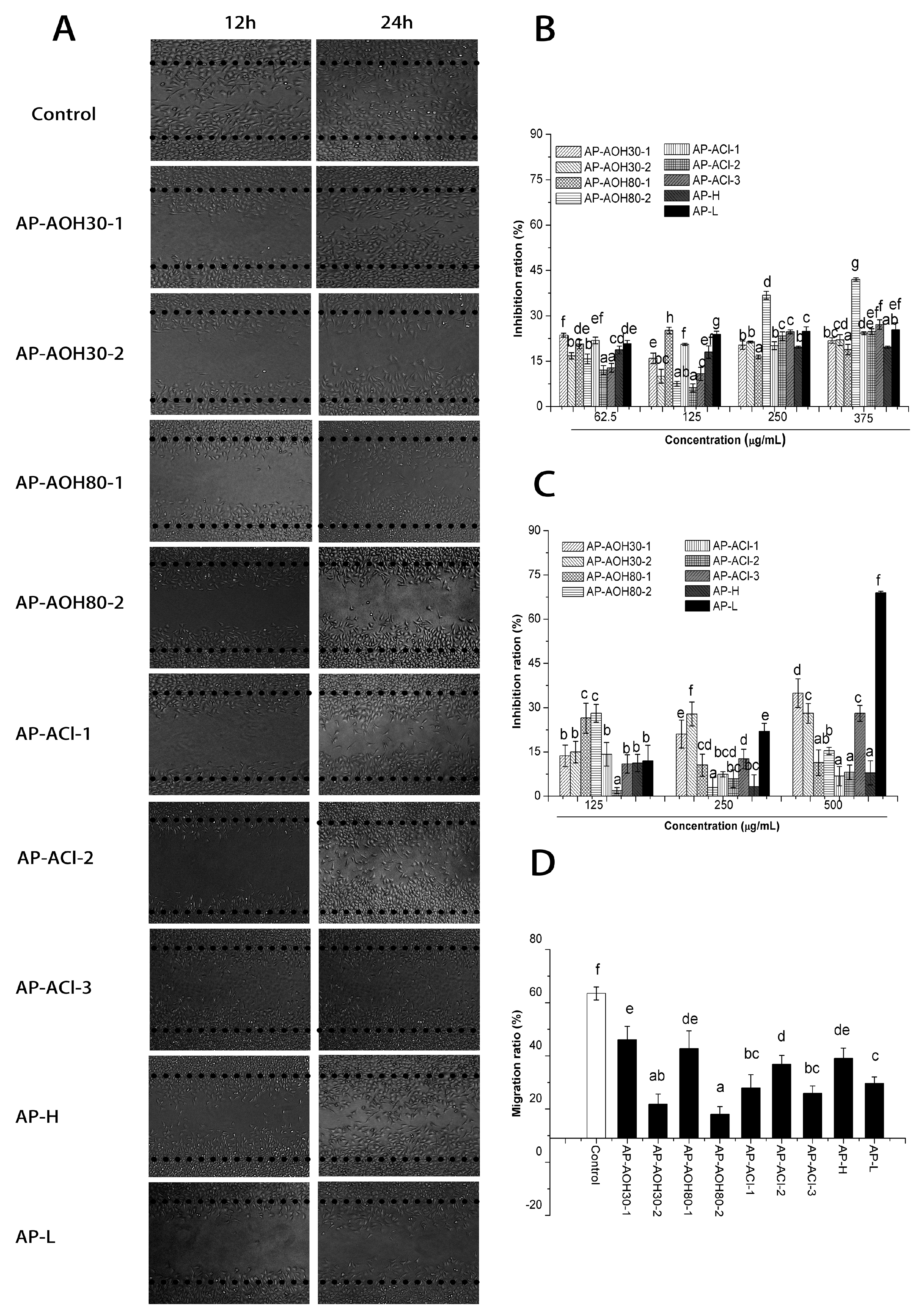
2.3.1. In Vitro Cell Proliferation Assay
2.3.2. In Vitro Inhibitory Effects of APF on Migration of MCF-7 Cells
2.4. In Vitro Immunomodulatory Activities
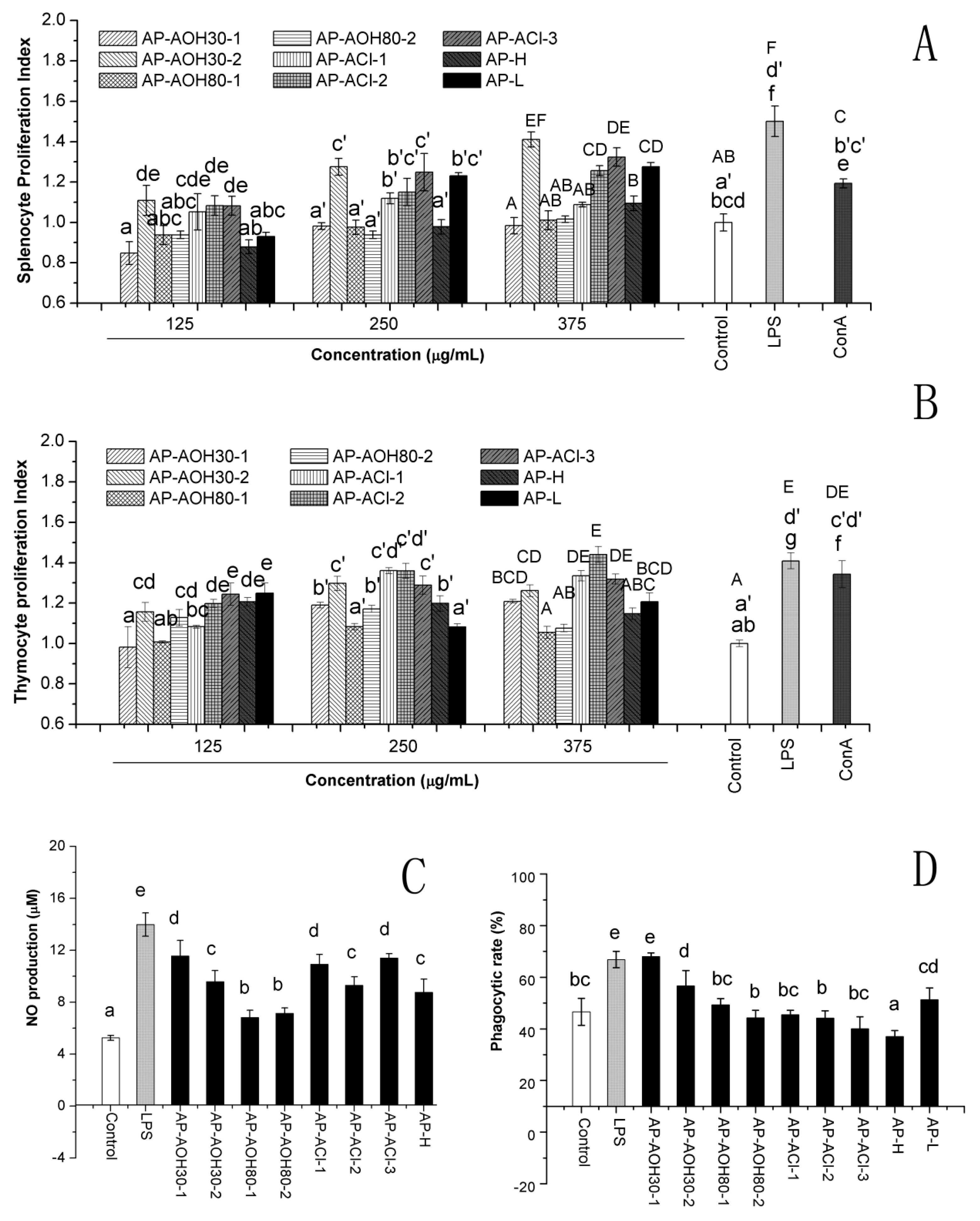
2.4.1. In Vitro Effect of APF on Lymphocyte Proliferation
2.4.2. Nitric Oxide (NO) Production
2.4.3. Peritoneal Macrophage-Mediated Cytotoxicity Assay
2.5. Comprehensive Comparison by Rank-Sum Ratio (RSR) Method
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemicals
4.3. Preparation of Crude Polysaccharides from the A. cantoniensis
4.4. Fractionation and Isolation of A. cantoniensis Polysaccharide
4.4.1. Fractionation of AP by Membrane Separation
4.4.2. Fractionation of AP by DEAE-52 Cellulose Column Combined with Ethanol Stepwise Precipitation
4.4.3. Fractionation of AP by DEAE-Sepharose Fast Flow Column
4.5. Characterization Comparison for APF
4.5.1. Chemical Composition of APF
4.5.2. Determination of Molecular Weight
4.5.3. FT-IR Spectral Analysis
4.5.4. Determination of Monosaccharide Composition
4.6. Cell Line Cultures
4.7. Anti-Tumor Assay
4.7.1. Antiproliferation Activity
4.7.2. Wound Healing Assay
4.8. Immunomodulatory Activities
4.8.1. Splenocyte and Thymocyte Proliferation Test
4.8.2. Determination of Nitric Oxide (NO) Production
4.8.3. Mouse Peritoneal Macrophage-Mediated Cytotoxicity
4.9. Rank Sum Ratio Analysis
4.10. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chiang, T.C.; Chang, H.M. Isolation and structural elucidation of some sapogenols from Abrus cantoniensis. Planta Medica 1982, 46, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Reddy Palvai, V.; Mahalingu, S.; Urooj, A. Abrus precatorius leaves: Antioxidant activity in food and biological systems, pH, and temperature stability. Int. J. Med. Chem. 2014, 2014, 7. [Google Scholar]
