Hepatotoxicity Induced by “the 3Ks”: Kava, Kratom and Khat
Abstract
:1. Introduction
1.1. Kava: General Concepts
1.2. Kratom: General Concepts
1.3. Khat: General Concepts
1.4. Aims
2. Results
2.1. Constituents
2.1.1. Kava
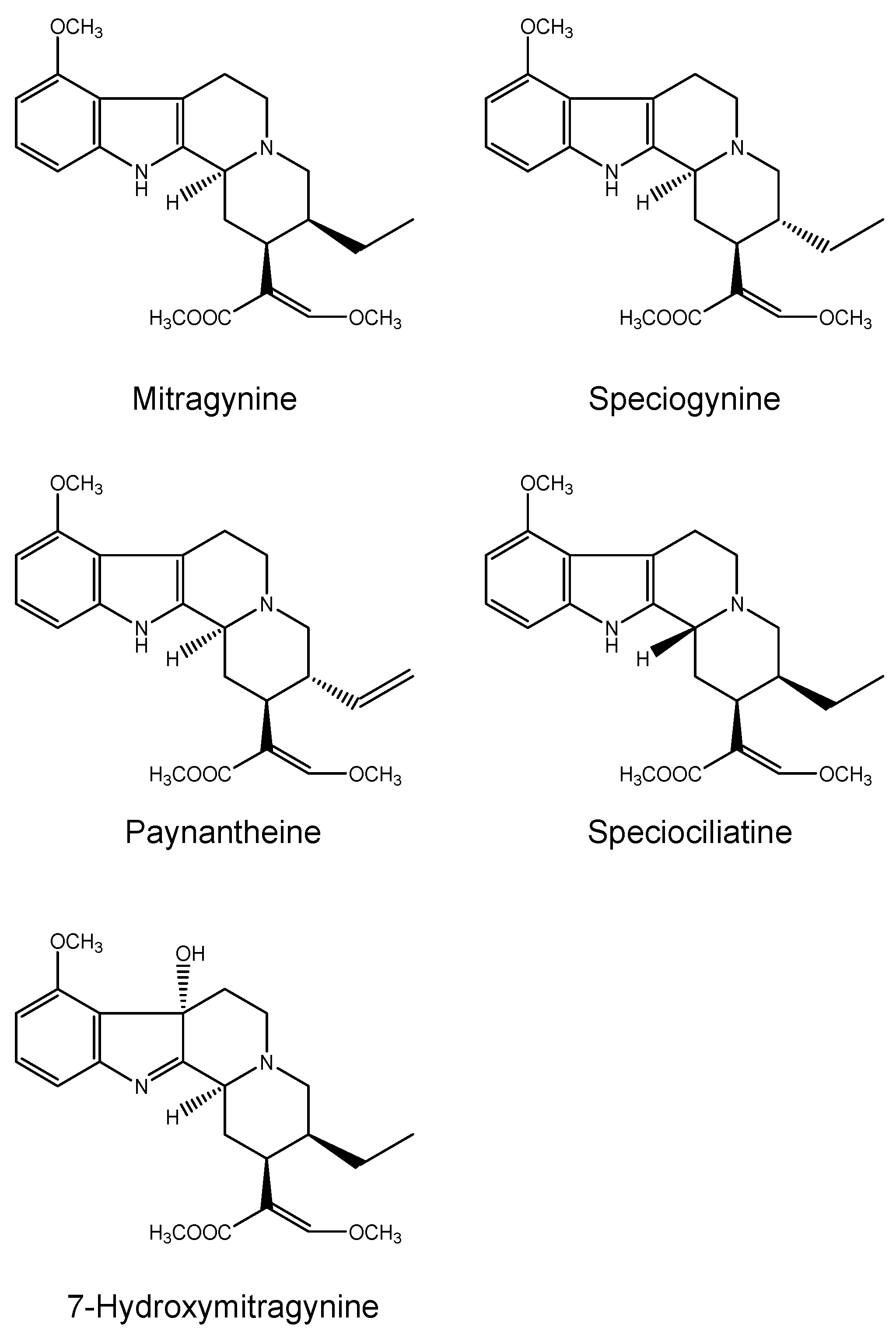
2.1.2. Kratom
2.1.3. Khat
2.2. Hepatotoxicity
2.2.1. Kava
Human Studies on Kava Intake
Toxicity Studies
Carcinogenicity, Mutagenicity and Toxicity
Glutathione (GSH) Depletion, Enzymatic Polymorphisms and Cyclooxygenase-Inhibition
P450 Activity Alteration
Reactive Metabolites
Mould Hepatotoxins and Contaminants
Inflammation
Kava Hepatotoxicity Reports
2.2.2. Kratom
Toxicity Studies in Cell Lines and in Animal Models
Kratom Hepatotoxicity Reports in Literature
2.2.3. Khat
Hepatotoxicity
Inhibiting Action
3. Materials and Methods
4. Conclusions
5. Limitations of the Review
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Teschke, R.; Frenzel, C.; Glass, X.; Schulze, J.; Eickhoff, A. Herbal hepatotoxicity: A critical review. Br. J. Clin. Pharmacol. 2013, 75, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Frenzel, C.; Schulze, J.; Eickhoff, A. Herbal hepatotoxicity: Challenges and pitfalls of causality assessment methods. World J. Gastroenterol. 2013, 19, 2864–2882. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Wolff, A. Kava hepatotoxicity: Regulatory data selection and causality assessment. Dig. Liver Dis. 2009, 41, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Danan, G.; Teschke, R. RUCAM in drug and herb induced liver injury: The update. Int. J. Mol. Sci. 2015, 17. [Google Scholar] [CrossRef]
- Teschke, R.; Genthner, A.; Wolff, A.; Frenzel, C.; Schulze, J.; Eickhoff, A. Herbal hepatotoxicity: Analysis of cases with initially reported positive re-exposure tests. Dig. Liver Dis. 2014, 46, 264–269. [Google Scholar] [CrossRef]
- Showman, A.F.; Baker, J.D.; Linares, C.; Naeole, C.K.; Borris, R.; Johnston, E.; Konanui, J.; Turner, H. Contemporary Pacific and Western perspectives onawa (Piper methysticum) toxicology. Fitoterapia 2015, 100, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, L.M.; Park, C.; Stokes, A.J.; Gomes, H.H.; Turner, H. Pacific island ′Awa (Kava) extracts, but not isolated kavalactones, promote proinflammatory responses in model mast cells. Phytother. Res. 2012, 26, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Ramzan, I. Role of ethanol in kava hepatotoxicity. Phytother. Res. 2010, 24, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Faolex. Available online: http://faolex.fao.org/docs/html/van38473.htm (accessed on 19 December 2015).
