Prodifferentiation Activity of Novel Vitamin D2 Analogs PRI-1916 and PRI-1917 and Their Combinations with a Plant Polyphenol in Acute Myeloid Leukemia Cells
Abstract
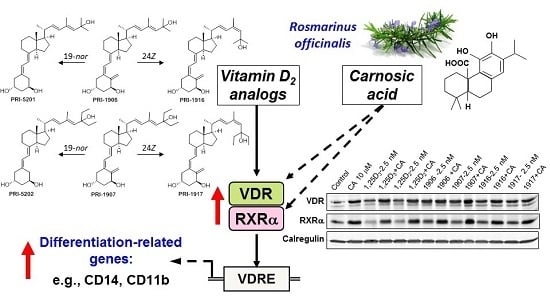
:1. Introduction
2. Results
2.1. Comparison of the Differentiation-Inducing Effects of Different Vitamin D Derivatives in a Panel of AML Cell Lines
2.2. Effects of Combinations of Vitamin D Derivatives and Carnosic Acid on Cell Differentiation and Cell Cycle Distribution in AML Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals, Antibodies and Plasmids
4.2. Cell Culture
4.3. Determination of Cell Differentiation and Cell Cycle Distribution by Flow Cytometry
4.4. Preparation of Whole Cell Lysates and Western Blotting
4.5. Transient Transfection and Reporter Gene Assay
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Abbreviations
| 1,25D2 | 1α,25-dihydroxyvitamin D2 |
| 1,25D3 | 1α,25-dihydroxyvitamin D3 |
| 25D3 | 25-hydroxyvitamin D3 |
| AML | acute myeloid leukemia |
| CA | carnosic acid |
| DR3 | direct repeat 3 |
| ER | everted repeat |
| LBD | ligand binding domain |
| RXRα | retinoid X receptor |
| VDDs | vitamin D derivatives |
| VDR | vitamin D receptor |
| VDRE | vitamin D response element |
References
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Hunter, A.E.; Kjeldsen, L.; Yin, J.; Gibson, B.E.; Wheatley, K.; Milligan, D. Optimization of chemotherapy for younger patients with acute myeloid leukemia: Results of the medical research council AML15 trial. J. Clin. Oncol. 2013, 31, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Erba, H.P. Finding the optimal combination therapy for the treatment of newly diagnosed AML in older patients unfit for intensive therapy. Leuk. Res. 2015, 39, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E. New agents: Great expectations not realized. Best Pract. Res. Clin. Haematol. 2013, 26, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Gocek, E.; Studzinski, G.P. Vitamin D and differentiation in cancer. Crit. Rev. Clin. Lab. Sci. 2009, 46, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Vanoirbeek, E.; Krishnan, A.; Eelen, G.; Verlinden, L.; Bouillon, R.; Feldman, D.; Verstuyf, A. The anti-cancer and anti-inflammatory actions of 1,25OH2D3. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Radujkovic, A.; Schnitzler, P.; Ho, A.D.; Dreger, P.; Luft, T. Low serum vitamin D levels are associated with shorter survival after first-line azacitidine treatment in patients with myelodysplastic syndrome and secondary oligoblastic acute myeloid leukemia. Clin. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Weinstein, S.J.; Moy, K.A.; Mannisto, S.; Albanes, D. Circulating 25-hydroxyvitamin D and prostate cancer survival. Cancer Epidemiol. Biomark. Prev. 2016, 25, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Muindi, J.R.; Tan, W.; Hu, Q.; Wang, D.; Liu, S.; Wilding, G.E.; Ford, L.A.; Sait, S.N.; Block, A.W.; et al. Low 25(OH) vitamin D3 levels are associated with adverse outcome in newly diagnosed, intensively treated adult acute myeloid leukemia. Cancer 2014, 120, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Cheung, F.S.; Lovicu, F.J.; Reichardt, J.K. Current progress in using vitamin D and its analogs for cancer prevention and treatment. Expert Rev. Anticancer Ther. 2012, 12, 811–837. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mirandola, L.; Pandey, A.; Nguyen, D.D.; Jenkins, M.R.; Turcel, M.; Cobos, E.; Chiriva-Internati, M. Application of vitamin D and derivatives in hematological malignancies. Cancer Lett. 2012, 319, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Gocek, E.; Studzinski, G.P. DNA repair in despair—Vitamin D is not fair. J. Cell. Biochem. 