- Shafi Sofi, M.; Sateesh, M.K.; Bashir, M.; Harish, G.; Lakshmeesha, T.R.; Vedashree, S.; Vedamurthy, A.B. Cytotoxic and pro-apoptotic effects of Abrus precatorius L. on human metastatic breast cancer cell line, mda-mb-231. Cytotechnology 2013, 65, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.A.; Samoylenko, V.; Jain, S.K.; Tekwani, B.L.; Khan, S.I.; Jacob, M.R.; Midiwo, J.O.; Hester, J.P.; Walker, L.A.; Muhammad, I. Antiparasitic and antimicrobial isoflavanquinones from Abrus schimperi. Nat. Prod. Commun. 2011, 6, 1645–1650. [Google Scholar] [PubMed]
- Wong, V.W.S.; Law, M.Y.; Hui, A.Y.; Lo, A.O.S.; Li, C.Y.; Soo, M.T.; Leung, H.Y.; Chan, H.L.Y. A hospital clinic-based survey on traditional chinese medicine usage among chronic hepatitis B patients. Complement. Ther. Med. 2005, 13, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, S.K.; Mallick, S.K.; Maiti, T.K. In vitro immunostimulatory properties of Abrus lectins derived peptides in tumor bearing mice. Phytomedicine 2009, 16, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Nie, S.; Huang, D.; Feng, Y.; Xie, M. A novel polysaccharide from ganoderma atrum exerts antitumor activity by activating mitochondria-mediated apoptotic pathway and boosting the immune system. J. Agric. Food Chem. 2014, 62, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, S.; Raghavendran, H.R.B.; Sunil, A.G.; Gayathri, V.; Ramakrishnan, G.; Vasanthi, H.R. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (marine brown alga). Food Chem. Toxicol. 2010, 48, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kouakou, K.; Yapi, A.; Kirpotina, L.N.; Jutila, M.A.; Quinn, M.T. Immunomodulatory and hemagglutinating activities of acidic polysaccharides isolated from Combretum racemosum. Int. Immunopharmacol. 2013, 15, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.Q.; Xiao, J.J.; Zhang, H.N.; Wang, J.H.; Pan, L.H.; Yang, X.F.; Luo, J.P. Polysaccharides in Laminaria japonica (LP): Extraction, physicochemical properties and their hypolipidemic activities in diet-induced mouse model of atherosclerosis. Food Chem. 2012, 134, 244–252. [Google Scholar] [CrossRef]