- Teschke, R.; Schulze, J. Risk of kava hepatotoxicity and the FDA consumer advisory. JAMA 2010, 304, 2174–2175. [Google Scholar] [CrossRef] [PubMed]
- Ehtpa. Available online: http://www.ehtpa.eu/pdf/EWG%20on%20Kava,%20Jul06.pdf (accessed on 29 January 2015).
- Olsen, L.R.; Grillo, M.P.; Skonberg, C. Constituents in kava extracts potentially involved in hepatotoxicity: A review. Chem. Res. Toxicol. 2011, 24, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.N. Kava: An overview. J. Ethnopharmacol. 1992, 37, 13–45. [Google Scholar] [CrossRef]
- Moulds, R.F.; Malani, J. Kava: Herbal panacea or liver poison? Med. J. Aust. 2003, 178, 451–453. [Google Scholar] [PubMed]
- Mathews, J.D.; Riley, M.D.; Fejo, L.; Munoz, E.; Milns, N.R.; Gardner, I.D.; Powers, J.R.; Ganygulpa, E.; Gununuwawuy, B.J. Effects of the heavy usage of kava on physical health: Summary of a pilot survey in an aboriginal community. Med. J. Aust. 1988, 148, 548–555. [Google Scholar] [PubMed]
- Clough, A.R.; Jacups, S.P.; Wang, Z.; Burns, C.B.; Bailie, R.S.; Cairney, S.J.; Collie, A.; Guyula, T.; McDonald, S.P.; Currie, B.J. Health effects of kava use in an eastern Arnhem Land Aboriginal community. Intern. Med. J. 2003, 33, 336–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.C.; Fricker, P.A.; Thomson, N.J.; Lee, K.A. Sudden death due to ischaemic heart disease in young aboriginal sportsmen in the Northern Territory, 1982–1996. Med. J. Aust. 1999, 170, 425–428. [Google Scholar] [PubMed]
- Weeramanthri, T.; Spillane, P.; Currie, B. Kava and Sudden Cardiac Death. In Kava Research Workshop, Proceedings of a Workshop Convened by the Miwatj Health Aboriginal Corporation, Nhulunbuy, Australia, 10–11 May 1994.
- Bilia, A.R.; Gallon, S.; Vincieri, F.F. Kava-kava and anxiety: Growing knowledge about the efficacy and safety. Life Sci. 2002, 70, 2581–2597. [Google Scholar] [CrossRef]
- World Health Organization. Assessments of the Risk of Hepatotoxicity with Kava Products; WHO Document Production Services: Geneva, Switzerland, 2007. [Google Scholar]
- Teschke, R.; Genthner, A.; Wolff, A. Kava hepatotoxicity: Comparison of aqueous, ethanolic, acetonic kava extracts and kava-herbs mixtures. J. Ethnopharmacol. 2009, 123, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Sarris, J.; Lebot, V. Kava hepatotoxicity solution: A six-point plan for new kava standardization. Phytomedicine 2011, 18, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Sarris, J.; Schweitzer, I. Kava hepatotoxicity in traditional and modern use: The presumed Pacific kava paradox hypothesis revisited. Br. J. Clin. Pharmacol. 2012, 73, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Ahpa. Available online: http://ventquery.com/1975428.html (accessed on 13 March 2016).
- Lebot, V. The Quality of Kava Consumed in the South Pacific. HerbalGram 2006, 71, 34–37. Available online: https://ewsd.wiv-isp.be/Publications%20on%20new%20psychoactive%20substances/Kava/Lebot2006_kava-quality-control.pdf (accessed on 13 March 2016). [Google Scholar]
- Sorrentino, L.; Capasso, A.; Schmidt, M. Safety of ethanolic kava extract: Results of a study of chronic toxicity in rats. Phytomedicine 2006, 13, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F. Response to Aghdassi et al., Letter to the editor “Genetic polymorphisms in the UDP-glucuronosyltransferase UGT1A7 gene in patients with acute liver failure after kava-kava consumption”. Arch. Toxicol. 2015, 489, 2175–2176. [Google Scholar] [CrossRef] [PubMed]
- Strahl, S.; Ehret, V.; Dahm, H.H.; Maier, K.P. Necrotizing hepatitis after taking herbal remedies. Dtsch. Med. Wochenschr. 1998, 123, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- FDA. Available online: http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm085482.htm (accessed on 19 December 2015).
- CDC. Available online: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5147a1.htm (accessed on 30 November 2015).
- Gazzetta Ufficiale. Available online: http://www.gazzettaufficiale.it/atto/vediMenuHTML;jsessionid=UQJ6BSGTdfr4T2No+AmHzg__.ntc-as1-guri2b?atto.dataPubblicazioneGazzetta=2002-06-18&atto.codiceRedazionale=02A07856&tipoSerie=serie_generale&tipoVigenza=originario (accessed on 19 December 2015).