2016, 117, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, M.; Studzinski, G.P. Enhancement by other compounds of the anti-cancer activity of vitamin D3 and its analogs. Exp. Cell Res. 2004, 298, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Stefan, T.; Ignatz-Hoover, J.; Moreton, S.; Parizher, G.; Saunthararajah, Y.; Wald, D.N. GSK-3 inhibition sensitizes acute myeloid leukemia cells to 1,25D-mediated differentiation. Cancer Res. 2016, 76, 2743–2753. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pesakhov, S.; Harrison, J.S.; Kafka, M.; Danilenko, M.; Studzinski, G.P. The MAPK ERK5, but not ERK1/2, inhibits the progression of monocytic phenotype to the functioning macrophage. Exp. Cell Res. 2015, 330, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Falzon, M. Restoration of the anti-proliferative and anti-migratory effects of 1,25-dihydroxyvitamin D by silibinin in vitamin D-resistant colon cancer cells. Cancer Lett. 2015, 362, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Nachliely, M.; Sharony, E.; Kutner, A.; Danilenko, M. Novel analogs of 1,25-dihydroxyvitamin D2 combined with a plant polyphenol as highly efficient inducers of differentiation in human acute myeloid leukemia cells. J. Steroid Biochem. Mol. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.; Priel, I.; Giat, J.; Levy, J.; Sharoni, Y.; Danilenko, M. Carnosic acid inhibits proliferation and augments differentiation of human leukemic cells induced by 1,25-dihydroxyvitamin D3 and retinoic acid. Nutr. Cancer 2001, 41, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Bobilev, I.; Novik, V.; Levi, I.; Shpilberg, O.; Levy, J.; Sharoni, Y.; Studzinski, G.P.; Danilenko, M. The Nrf2 transcription factor is a positive regulator of myeloid differentiation of acute myeloid leukemia cells. Cancer Biol. Ther. 2011, 11, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Harrison, J.S.; Uskokovic, M.; Danilenko, M.; Studzinski, G.P. Silibinin can induce differentiation as well as enhance vitamin D3-induced differentiation of human AML cells ex vivo and regulates the levels of differentiation-related transcription factors. Hematol. Oncol. 2010, 28, 124–132. [Google Scholar] [PubMed]
- Danilenko, M.; Wang, Q.; Wang, X.; Levy, J.; Sharoni, Y.; Studzinski, G.P. Carnosic acid potentiates the antioxidant and prodifferentiation effects of 1α,25-dihydroxyvitamin D3 in leukemia cells but does not promote elevation of basal levels of intracellular calcium. Cancer Res. 2003, 63, 1325–1332. [Google Scholar] [PubMed]
- Kang, S.N.; Lee, M.H.; Kim, K.M.; Cho, D.; Kim, T.S. Induction of human promyelocytic leukemia HL-60 cell differentiation into monocytes by silibinin: Involvement of protein kinase C. Biochem. Pharmacol. 2001, 61, 1487–1495. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, R.L.; Cui, X.X.; Newmark, H.L.; Conney, A.H. Synergistic effects of curcumin on all-trans retinoic acid- and 1α,25-dihydroxyvitamin D3-induced differentiation in human promyelocytic leukemia HL-60 cells. Oncol. Res. 1997, 9, 19–29. [Google Scholar] [PubMed]
- Jones, G. Extrarenal vitamin D activation and interactions between vitamin D2, vitamin D3, and vitamin D analogs. Annu. Rev. Nutr. 2013, 33, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.D.; Garcia, F.G.; Walsh, R.J. A comparison of the toxicity of ergocalciferol and cholecalciferol in rhesus monkeys (Macaca mulatta). J. Nutr. 1972, 102, 975–986. [Google Scholar] [PubMed]