- Monobe, M.; Ema, K.; Azuma, K.; Maeda-Yamamoto, M. Enhancement of phagocytic activity by a crude polysaccharide from tea (Camellia sinensis) extract. In Animal Cell Technology: Basic & Applied Aspects; Kamihira, M., Katakura, Y., Ito, A., Eds.; Springer Netherlands: Shizuoka, Japan, 2010; Volume 16, pp. 333–338. [Google Scholar]
- Na, Y.S.; Kim, W.J.; Kim, S.M.; Park, J.K.; Lee, S.M.; Kim, S.O.; Synytsya, A.; Park, Y.I. Purification, characterization and immunostimulating activity of water-soluble polysaccharide isolated from capsosiphon fulvescens. Int. Immunopharmacol. 2010, 10, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, X.; Fu, Z.; Wang, Z.; Zhang, J. Sulphated modification of a polysaccharide obtained from fresh persimmon (Diospyros kaki l.) fruit and antioxidant activities of the sulphated derivatives. Food Chem. 2011, 127, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Sekkal, M.; Dincq, V.; Legrand, P.; Huvenne, J.P. Investigation of the glycosidic linkages in several oligosaccharides using FT-IR and FT raman spectroscopies. J. Mol. Struct. 1995, 349, 349–352. [Google Scholar] [CrossRef]
- Lee, K.S.; Shin, J.S.; Nam, K.S. Cancer chemopreventive effects of starfish polysaccharide in human breast cancer cells. Biotechnol. Bioprocess Eng. 2011, 16, 987–991. [Google Scholar] [CrossRef]
- Xie, X.F.; Wang, J.W.; Zhang, H.P. Characterization and antitumor activities of a water-soluble polysaccharide from Ampelopsis megalophylla. Carbohydr. Polym. 2015, 129, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.S.; Wu, Y.W.; Xu, S.F.; Sun, H.X.; Chen, F.Y.; Yao, L. Antitumor and immunomodulatory activity of polysaccharides from the roots of Actinidia eriantha. J. Ethnopharmacol. 2009, 125, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Xu, H.M.; Suo, Y.R. Raspberry pulp polysaccharides inhibit tumor growth via immunopotentiation and enhance docetaxel chemotherapy against malignant melanoma in vivo. Food Funct. 2015, 6, 3022–3034. [Google Scholar] [CrossRef] [PubMed]