- Hassan, Z.; Muzaimi, M.; Navaratnam, V.; Yusoff, N.H.; Suhaimi, F.W.; Vadivelu, R.; Vicknasingam, B.K.; Amato, D.; von Horsten, S.; Ismail, N.I.; et al. From Kratom to mitragynine and its derivatives: Physiological and behavioural effects related to use, abuse, and addiction. Neurosci. Biobehav. Rev. 2013, 37, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.L.; Prast, C.J. Ethnopharmacology of kratom and the Mitragyna alkaloids. J. Ethnopharmacol. 1988, 23, 115–119. [Google Scholar] [CrossRef]
- Ingsathit, A.; Woratanarat, P.; Anukarahanonta, T.; Rattanasiri, S.; Chatchaipun, P.; Wattayakorn, K.; Lim, S.; Suriyawongpaisal, P. Prevalence of psychoactive drug use among drivers in Thailand: A roadside survey. Accid. Anal. Prev. 2009, 41, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Kawamura, M.; Kikura-Hanajiri, R.; Takayama, H.; Goda, Y. The botanical origin of kratom (Mitragyna speciosa; Rubiaceae) available as abused drugs in the Japanese markets. J. Nat. Med. 2009, 63, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Adkins, J.E.; Boyer, E.W.; McCurdy, C.R. Mitragyna speciosa, a psychoactive tree from Southeast Asia with opioid activity. Curr. Top. Med. Chem. 2011, 11, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.L.; Kaufman, N.C.; Grundmann, O. The pharmacology and toxicology of kratom: From traditional herb to drug of abuse. Int. J. Legal Med. 2016, 130, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Shellard, E.J. Ethnopharmacology of kratom and the Mitragyna alkaloids. J. Ethnopharmacol. 1989, 25, 123–124. [Google Scholar] [CrossRef]
- Gong, F.; Gu, H.; Xu, Q.; Kang, W. Genus Mitragyna: Ethnomedicinal uses and pharmacological studies. Phytopharmacology 2012, 3, 263–272. [Google Scholar]
- Suwanlert, S. A study of kratom eaters in Thailand. Bull. Narc. 1975, 27, 21–27. [Google Scholar] [PubMed]
- Vicknasingam, B.; Narayanan, S.; Beng, G.T.; Mansor, S.M. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int. J. Drug Policy 2010, 21, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Jivan, J.K.; Andurkar, S.V. Pharmacology of kratom: An emerging botanical agent with stimulant, analgesic and opioid-like effects. J. Am. Osteopath. Assoc. 2012, 112, 792–799. [Google Scholar] [PubMed]
- Burkill, I.H.; Birtwistle, W.; Foxworthy, F.W.; Scrivenor, J.B.; Watson, J.B. A Dictionary of the Economic Products of the Malay peninsula; Oxford University Press: London, UK, 1935; Volume 2, pp. 1480–1483. [Google Scholar]
- Wray, L. Biak: An opium substitute. J. Fed. Malay States Mus. 1907, 2, 53. [Google Scholar]
- Harizal, S.N.; Mansor, S.M.; Hasnan, J.; Tharakan, J.K.; Abdullah, J. Acute toxicity study of the standardized methanolic extract of Mitragyna speciosa Korth in rodent. J. Ethnopharmacol. 2010, 131, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, J.; Olszewski, D.; Sedefov, R. Legal highs on the Internet. Subst. Use Misuse 2010, 45, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Arndt, T.; Claussen, U.; Gussregen, B.; Schrofel, S.; Sturzer, B.; Werle, A.; Wolf, G. Kratom alkaloids and O-desmethyltramadol in urine of a “Krypton” herbal mixture consumer. Forensic Sci. Int. 2011, 208, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, C.; Costa, D.; Dao, J.; Isaac, R.; LeBlanc, Y.C.; Rhoades, J.; Windsor, R.C. An evidence-based systematic review of kratom (Mitragyna speciosa) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2013, 10, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Kronstrand, R.; Roman, M.; Thelander, G.; Eriksson, A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J. Anal. Toxicol. 2011, 35, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Tungtananuwat, W.; Lawanprasert, S. Fatal 4 × 100; home-made kratom juice cocktail. J. Health Res. 2010, 24, 43–47. [Google Scholar]
- Kowalczuk, A.P.; Lozak, A.; Zjawiony, J.K. Comprehensive methodology for identification of Kratom in police laboratories. Forensic Sci. Int. 2013, 233, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B. Kratom and Other Mitragynines: The Chemistry and Pharmacology of Opioids from a Non-Opium Source; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014. [Google Scholar]
- Sabetghadam, A.; Ramanathan, S.; Sasidharan, S.; Mansor, S.M. Subchronic exposure to mitragynine, the principal alkaloid of Mitragyna speciosa, in rats. J. Ethnopharmacol. 2013, 146, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Kapp, F.G.; Maurer, H.H.; Auwarter, V.; Winkelmann, M.; Hermanns-Clausen, M. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J. Med. Toxicol. 2011, 7, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Erowid. Available online: https://www.erowid.org/plants/kratom/kratom_health.shtml (accessed on 28 December 2015).
- El-Menyar, A.; Mekkodathil, A.; Al-Thani, H.; Al-Motarreb, A. Khat use: History and heart failure. Oman Med. J. 2015, 30, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.B. “Natural Amphetamine” Khat: A cultural tradition or a drug of abuse? Int. Rev. Neurobiol. 2015, 120, 235–255. [Google Scholar] [PubMed]
- Balint, E.E.; Falkay, G.; Balint, G.A. Khat—A controversial plant. Wien. Klin. Wochenschr. 2009, 121, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Toennes, S.W.; Harder, S.; Schramm, M.; Niess, C.; Kauert, G.F. Pharmacokinetics of cathinone, cathine and norephedrine after the chewing of khat leaves. Br. J. Clin. Pharmacol. 2003, 56, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.D.; Le Roux, C.W.; Emmanuel, A.V.; Halket, J.M.; Przyborowska, A.M.; Kamm, M.A.; Murray-Lyon, I.M. The effect of Khat (Catha edulis) as an appetite suppressant is independent of ghrelin and PYY secretion. Appetite 2008, 51, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Girma, T.; Mossie, A.; Getu, Y. Association between body composition and khat chewing in Ethiopian adults. BMC Res. Notes 2015, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Al-Habori, M. The potential adverse effects of habitual use of Catha edulis (khat). Expert Opin. Drug Saf. 2005, 4, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Pateria, P.; de Boer, B.; MacQuillan, G. Liver abnormalities in drug and substance abusers. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 577–596. [Google Scholar] [CrossRef] [PubMed]