- Sjoden, G.; Smith, C.; Lindgren, U.; DeLuca, H.F. 1α-hydroxyvitamin D2 is less toxic than 1α-hydroxyvitamin D3 in the rat. Proc. Soc. Exp. Biol. Med. 1985, 178, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Opolski, A.; Wietrzyk, J.; Chrobak, A.; Marcinkowska, E.; Wojdat, E.; Kutner, A.; Radzikowski, C. Antiproliferative activity in vitro of side-chain analogues of calcitriol against various human normal and cancer cell lines. Anticancer Res. 1999, 19, 5217–5222. [Google Scholar] [PubMed]
- Pietraszek, A.; Malinska, M.; Chodynski, M.; Krupa, M.; Krajewski, K.; Cmoch, P.; Wozniak, K.; Kutner, A. Synthesis and crystallographic study of 1,25-dihydroxyergocalciferol analogs. Steroids 2013, 78, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Bolla, N.R.; Corcoran, A.; Yasuda, K.; Chodynski, M.; Krajewski, K.; Cmoch, P.; Marcinkowska, E.; Brown, G.; Sakaki, T.; Kutner, A. Synthesis and evaluation of geometric analogs of 1α,25-dihydroxyvitamin D as potential therapeutics. J. Steroid Biochem. Mol. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Baurska, H.; Marchwicka, A.; Klopot, A.; Kutner, A.; Marcinkowska, E. Studies on the mechanisms of superagonistic pro-differentiating activities of side-chain modified analogs of vitamin D2. Oncol. Rep. 2012, 28, 1110–1116. [Google Scholar] [PubMed]
- Kojima, K.; Konopleva, M.; Samudio, I.J.; Shikami, M.; Cabreira-Hansen, M.; McQueen, T.; Ruvolo, V.; Tsao, T.; Zeng, Z.; Vassilev, L.T.; et al. MDM2 antagonists induce p53-dependent apoptosis in AML: Implications for leukemia therapy. Blood 2005, 106, 3150–3159. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Rotter, V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc. Natl. Acad. Sci. USA 1985, 82, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Durland-Busbice, S.; Reisman, D. Lack of p53 expression in human myeloid leukemias is not due to mutations in transcriptional regulatory regions of the gene. Leukemia 2002, 16, 2165–2167. [Google Scholar] [CrossRef] [PubMed]
- Bastie, J.N.; Balitrand, N.; Guidez, F.; Guillemot, I.; Larghero, J.; Calabresse, C.; Chomienne, C.; Delva, L. 1α,25-dihydroxyvitamin D3 transrepresses retinoic acid transcriptional activity via vitamin D receptor in myeloid cells. Mol. Endocrinol. 2004, 18, 2685–2699. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, M.; Wang, X.; Studzinski, G.P. Carnosic acid and promotion of monocytic differentiation of HL60-G cells initiated by other agents. J. Natl. Cancer Inst. 2001, 93, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, L.; Li, X.; Yu, C.; Zhang, K.; Jiang, Y.; Wu, L.; Lu, W.; Tu, P. High-performance liquid chromatography method for determination of carnosic acid in rat plasma and its application to pharmacokinetic study. Biomed. Chromatogr. 2009, 23, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Wietrzyk, J.; Nevozhay, D.; Milczarek, M.; Filip, B.; Kutner, A. Toxicity and antitumor activity of the vitamin D analogs PRI-1906 and PRI-1907 in combined treatment with cyclophosphamide in a mouse mammary cancer model. Cancer Chemother. Pharmacol. 2008, 62, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Trynda, J.; Turlej, E.; Milczarek, M.; Pietraszek, A.; Chodynski, M.; Kutner, A.; Wietrzyk, J. Antiproliferative activity and in vivo toxicity of double-point modified analogs of 1,25-dihydroxyergocalciferol. Int. J. Mol. Sci. 2015, 16, 24873–24894. [Google Scholar] [CrossRef] [PubMed]