- Katsiari, C.G.; Liossis, S.N.C.; Sfikakis, P.P. The pathophysiologic role of monocytes and macrophages in systemic lupus erythematosus: A reappraisal. Semin. Arthritis Rheum. 2010, 39, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Helsper, J.P.F.G.; Wei, S.; Baars, J.J.P.; van Griensven, L.J.L.D.; Sonnenberg, A.S.M.; Mensink, R.P.; Plat, J. Effects of mushroom-derived β-glucan-rich polysaccharide extracts on nitric oxide production by bone marrow-derived macrophages and nuclear factor-κB transactivation in Caco-2 reporter cells: Can effects be explained by structure? Mol. Nutr. Food Res. 2010, 54, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Fan, L.; Ai, L.; Shan, L. Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus jujuba CV. Jinsixiaozao. Carbohydr. Polym. 2011, 84, 390–394. [Google Scholar] [CrossRef]
- Yang, F.; Ito, Y. Method for the fractionation of dextran by centrifugal precipitation chromatography. Anal. Chem. 2002, 74, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.C. An application of rsr method in environmental pollution health damage. Adv. Mater. Res. 2012, 518–523, 4839–4842. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Tvete Inngjerdingen, K.; Ballo, N.G.; Zhang, B.Z.; Malterud, K.E.; Michaelsen, T.E.; Diallo, D.; Smestad Paulsen, B. A comparison of bioactive aqueous extracts and polysaccharide fractions from roots of wild and cultivated Cochlospermum tinctorium a. Rich. Phytochemistry 2013, 93, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Park, H.R.; Lee, H.S.; Sun, Y.C.; Kim, Y.S.; Shin, K.S. Anti-metastatic effect of polysaccharide isolated from Colocasia esculenta is exerted through immunostimulation. Int. J. Mol. Med. 2012, 31, 361–368. [Google Scholar] [PubMed]
- Zhao, T.; Mao, G.; Mao, R.; Zou, Y.; Zheng, D.; Feng, W.; Ren, Y.; Wang, W.; Zheng, W.; Song, J.; et al. Antitumor and immunomodulatory activity of a water-soluble low molecular weight polysaccharide from Schisandra chinensis (Turcz.) baill. Food Chem. Toxicol. 2013, 55, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Lerouge, P.; Loutelier-Bourhis, C.; Driouich, A.; Ray, B. Structural characterisation of hemicellulosic polysaccharides from Benincasa hispida using specific enzyme hydrolysis, ion exchange chromatography and maldi-tof mass spectroscopy. Carbohydr. Polym. 2005, 59, 231–238. [Google Scholar] [CrossRef]