- Riyaz, S.; Imran, M.; Gleeson, D.; Karajeh, M.A. Khat (Catha edulis) as a possible cause of autoimmune hepatitis. World J. Hepatol. 2014, 6, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Roelandt, P.; George, C.; d’Heygere, F.; Aerts, R.; Monbaliu, D.; Laleman, W.; Cassiman, D.; Verslype, C.; van Steenbergen, W.; Pirenne, J.; et al. Acute liver failure secondary to khat (Catha edulis)-induced necrotic hepatitis requiring liver transplantation: Case report. Transpl. Proc. 2011, 43, 3493–3495. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, H.; Komuta, M.; Monsalve, C.; Starkel, P.; Lefebvre, C. To chew or not to chew: That’s the question. Acta Clin. Belg. 2015. [Google Scholar] [CrossRef] [PubMed]
- Soboka, M.; Tesfaye, M.; Feyissa, G.T.; Hanlon, C. Khat use in people living with HIV: A facility-based cross-sectional survey from South West Ethiopia. BMC Psychiatry 2015, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Ketema, T.; Yohannes, M.; Alemayehu, E.; Ambelu, A. Effect of chronic khat (Catha edulis, Forsk) use on outcome of Plasmodium berghei ANKA infection in Swiss albino mice. BMC Infect. Dis. 2015, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Ketema, T.; Bacha, K.; Alemayehu, E.; Ambelu, A. Incidence of severe malaria syndromes and status of immune responses among Khat chewer Malaria patients in Ethiopia. PLoS ONE 2015, 10, e0131212. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Qiu, S.X.; Lebot, V. Herbal hepatotoxicity by kava: Update on pipermethystine, flavokavain B, and mould hepatotoxins as primarily assumed culprits. Dig. Liver Dis. 2011, 43, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Whitton, P.A.; Lau, A.; Salisbury, A.; Whitehouse, J.; Evans, C.S. Kava lactones and the kava-kava controversy. Phytochemistry 2003, 64, 673–679. [Google Scholar] [CrossRef]
- Clouatre, D.L. Kava kava: Examining new reports of toxicity. Toxicol. Lett. 2004, 150, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Denham, A.; McIntyre, M.; Whitehouse, J. Kava—The unfolding story: Report on a work-in-progress. J. Altern. Complement. Med. 2002, 8, 237–263. [Google Scholar] [CrossRef] [PubMed]
- Dragull, K.; Yoshida, W.Y.; Tang, C.S. Piperidine alkaloids from Piper methysticum. Phytochemistry 2003, 63, 193–198. [Google Scholar] [CrossRef]
- Siméoni, P.; Lebot, V. Identification of factors determining kavalactones content and chemotype in Kava (Piper methysticum Forst. f.). Biochem. Syst. Ecol. 2002, 30, 413–424. [Google Scholar] [CrossRef]
- Rowe, A.; Zhang, L.Y.; Ramzan, I. Toxicokinetics of kava. Adv. Pharmacol. Sci. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Leon, F.; Habib, E.; Adkins, J.E.; Furr, E.B.; McCurdy, C.R.; Cutler, S.J. Phytochemical characterization of the leaves of Mitragyna speciosa grown in U.S.A. Nat. Prod. Commun. 2009, 4, 907–910. [Google Scholar] [PubMed]
- Takayama, H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem. Pharm. Bull. 2004, 52, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Horie, S.; Ishikawa, H.; Takayama, H.; Aimi, N.; Ponglux, D.; Watanabe, K. Antinociceptive effect of 7-hydroxymitragynine in mice: Discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sci. 2004, 74, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D. The anti-opium leaf. Pharm. J. 1907, 78, 453. [Google Scholar]
- Zacharias, D.E.; Rosenstein, R.D.; Jeffrey, E.A. The structure of mitragynine hydroiodide. Acta Crystallogr. 1965, 18, 1039–1043. [Google Scholar] [CrossRef]
- Matsumoto, K.; Mizowaki, M.; Suchitra, T.; Takayama, H.; Sakai, S.; Aimi, N.; Watanabe, H. Antinociceptive action of mitragynine in mice: Evidence for the involvement of supraspinal opioid receptors. Life Sci. 1996, 59, 1149–1155. [Google Scholar] [CrossRef]
- Watanabe, K.; Yano, S.; Horie, S.; Yamamoto, L.T. Inhibitory effect of mitragynine, an alkaloid with analgesic effect from Thai medicinal plant Mitragyna speciosa, on electrically stimulated contraction of isolated guinea-pig ileum through the opioid receptor. Life Sci. 1997, 60, 933–942. [Google Scholar] [CrossRef]
- Rosenbaum, C.D.; Carreiro, S.P.; Babu, K.M. Here today, gone tomorrow...and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J. Med. Toxicol. 2012, 8, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Hatori, Y.; Murayama, T.; Tashima, K.; Wongseripipatana, S.; Misawa, K.; Kitajima, M.; Takayama, H.; Horie, S. Involvement of mu-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur. J. Pharmacol. 2006, 549, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Misawa, K.; Kogure, N.; Said, I.M.; Horie, S.; Hatori, Y.; Murayama, T.; Takayama, H. A new indole alkaloid, 7-hydroxyspeciociliatine, from the fruits of Malaysian Mitragyna speciosa and its opioid agonistic activity. J. Nat. Med. 2006, 60, 28–35. [Google Scholar] [CrossRef]
- Erowid. Available online: https://www.erowid.org/plants/kratom/kratom_dose.shtml (accessed on 2 January 2016).
- European Monitoring Center for Drugs and Drug Addiction. Available online: http://www.emcdda.europa.eu/publications/drug-profiles/kratom (accessed on 2 January 2016).