- Malinska, M.; Kutner, A.; Wozniak, K. Predicted structures of new Vitamin D Receptor agonists based on available X-ray structures. Steroids 2015, 104, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, S.; Danielsson, C.; Kahlen, J.P.; Schrader, M.; Mathiasen, I.S.; Binderup, L.; Carlberg, C. The anti-proliferative effect of vitamin D3 analogues is not mediated by inhibition of the AP-1 pathway, but may be related to promoter selectivity. Oncogene 1995, 11, 1853–1858. [Google Scholar] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2 vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Yu, H.; Kim, J.J.; Lee, M.J.; Park, S.K. Vitamin D-induced ectodomain shedding of TNF receptor 1 as a nongenomic action: D3 vs. D2 derivatives. J. Steroid Biochem. Mol. Biol. 2016, 155, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, R.; Novik, V.; Danilenko, M. Cell-type-specific effects of silibinin on vitamin D-induced differentiation of acute myeloid leukemia cells are associated with differential modulation of RXRα levels. Leuk. Res. Treat. 2012, 2012. [Google Scholar] [CrossRef]
- Shabtay, A.; Sharabani, H.; Barvish, Z.; Kafka, M.; Amichay, D.; Levy, J.; Sharoni, Y.; Uskokovic, M.R.; Studzinski, G.P.; Danilenko, M. Synergistic antileukemic activity of carnosic acid-rich rosemary extract and the 19-nor gemini vitamin D analogue in a mouse model of systemic acute myeloid leukemia. Oncology 2008, 75, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Sharabani, H.; Izumchenko, E.; Wang, Q.; Kreinin, R.; Steiner, M.; Barvish, Z.; Kafka, M.; Sharoni, Y.; Levy, J.; Uskokovic, M.; et al. Cooperative antitumor effects of vitamin D3 derivatives and rosemary preparations in a mouse model of myeloid leukemia. Int. J. Cancer 2006, 118, 3012–3021. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Freedman, L.P. Transcriptional synergism between the vitamin D3 receptor and other nonreceptor transcription factors. Mol. Endocrinol. 1994, 8, 1593–1604. [Google Scholar] [PubMed]







| Compounds | HL60 | U937 | MOLM-13 | KG-1a |
|---|---|---|---|---|
| 1,25D3 | 4.58 ± 0.34 | 1.97 ± 0.19 | 1.45 ± 0.09 | 8.65 ± 0.42 |
| 1,25D2 | 8.71 ± 0.62 * | 4.31 ± 0.66 * | 1.86 ± 0.15 | 32.29 ± 4.52 ** |
| PRI-1906 | 4.88 ± 0.02 | 2.51 ± 0.28 | 1.67 ± 0.10 | 4.72 ± 0.97 |
| PRI-1916 | 3.45 ± 0.07 * | 2.87 ± 0.43 | 1.19 ± 0.08 | 5.85 ± 1.24 |
| PRI-1907 | 0.42 ± 0.06 | 0.56 ± 0.08 | 0.19 ± 0.02 | 1.27 ± 0.04 |
| PRI-1917 | 3.90 ± 0.15 ## | 2.92 ± 0.51 ## | 0.57 ± 0.04 # | 4.87 ± 0.42 # |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nachliely, M.; Sharony, E.; Bolla, N.R.; Kutner, A.; Danilenko, M. Prodifferentiation Activity of Novel Vitamin D2 Analogs PRI-1916 and PRI-1917 and Their Combinations with a Plant Polyphenol in Acute Myeloid Leukemia Cells. Int. J. Mol. Sci. 2016, 17, 1068. https://doi.org/10.3390/ijms17071068
Nachliely M, Sharony E, Bolla NR, Kutner A, Danilenko M. Prodifferentiation Activity of Novel Vitamin D2 Analogs PRI-1916 and PRI-1917 and Their Combinations with a Plant Polyphenol in Acute Myeloid Leukemia Cells. International Journal of Molecular Sciences. 2016; 17(7):1068. https://doi.org/10.3390/ijms17071068
Chicago/Turabian StyleNachliely, Matan, Ehud Sharony, Narasimha Rao Bolla, Andrzej Kutner, and Michael Danilenko. 2016. "Prodifferentiation Activity of Novel Vitamin D2 Analogs PRI-1916 and PRI-1917 and Their Combinations with a Plant Polyphenol in Acute Myeloid Leukemia Cells" International Journal of Molecular Sciences 17, no. 7: 1068. https://doi.org/10.3390/ijms17071068
APA StyleNachliely, M., Sharony, E., Bolla, N. R., Kutner, A., & Danilenko, M. (2016). Prodifferentiation Activity of Novel Vitamin D2 Analogs PRI-1916 and PRI-1917 and Their Combinations with a Plant Polyphenol in Acute Myeloid Leukemia Cells. International Journal of Molecular Sciences, 17(7), 1068. https://doi.org/10.3390/ijms17071068