- Paulsen, B.S.; Olafsdóttir, E.N.S.; Ingólfsdóttir, K. Chromatography and electrophoresis in separation and characterization of polysaccharides from lichens. J. Chromatogr. A 2002, 967, 163–171. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Wang, Q.; Wang, D.; Dong, D.; Mu, H.; Ye, X.S.; Duan, J. Purification, antioxidant and immunological activities of polysaccharides from actinidia chinensis roots. Int. J. Biol. Macromol. 2015, 72, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.C.C.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, C.; Ma, L.; Zhang, Z.; Cao, L.; Liu, J.; Zeng, X. Preparation, preliminary characterization and immunostimulatory activity of polysaccharide fractions from the peduncles of Hovenia dulcis. Food Chem. 2013, 138, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, H.; Zhang, X. Structure and immuno-stimulating activities of a new heteropolysaccharide from Lentinula edodes. J. Agric. Food Chem. 2012, 60, 11560–11566. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, J.; Zhang, Y.; Chen, H.; Wang, Y. Physicochemical characterization of puerh tea polysaccharides and their antioxidant and α-glycosidase inhibition. J. Funct. Foods 2014, 6, 545–554. [Google Scholar] [CrossRef]
- Li, J.E.; Nie, S.P.; Xie, M.Y.; Li, C. Isolation and partial characterization of a neutral polysaccharide from mosla chinensis maxim. CV. Jiangxiangru and its antioxidant and immunomodulatory activities. J. Funct. Foods 2014, 6, 410–418. [Google Scholar] [CrossRef]
- Liao, N.B.; Chen, S.G.; Ye, X.Q.; Zhong, J.J.; Wu, N.; Dong, S.; Yang, B.; Liu, D.H. Antioxidant and anti-tumor activity of a polysaccharide from freshwater clam, corbicula fluminea. Food Funct. 2013, 4, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Imagej. Available online: http://rsb.info.nih.gov/ij/ (accessed on 31 March 2016).