- Matsumoto, K.; Horie, S.; Takayama, H.; Ishikawa, H.; Aimi, N.; Ponglux, D.; Murayama, T.; Watanabe, K. Antinociception, tolerance and withdrawal symptoms induced by 7-hydroxymitragynine, an alkaloid from the Thai medicinal herb Mitragyna speciosa. Life Sci. 2005, 78, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.M.; Chik, Z.; Ramachandra, M.; Subramaniam, U.; Aziddin, R.E.; Mohamed, Z. Evaluation of the effects of Mitragyna speciosa alkaloid extract on cytochrome P450 enzymes using a high throughput assay. Molecules 2011, 16, 7344–7356. [Google Scholar] [CrossRef] [PubMed]
- Boyer, E.W.; Babu, K.M.; Adkins, J.E.; McCurdy, C.R.; Halpern, J.H. Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa Korth). Addiction 2008, 103, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Holler, J.M.; Vorce, S.P.; McDonough-Bender, P.C.; Magluilo, J., Jr.; Solomon, C.J.; Levine, B. A drug toxicity death involving propylhexedrine and mitragynine. J. Anal. Toxicol. 2011, 35, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, J.L.; Lapoint, J.; Hodgman, M.J.; Aldous, K.M. Seizure and coma following Kratom (Mitragynina speciosa Korth) exposure. J. Med. Toxicol. 2010, 6, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Neerman, M.F.; Frost, R.E.; Deking, J. A drug fatality involving Kratom. J. Forensic Sci. 2013, 58 (Suppl. S1), S278–S279. [Google Scholar] [CrossRef] [PubMed]
- Dorman, C.; Wong, M.; Khan, A. Cholestatic hepatitis from prolonged kratom use: A case report. Hepatology 2015, 61, 1086–1087. [Google Scholar] [CrossRef] [PubMed]
- Kite, G.C.; Ismail, M.; Simmonds, M.S.; Houghton, P.J. Use of doubly protonated molecules in the analysis of cathedulins in crude extracts of khat (Catha edulis) by liquid chromatography/serial mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Wabe, N.T. Chemistry, pharmacology, and toxicology of khat (Catha edulis forsk): A review. Addict. Health 2011, 3, 137–149. [Google Scholar] [PubMed]
- Groves, R.A.; Hagel, J.M.; Zhang, Y.; Kilpatrick, K.; Levy, A.; Marsolais, F.; Lewinsohn, E.; Sensen, C.W.; Facchini, P.J. Transcriptome profiling of khat (Catha edulis) and Ephedra sinica reveals gene candidates potentially involved in amphetamine-type alkaloid biosynthesis. PLoS ONE 2015, 10, e0119701. [Google Scholar] [CrossRef] [PubMed]
- Hagel, J.M.; Krizevski, R.; Kilpatrick, K.; Sitrit, Y.; Marsolais, F.; Lewinsohn, E.; Facchini, P.J. Expressed sequence tag analysis of khat (Catha edulis) provides a putative molecular biochemical basis for the biosynthesis of phenylpropylamino alkaloids. Genet. Mol. Biol. 2011, 34, 640–646. [Google Scholar] [PubMed]
- Krizevski, R.; Dudai, N.; Bar, E.; Lewinsohn, E. Developmental patterns of phenylpropylamino alkaloids accumulation in khat (Catha edulis, Forsk.). J. Ethnopharmacol. 2007, 114, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Kalix, P.; Braenden, O. Pharmacological aspects of the chewing of khat leaves. Pharmacol. Rev. 1985, 37, 149–164. [Google Scholar] [PubMed]
- UN. Étude sûr la Composition Chimique du Khat: Recherches sûr la Fraction Phenylalkylamine; UN Document MNAR/11/1975; United Nations: New York, NY, USA, 1975. [Google Scholar]
- Russmann, S.; Barguil, Y.; Cabalion, P.; Kritsanida, M.; Duhet, D.; Lauterburg, B.H. Hepatic injury due to traditional aqueous extracts of kava root in New Caledonia. Eur. J. Gastroenterol. Hepatol. 2003, 15, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Rychetnik, L.; Madronio, C.M. The health and social effects of drinking water-based infusions of kava: A review of the evidence. Drug Alcohol Rev. 2011, 30, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Clough, A. Enough! Or too much. What is “excessive” kava use in Arnhem Land? Drug Alcohol Rev. 2003, 22, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Onopa, J.; Holck, P.; Kaufusi, P.; Kabasawa, D.; Craig, W.J.; Dragull, K.; Levine, A.M.; Baker, J.D. Traditional kava beverage consumption and liver function tests in a predominantly Tongan population in Hawaii. Clin. Toxicol. 2007, 45, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.H.; Ernst, E. Kava extract for treating anxiety. Cochrane Database Syst. Rev. 2003, 195, CD003383. [Google Scholar]
- Sarris, J.; Kavanagh, D.J.; Byrne, G.; Bone, K.M.; Adams, J.; Deed, G. The Kava Anxiety Depression Spectrum Study (KADSS): A randomized, placebo-controlled crossover trial using an aqueous extract of Piper methysticum. Psychopharmacology 2009, 205, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Stough, C.; Teschke, R.; Wahid, Z.T.; Bousman, C.A.; Murray, G.; Savage, K.M.; Mouatt, P.; Ng, C.; Schweitzer, I. Kava for the treatment of generalized anxiety disorder RCT: Analysis of adverse reactions, liver function, addiction, and sexual effects. Phytother. Res. 2013, 27, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, P.V.; Dragull, K.; Tang, C.S. In vitro toxicity of kava alkaloid, pipermethystine, in HepG2 cells compared to kavalactones. Toxicol. Sci. 2004, 79, 106–111. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, R.A.; Zhang, W.; DiSilvestro, D.J. Kava feeding in rats does not cause liver injury nor enhance galactosamine-induced hepatitis. Food Chem. Toxicol. 2007, 45, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Lechtenberg, M.; Quandt, B.; Schmidt, M.; Nahrstedt, A. Is the alkaloid pipermethystine connected with the claimed liver toxicity of Kava products? Pharmazie 2008, 63, 71–74. [Google Scholar] [PubMed]
