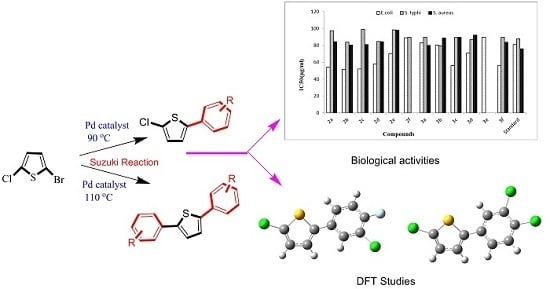
One Pot Selective Arylation of 2-Bromo-5-Chloro Thiophene; Molecular Structure Investigation via Density Functional Theory (DFT), X-ray Analysis, and Their Biological Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparations
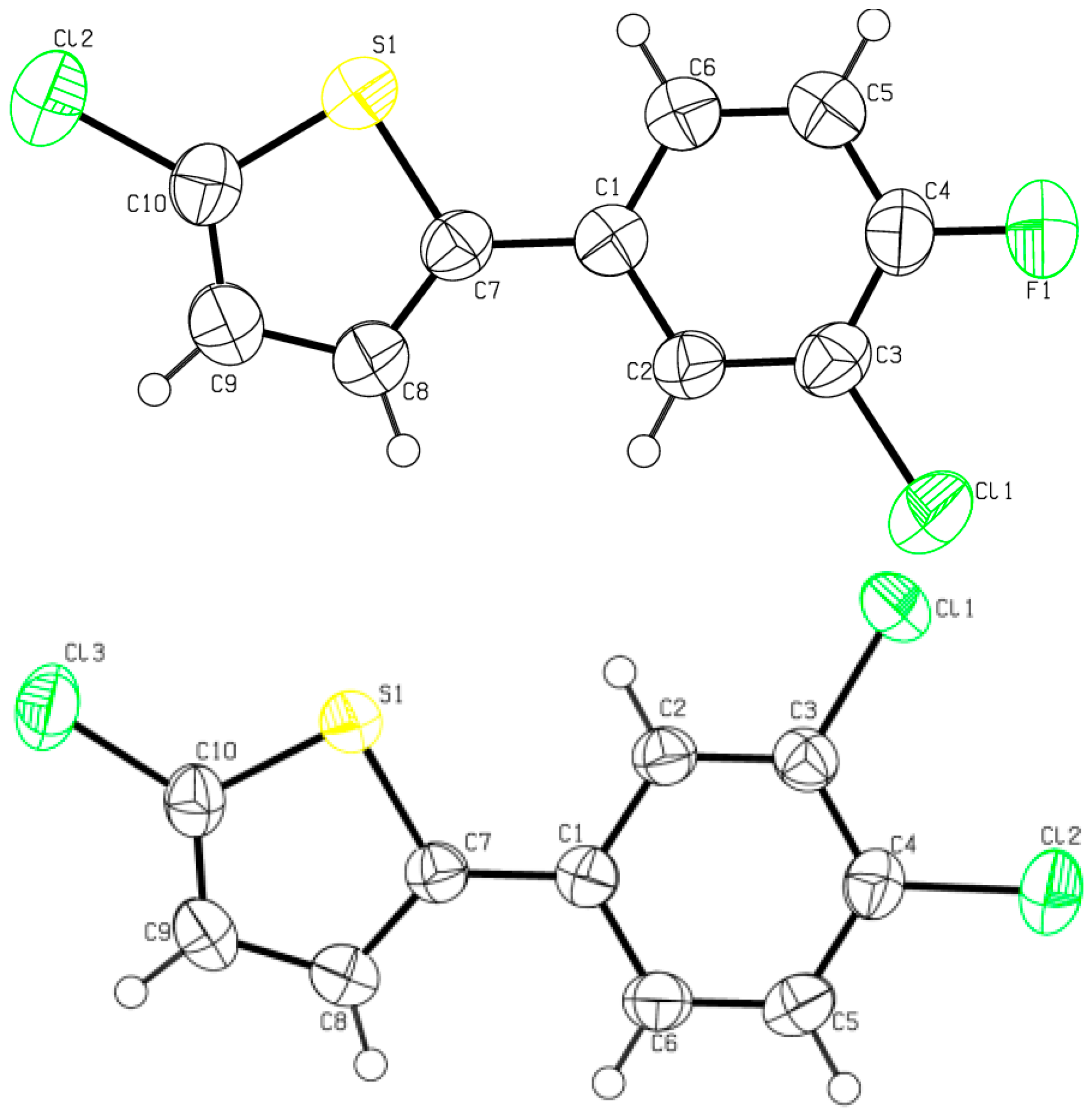
2.2. Crystal Structure Determinations
2.3. Density Functional Theory (DFT) Studies
2.3.1. Molecular Geometries
2.3.2. Frontier Molecular Orbital (FMOs) Analysis
2.3.3. Molecular Electrostatic Potential (MEP)
3. Biological Studies
3.1. Antibacterial Activity
3.2. Antioxidant Activity
4. Materials and Methods
4.1. Synthesis of 2-Aryl-5-chloro thiophenes (2a–f)
4.1.1. 2-Chloro-5-(4-methylphenyl) thiophene (2a)
4.1.2. 2-Chloro-5-(4-methoxyphenyl) thiophene (2b)
4.1.3. 2-Chloro-5-(4-chlorophenyl) thiophene (2c)
4.1.4. 2-Chloro-5-(3-chloro-4-fluorophenyl) thiophene (2d)
4.1.5. 2-Chloro-5-(3,5-dimethylphenyl) thiophene (2e)
4.1.6. 2-Chloro-5-(3,4-dichlorophenyl) thiophene (2f)
4.2. Synthesis of Biarylthiophenes (3a–f)
4.2.1. 2,5-Bis(4-methylphenyl) thiophene (3a)
4.2.2. 2,5-Bis(4-methoxyphenyl) thiophene (3b)
4.2.3. 2,5-Bis(4-chlorophenyl) thiophene (3c)
4.2.4. 2,5-Bis(3-chloro-4-fluorophenyl) thiophene (3d)
4.2.5. 2,5-Bis(3,5-dimethylphenyl) thiophene (3e)
4.2.6. 2,5-Bis(3,4-dichlorophenyl) thiophene (3f)
4.3. X-ray Diffraction Analysis
4.4. Computational Methods
4.5. Antibacterial Assay
4.6. Nitric Oxide Scavenging Activity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parry, P.R.; Wang, C.; Batsanov, A.S.; Bryce, M.R.; Tarbit, B. Functionalized pyridylboronic acids and their Suzuki cross-coupling reactions to yield novel heteroarylpyridines. J. Org. Chem. 2002, 67, 7541–7543. [Google Scholar] [CrossRef] [PubMed]
- Proutiere, F.; Aufiero, M.; Schoenebeck, F. Reactivity and stability of dinuclear Pd(I) complexes: Studies on the active catalytic species, insights into precatalyst activation and deactivation, and application in highly selective cross-coupling reactions. J. Am. Chem. Soc. 2011, 134, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liebscher, J. Carbon–carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Joshaghani, M.; Daryanavard, M.; Rafiee, E.; Nadri, S. Synthesis and applications of a new palladacycle as a high active catalyst in the Suzuki couplings. J. Organomet. Chem. 2008, 693, 3135–3140. [Google Scholar] [CrossRef]