- Park, K.; Park, H.S.; Kim, M.K.; Hong, G.E.; Nagappan, A.; Lee, H.J.; Yumnam, S.; Lee, W.S.; Won, C.K.; Shin, S.C.; et al. Flavonoids identified from korean citrus aurantium l. Inhibit non-small cell lung cancer growth in vivo and in vitro. J. Funct. Foods 2014, 7, 287–297. [Google Scholar] [CrossRef]
- Kruisbeek, A.M. Isolation of mouse mononuclear cells. Curr. Protoc. Immunol. 2001. [Google Scholar] [CrossRef]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008, 14.1.1–14.1.14. [Google Scholar] [CrossRef]
- Chen, H.; Xing, B.Z.; Liu, X.H.; Zhan, B.Y.; Zhou, J.Q.; Zhu, H.C.; Chen, Z.Y. Similarities between ozone oxidative preconditioning and ischemic preconditioning in renal ischemia/reperfusion injury. Arch. Med. Res. 2008, 39, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Nie, S.P.; Huang, D.F.; Huang, J.Q.; Wang, Y.W.; Xie, M.Y. Polysaccharide from Ganoderma atrum evokes antitumor activity via toll-like receptor 4-mediated NF-κB and mitogen-activated protein kinase signaling pathways. J. Agric. Food Chem. 2013, 61, 3676–3682. [Google Scholar] [CrossRef] [PubMed]
| Items | Yield (mg/kg) | Total Sugar 1 (w/w, %) | Uronic Acid 1 (w/w, %) | Sulfate 1 (w/w, %) | Distribution of Molecular Weight (×104 Da) | |||
|---|---|---|---|---|---|---|---|---|
| Peak 1 | Content (%) | Peak 2 | Content (%) | |||||
| AP-AOH30-1 | 237.1 | 74.28 ± 1.08 e | 20.33 ± 1.70 f | 8.48 ± 0.43 e | 11.27 | 62.27 | 1.69 | 15.97 |
| AP-AOH30-2 | 64.8 | 67.51 ± 1.91 cd | 22.57 ± 1.48 g | 11.05 ± 0.40 f | 12.98 | 59.77 | 1.67 | 34.55 |
| AP-AOH80-1 | 301.6 | 71.08 ± 1.67 de | 1.84 ± 0.92 a | 0.83 ± 0.77 a | 5.73 | 14.96 | 0.51 | 78.93 |
| AP-AOH80-2 | 93.7 | 66.97 ± 4.21 c | 0.75 ± 0.30 a | 0.98 ± 0.31 ab | 2.61 | 85.16 | n.d.2 | n.d. |
| AP-ACl-1 | 702.3 | 82.19 ± 2.36 f | 6.05 ± 0.38 b | 1.93 ± 0.38 c | 2.68 | 84.91 | n.d. | n.d. |
| AP-ACl-2 | 504.5 | 84.78 ± 2.28 f | 10.87 ± 2.01 d | 1.62 ± 0.25 bc | 14.05 | 52.06 | 1.97 | 22.36 |
| AP-ACl-3 | 613.5 | 83.93 ± 1.60 f | 13.62 ± 0.57 e | 3.58 ± 0.16 d | 4.4 | 83.31 | n.d. | n.d. |
| AP-H | 865.2 | 55.78 ± 1.18 a | 2.52 ± 1.08 a | 1.06 ± 0.29 ab | 8.54 | 36.45 | 5.44 | 53.20 |
| AP-L | 121.4 | 59.93 ± 1.54 b | 9.31 ± 1.03 c | 3.88 ± 0.32 d | 1.45 | 75.57 | n.d. | n.d. |
| Items | AP-AOH30-1 | AP-AOH30-2 | AP-AOH80-1 | AP-AOH80-2 | AP-ACl-1 | AP-ACl-2 | AP-ACl-3 | AP-H | AP-L | Assignments 2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Wavelength (cm−1) | 3435 | 3425 | 3421 | 3440 | 3435 | 3429 | 3431 | 3426 | 3656 | v O–H |
| 2927 | 2934 | 2934 | 2933 | 2942 | 2930 | 2925 | 2939 | 2941 | v C–H | |
| 1743 | 1743 | n.d.1 | n.d. | n.d. | 1735 | 1723 | n.d. | 1739 | δ C=O | |
| 1421 | 1420 | n.d. | n.d. | n.d. | 1424 | 1424 | n.d. | 1415 | δ O–C=O | |
| 1371 | 1373 | 1238 | 1334 | 1334 | 1373 | 1370 | 1334 | 1367 | Sulpates | |
| 1240 | 1240 | n.d. | 1236 | 1236 | 1241 | 1240 | 1236 | 1241 | v O=S=O | |
| 578 | 579 | n.d. | 587 | n.d. | 578 | 576 | 585 | n.d. | δ O=S=O | |