- Tang, J.; Dunlop, R.A.; Rowe, A.; Rodgers, K.J.; Ramzan, I. Kavalactones Yangonin and Methysticin induce apoptosis in human hepatocytes (HepG2) in vitro. Phytother. Res. 2011, 25, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Gross, S.; Liu, J.H.; Yu, B.Y.; Feng, L.L.; Nolta, J.; Sharma, V.; Piwnica-Worms, D.; Qiu, S.X. Flavokawain B, the hepatotoxic constituent from kava root, induces GSH-sensitive oxidative stress through modulation of IKK/NF-κB and MAPK signaling pathways. FASEB J. 2010, 24, 4722–4732. [Google Scholar] [CrossRef] [PubMed]
- Lude, S.; Torok, M.; Dieterle, S.; Jaggi, R.; Buter, K.B.; Krahenbuhl, S. Hepatocellular toxicity of kava leaf and root extracts. Phytomedicine 2008, 15, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Jhoo, J.W.; Freeman, J.P.; Heinze, T.M.; Moody, J.D.; Schnackenberg, L.K.; Beger, R.D.; Dragull, K.; Tang, C.S.; Ang, C.Y. In vitro cytotoxicity of nonpolar constituents from different parts of kava plant (Piper methysticum). J. Agric. Food Chem. 2006, 54, 3157–3162. [Google Scholar] [CrossRef] [PubMed]
- Eskander, R.N.; Randall, L.M.; Sakai, T.; Guo, Y.; Hoang, B.; Zi, X. Flavokawain B, a novel, naturally occurring chalcone, exhibits robust apoptotic effects and induces G2/M arrest of a uterine leiomyosarcoma cell line. J. Obstet. Gynaecol. Res. 2012, 38, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Narayanapillai, S.C.; Leitzman, P.; O’Sullivan, M.G.; Xing, C. Flavokawains A and B in kava, not dihydromethysticin, potentiate acetaminophen-induced hepatotoxicity in C57BL/6 mice. Chem. Res. Toxicol. 2014, 27, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.C.; Johnston, E.; Xing, C.; Hegeman, A.D. Measuring the chemical and cytotoxic variability of commercially available kava (Piper methysticum G. Forster). PLoS ONE 2014, 9, e111572. [Google Scholar] [CrossRef] [PubMed]
- Lebot, V.; Do, T.K.; Legendre, L. Detection of flavokavins (A, B, C) in cultivars of kava (Piper methysticum) using high performance thin layer chromatography (HPTLC). Food Chem. 2014, 151, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, X.; Ji, T.; Liu, Z.; Gu, M.; Hoang, B.H.; Zi, X. Dietary feeding of Flavokawain A, a Kava chalcone, exhibits a satisfactory safety profile and its association with enhancement of phase II enzymes in mice. Toxicol. Rep. 2014, 1, 2–11. [Google Scholar] [PubMed]
- Singh, Y.N.; Devkota, A.K. Aqueous kava extracts do not affect liver function tests in rats. Planta Med. 2003, 69, 496–469. [Google Scholar] [PubMed]
- Clayton, N.P.; Yoshizawa, K.; Kissling, G.E.; Burka, L.T.; Chan, P.C.; Nyska, A. Immunohistochemical analysis of expressions of hepatic cytochrome P450 in F344 rats following oral treatment with kava extract. Exp. Toxicol. Pathol. 2007, 58, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Bone, K. The Essential Guide to Herbal Safety; Elsevier Inc.: Philadelphia, PA, USA, 2005; Available online: https://books.google.it/books?id=85M0N8UioCcC&pg=PA197&lpg=PA197&dq=is+kava+really+hepatotoxic?&source=bl&ots=nrZVROjep&sig=EEFj0MpPOFqvXTJu81BlwMB6jHA&hl=it&sa=X&ved=0ahUKEwjI6L2KlrvLAhXIVRQKHQA2DCwQ6AEINzAE#v=onepage&q=is%20kava%20really%20hepatotoxic%3F&f=false (accessed on 27 December 2015).
- Unimuenster. Available online: http://www.uni-muenster.de/imperia/md/content/pharmazeutische_biologie/_v/review.pdf (accessed on 22 December 2015).
- Whittaker, P.; Clarke, J.J.; San, R.H.; Betz, J.M.; Seifried, H.E.; de Jager, L.S.; Dunkel, V.C. Evaluation of commercial kava extracts and kavalactone standards for mutagenicity and toxicity using the mammalian cell gene mutation assay in L5178Y mouse lymphoma cells. Food Chem. Toxicol. 2008, 46, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Behl, M.; Nyska, A.; Chhabra, R.S.; Travlos, G.S.; Fomby, L.M.; Sparrow, B.R.; Hejtmancik, M.R.; Chan, P.C. Liver toxicity and carcinogenicity in F344/N rats and B6C3F1 mice exposed to Kava Kava. Food Chem. Toxicol. 2011, 49, 2820–2829. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Toxicology and carcinogenesis studies of kava kava extract (CAS No. 9000-38-8) in F344/N rats and B6C3F1 mice (Gavage Studies). Natl. Toxicol. Progr. Tech. Rep. Ser. 2012, 571, 1–186. [Google Scholar]
- Yang, X.; Salminen, W.F. Kava extract, an herbal alternative for anxiety relief, potentiates acetaminophen-induced cytotoxicity in rat hepatic cells. Phytomedicine 2011, 18, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; Abittan, C.S. Pharmacology and metabolism of alcohol, including its metabolic effects and interactions with other drugs. Clin. Dermatol. 1999, 17, 365–379. [Google Scholar] [CrossRef]
- Li, A.P. A review of the common properties of drugs with idiosyncratic hepatotoxicity and the “multiple determinant hypothesis” for the manifestation of idiosyncratic drug toxicity. Chem. Biol. Interact. 2002, 142, 7–23. [Google Scholar] [CrossRef]
- Duffield, A.M.; Jamieson, D.D.; Lidgard, R.O.; Duffield, P.H.; Bourne, D.J. Identification of some human urinary metabolites of the intoxicating beverage kava. J. Chromatogr. 1989, 475, 273–281. [Google Scholar] [CrossRef]
- Shen, W.W. The metabolism of psychoactive drugs: A review of enzymatic biotransformation and inhibition. Biol. Psychiatry 1997, 41, 814–826. [Google Scholar] [CrossRef]
- Russmann, S.; Lauterburg, B.H.; Helbling, A. Kava hepatotoxicity. Ann. Intern. Med. 2001, 135, 68–69. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): Clinical consequences, evolutionary aspects and functional diversity. Pharmacogenom. J. 2005, 5, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Aghdassi, A.A.; Kraft, M.; Domschke, W.; Lerch, M.M.; Weiss, F.U. Genetic polymorphisms in the UDP-glucuronosyltransferase UGT1A7 gene in patients with acute liver failure after kava-kava consumption. Arch. Toxicol. 2015, 89, 2173–2174. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yu, L.; Nair, M.G.; DeWitt, D.L.; Ramsewak, R.S. Cyclooxygenase enzyme inhibitory compounds with antioxidant activities from Piper methysticum (kava kava) roots. Phytomedicine 2002, 9, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Raman, P.; Dewitt, D.L.; Nair, M.G. Lipid peroxidation and cyclooxygenase enzyme inhibitory activities of acidic aqueous extracts of some dietary supplements. Phytother. Res. 2008, 22, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Mathews, J.M.; Etheridge, A.S.; Black, S.R. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab. Dispos. 2002, 30, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Henderson, G.L.; Harkey, M.R.; Sakai, Y.; Li, A. Effects of kava (Kava-kava, ′Awa, Yaqona, Piper methysticum) on c-DNA-expressed cytochrome P450 enzymes and human cryopreserved hepatocytes. Phytomedicine 2004, 11, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Cote, C.S.; Kor, C.; Cohen, J.; Auclair, K. Composition and biological activity of traditional and commercial kava extracts. Biochem. Biophys. Res. Commun. 2004, 322, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Mathews, J.M.; Etheridge, A.S.; Valentine, J.L.; Black, S.R.; Coleman, D.P.; Patel, P.; So, J.; Burka, L.T. Pharmacokinetics and disposition of the kavalactone kawain: Interaction with kava extract and kavalactones in vivo and in vitro. Drug Metab. Dispos. 2005, 33, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.T.; Dragull, K.; Tang, C.S.; Bittenbender, H.C.; Efird, J.T.; Nerurkar, P.V. Effects of kava alkaloid, pipermethystine, and kavalactones on oxidative stress and cytochrome P450 in F-344 rats. Toxicol. Sci. 2007, 97, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, Q.; Xia, Q.; Dial, S.; Chan, P.C.; Fu, P. Analysis of gene expression changes of drug metabolizing enzymes in the livers of F344 rats following oral treatment with kava extract. Food Chem. Toxicol. 2009, 47, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Hashida, H.; Arita, A.; Hamaguchi, K.; Shimura, F. High dose of commercial products of kava (Piper methysticum) markedly enhanced hepatic cytochrome P450 1A1 mRNA expression with liver enlargement in rats. Food Chem. Toxicol. 2008, 46, 3732–3738. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shi, Q.; Dial, S.; Xia, Q.; Mei, N.; Li, Q.Z.; Chan, P.C.; Fu, P. Gene expression profiling in male B6C3F1 mouse livers exposed to kava identifies—Changes in drug metabolizing genes and potential mechanisms linked to kava toxicity. Food Chem. Toxicol. 2010, 48, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Harkey, M.R.; Henderson, G.L. Synthesis, in vitro, reactivity, and identification of 6-phenyl-3-hexen-2-one in human urine after kava-kava (Piper methysticum) ingestion. Planta Med. 2005, 71, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.M.; Qiu, S.X.; Zhang, S.; Zhang, F.; Burdette, J.E.; Yu, L.; Bolton, J.L.; van Breemen, R.B. Identification of novel electrophilic metabolites of Piper methysticum Forst (Kava). Chem. Res. Toxicol. 2003, 16, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Zenger, K.; Agnolet, S.; Schneider, B.; Kraus, B. Biotransformation of Flavokawains A, B, and C, Chalcones from Kava (Piper methysticum), by Human Liver Microsomes. J. Agric. Food Chem. 2015, 63, 6376–6385. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.; Ramzan, I. Are mould hepatotoxins responsible for kava hepatotoxicity? Phytother. Res. 2012, 26, 1768–1770. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Sarris, J.; Lebot, V. Contaminant hepatotoxins as culprits for kava hepatotoxicity—Fact or fiction? Phytother. Res. 2013, 27, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Rowe, A.; Ramzan, I. Does inflammation play a role in kava hepatotoxicity? Phytother. Res. 2011, 25, 629–630. [Google Scholar] [PubMed]
- Zhang, L.; Rowe, A.; Braet, F.; Ramzan, I. Macrophage depletion ameliorates kavalactone damage in the isolated perfused rat liver. J. Toxicol. Sci. 2012, 37, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Gow, P.J.; Connelly, N.J.; Hill, R.L.; Crowley, P.; Angus, P.W. Fatal fulminant hepatic failure induced by a natural therapy containing kava. Med. J. Aust. 2003, 178, 442–443. [Google Scholar] [PubMed]
- Escher, M.; Desmeules, J.; Giostra, E.; Mentha, G. Hepatitis associated with Kava, a herbal remedy for anxiety. BMJ 2001, 322, 1097. [Google Scholar] [CrossRef]
- Campo, J.V.; McNabb, J.; Perel, J.M.; Mazariegos, G.V.; Hasegawa, S.L.; Reyes, J. Kava-induced fulminant hepatic failure. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 631–632. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.D.; Stolpman, D.; Olyaei, A.; Corless, C.L.; Ham, J.M.; Schwartz, J.M.; Orloff, S.L. High prevalence of potentially hepatotoxic herbal supplement use in patients with fulminant hepatic failure. Arch. Surg. 2003, 138, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Baumuller, H.M.; Seitz, K.; Vasilakis, D.; Seitz, G.; Seitz, H.K.; Schuppan, D. Hepatitis induced by Kava (Piper methysticum rhizoma). J. Hepatol. 2003, 39, 62–67. [Google Scholar] [CrossRef]
- Humberston, C.L.; Akhtar, J.; Krenzelok, E.P. Acute hepatitis induced by kava kava. J. Toxicol. Clin. Toxicol. 2003, 41, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Gaus, W.; Loew, D. Kava extracts: Safety and risks including rare hepatotoxicity. Phytomedicine 2003, 10, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Christl, S.U.; Seifert, A.; Seeler, D. Toxic hepatitis after consumption of traditional kava preparation. J. Travel Med. 2009, 16, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Scribd. Available online: http://www.scribd.com/doc/46179789/Cytotoxicity-of-Extract-of-Malaysian-Mitragyna-Speciosa-Korth-and-Its-Dominant-Alkaloid Mitragynine#scribd (accessed on 4 January 2016).