| 895 | 894 | 893 | 896 | 894 | 898 | 895 | 893 | 894 | β-linked | |
| 850 | 854 | n.d. | 853 | 854 | n.d. | 857 | n.d. | 855 | α-linked |
| Fractions | Sugar Components (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| GlcA | GalA | Rha | Ara | Xyl | Fuc | Glc | Gal | |
| AP-AOH30-1 | n.d.1 | 10.0 | 7.4 | 26.2 | n.d. | 8.1 | 9.0 | 39.3 |
| AP-AOH30-2 | 46.1 | 22.1 | 4.0 | 11.7 | n.d. | n.d. | 3.5 | 12.6 |
| AP-AOH80-1 | n.d. | n.d. | n.d. | 8.8 | 2.9 | 3.2 | 69.6 | 15.5 |
| AP-AOH80-2 | n.d. | 2.1 | 15.1 | 18.1 | 2.7 | 9.4 | 6.2 | 46.5 |
| AP-ACl-1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 83.7 | 16.3 |
| AP-ACl-2 | n.d. | 5.5 | 3.5 | 6.3 | 2.9 | 3.4 | 64.8 | 13.6 |
| AP-ACl-3 | 61.1 | 3.0 | 9.8 | 8.9 | n.d. | 3.0 | 4.3 | 9.9 |
| AP-H | n.d. | n.d. | n.d. | 30.9 | n.d. | n.d. | 12.3 | 56.8 |
| AP-L | n.d. | 7.6 | 9.1 | 17.0 | 3.0 | 5.2 | 30.3 | 27.7 |
| Items | RSR_anti 1 | RSR_immu | RSR_compr |
|---|---|---|---|
| AP-AOH30-1 | 0.6944 | 0.4583 | 0.5764 5 |
| AP-AOH30-2 | 0.5556 | 0.8194 | 0.6875 |
| AP-AOH80-1 | 0.5833 | 0.2778 | 0.4306 |
| AP-AOH80-2 | 0.5417 | 0.3194 | 0.4306 |
| AP-ACl-1 | 0.5278 | 0.6389 | 0.5833 |
| AP-ACl-2 | 0.3750 | 0.6944 | 0.5347 |
| AP-ACl-3 | 0.5417 | 0.7917 | 0.6667 |
| AP-H | 0.4028 | 0.3611 | 0.3819 |
| AP-L | 0.7778 | 0.5972 | 0.6875 |
| APF_30%EtOH 2 | 0.6250 | 0.6389 | 0.6319 |
| APF_80%EtOH | 0.5625 | 0.2986 | 0.4306 |
| APF_sepharosec 3 | 0.4815 | 0.7083 | 0.5949 |
| APF_cellulose | 0.5938 | 0.4687 | 0.5313 |
| APF_neutrald 4 | 0.6018 | 0.3148 | 0.5301 |
| APF_acidic | 0.5034 | 0.6563 | 0.5799 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Fu, X.; Brennan, M.A.; Brennan, C.S.; Chun, C. The Effects of Different Purifying Methods on the Chemical Properties, in Vitro Anti-Tumor and Immunomodulatory Activities of Abrus cantoniensis Polysaccharide Fractions. Int. J. Mol. Sci. 2016, 17, 511. https://doi.org/10.3390/ijms17040511
Wu S, Fu X, Brennan MA, Brennan CS, Chun C. The Effects of Different Purifying Methods on the Chemical Properties, in Vitro Anti-Tumor and Immunomodulatory Activities of Abrus cantoniensis Polysaccharide Fractions. International Journal of Molecular Sciences. 2016; 17(4):511. https://doi.org/10.3390/ijms17040511
Chicago/Turabian StyleWu, Shaowei, Xiong Fu, Margaret A. Brennan, Charles S. Brennan, and Chen Chun. 2016. "The Effects of Different Purifying Methods on the Chemical Properties, in Vitro Anti-Tumor and Immunomodulatory Activities of Abrus cantoniensis Polysaccharide Fractions" International Journal of Molecular Sciences 17, no. 4: 511. https://doi.org/10.3390/ijms17040511
APA StyleWu, S., Fu, X., Brennan, M. A., Brennan, C. S., & Chun, C. (2016). The Effects of Different Purifying Methods on the Chemical Properties, in Vitro Anti-Tumor and Immunomodulatory Activities of Abrus cantoniensis Polysaccharide Fractions. International Journal of Molecular Sciences, 17(4), 511. https://doi.org/10.3390/ijms17040511