- Moklas, M.A.M.; Nurul Raudzah, A.R.; Taufik Hidayat, M.; Sharida, F.; Farah Idayu, N.; Zulkhairi, A.; Shamima, A.R. A preliminary toxicity study of mitragynine, an alkaloid from Mitragyna speciosa Korth and its effects on locomotor activity in rats. Adv. Med. Dent. Sci. 2008, 2, 56–60. [Google Scholar]
- Ghazali, A.R.; Abdullah, R.; Ramli, N.; Rajab, N.F.; Ahmad-Kamal, M.S.; Yahya, N.A. Mutagenic and antimutagenic activities of Mitragyna speciosa Korth extract using Ames test. J. Med. Plants Res. 2011, 5, 1345–1348. [Google Scholar]
- Macko, E.; Weisbach, J.A.; Douglas, B. Some observations on the pharmacology of mitragynine. Arch. Int. Pharmacodyn. Ther. 1972, 198, 145–161. [Google Scholar] [PubMed]
- De Moraes, N.V.; Moretti, R.A.; Furr, E.B., 3rd; McCurdy, C.R.; Lanchote, V.L. Determination of mitragynine in rat plasma by LC-MS/MS: Application to pharmacokinetics. J. Chromatogr. B 2009, 877, 2593–2597. [Google Scholar] [CrossRef] [PubMed]
- Janchawee, B.; Keawpradub, N.; Chittrakarn, S.; Prasettho, S.; Wararatananurak, P.; Sawangjareon, K. A high-performance liquid chromatographic method for determination of mitragynine in serum and its application to a pharmacokinetic study in rats. Biomed. Chromatogr. 2007, 21, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Azizi, J.; Ismail, S.; Mordi, M.N.; Ramanathan, S.; Said, M.I.; Mansor, S.M. In vitro and in vivo effects of three different Mitragyna speciosa korth leaf extracts on phase II drug metabolizing enzymes—Glutathione transferases (GSTs). Molecules 2010, 15, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Kalix, P. Khat, an amphetamine-like stimulant. J. Psychoact. Drugs 1994, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Nencini, P.; Ahmed, A.M. Khat consumption: A pharmacological review. Drug Alcohol Depend. 1989, 23, 19–29. [Google Scholar] [CrossRef]
- Chapman, M.H.; Kajihara, M.; Borges, G.; O’Beirne, J.; Patch, D.; Dhillon, A.P.; Crozier, A.; Morgan, M.Y. Severe, acute liver injury and khat leaves. N. Engl. J. Med. 2010, 362, 1642–1644. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.D.; Chen, J.; Xiang, M.; Zhou, J.; Chen, X.; Gong, F. Khat (Catha edulis) generates reactive oxygen species and promotes hepatic cell apoptosis via MAPK activation. Int. J. Mol. Med. 2013, 32, 389–395. [Google Scholar] [PubMed]
- Aklillu, E.; Herrlin, K.; Gustafsson, L.L.; Bertilsson, L.; Ingelman-Sundberg, M. Evidence for environmental influence on CYP2D6-catalysed debrisoquine hydroxylation as demonstrated by phenotyping and genotyping of Ethiopians living in Ethiopia or in Sweden. Pharmacogenetics 2002, 12, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Cascorbi, I. Pharmacogenetics of cytochrome p4502D6: Genetic background and clinical implication. Eur. J. Clin. Investig. 2003, 33 (Suppl. S2), 17–22. [Google Scholar] [CrossRef]
- Pedersen, A.J.; Reitzel, L.A.; Johansen, S.S.; Linnet, K. In vitro metabolism studies on mephedrone and analysis of forensic cases. Drug Test. Anal. 2013, 5, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.J.; Petersen, T.H.; Linnet, K. In vitro metabolism and pharmacokinetic studies on methylone. Drug Metab. Dispos. 2013, 41, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Bedada, W.; de Andres, F.; Engidawork, E.; Pohanka, A.; Beck, O.; Bertilsson, L.; Llerena, A.; Aklillu, E. The Psychostimulant khat (Catha edulis) Inhibits CYP2D6 enzyme activity in humans. J. Clin. Psychopharmacol. 2015, 35, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Kava hepatotoxicity: Pathogenetic aspects and prospective considerations. Liver Int. 2010, 30, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, K.; Schmidt, M.; Nahrstedt, A. German kava ban lifted by court: The alleged hepatotoxicity of kava (Piper methysticum) as a case of ill-defined herbal drug identity, lacking quality control, and misguided regulatory politics. Planta Med. 2015, 81, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Herbalgram. Available online: http://cms.herbalgram.org/heg/volume11/07July/GermanKavaBanReversal.html?ts=1457796407&signature=f5ff90a5a274ae67fa1fc62f1ed7ee40%3Cspan&ts=1458062348&signature=91d4418f692013ef2178b44d285f2169 (accessed on 13 March 2016).
- Savage, K.M.; Stough, C.K.; Byrne, G.J.; Scholey, A.; Bousman, C.; Murphy, J.; Macdonald, P.; Suo, C.; Hughes, M.; Thomas, S.; et al. Kava for the treatment of generalised anxiety disorder (K-GAD): Study protocol for a randomised controlled trial. Trials 2015, 16. [Google Scholar] [CrossRef] [PubMed]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantano, F.; Tittarelli, R.; Mannocchi, G.; Zaami, S.; Ricci, S.; Giorgetti, R.; Terranova, D.; Busardò, F.P.; Marinelli, E. Hepatotoxicity Induced by “the 3Ks”: Kava, Kratom and Khat. Int. J. Mol. Sci. 2016, 17, 580. https://doi.org/10.3390/ijms17040580
Pantano F, Tittarelli R, Mannocchi G, Zaami S, Ricci S, Giorgetti R, Terranova D, Busardò FP, Marinelli E. Hepatotoxicity Induced by “the 3Ks”: Kava, Kratom and Khat. International Journal of Molecular Sciences. 2016; 17(4):580. https://doi.org/10.3390/ijms17040580
Chicago/Turabian StylePantano, Flaminia, Roberta Tittarelli, Giulio Mannocchi, Simona Zaami, Serafino Ricci, Raffaele Giorgetti, Daniela Terranova, Francesco P. Busardò, and Enrico Marinelli. 2016. "Hepatotoxicity Induced by “the 3Ks”: Kava, Kratom and Khat" International Journal of Molecular Sciences 17, no. 4: 580. https://doi.org/10.3390/ijms17040580