- Mora, M.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Suzuki cross-coupling reactions over Pd(II)-hydrotalcite catalysts in water. J. Mol. Catal. A Chem. 2008, 285, 79–83. [Google Scholar] [CrossRef]
- Zim, D.; Nobre, S.M.; Monteiro, A.L. Suzuki cross-coupling reaction catalyzed by sulfur-containing palladacycles: Formation of palladium active species. J. Mol. Catal. A Chem. 2008, 287, 16–23. [Google Scholar] [CrossRef]
- Guan, R.F.; Zhou, C.J.; Feng, S.Y.; Berg, D.J.; Stobart, S.R. The application of Suzuki coupling reaction on the preparation of carbosilane dendrimers with 4-(naphthalen-1-yl) phenyl core. Chin. Chem. Lett. 2006, 17, 293–295. [Google Scholar]
- Abbas, S.; Hussain, M.; Ali, S.; Parvez, M.; Raza, A.; Haider, A.; Iqbal, J. Structural, enzyme inhibition, antibacterial and DNA protection studies of organotin(IV) derivatives of thiophene-2-carboxylic acid. J. Organomet. Chem. 2013, 724, 255–261. [Google Scholar] [CrossRef]
- Gouda, M.A.; Berghot, M.A.; Baz, E.A.; Hamama, W.S. Synthesis, antitumor and antioxidant evaluation of some new thiazole and thiophene derivatives incorporated coumarin moiety. Med. Chem. Res. 2012, 21, 1062–1070. [Google Scholar] [CrossRef]
- Rizwan, K.; Zubair, M.; Rasool, N.; Ali, S.; Zahoor, A.F.; Rana, U.A.; Khan, S.U.-D.; Shahid, M.; Zia-Ul-Haq, M.; Jaafar, H.Z. Regioselective synthesis of 2-(bromomethyl)-5-aryl-thiophene derivatives via palladium(0) catalyzed suzuki cross-coupling reactions: As antithrombotic and haemolytically active molecules. Chem. Cent. J. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Gull, Y.; Rasool, N.; Noreen, M.; Nasim, F.-u.-H.; Yaqoob, A.; Kousar, S.; Rashid, U.; Bukhari, I.H.; Zubair, M.; Islam, M.S. Efficient synthesis of 2-amino-6-arylbenzothiazoles via Pd(0) Suzuki cross coupling reactions: Potent urease enzyme inhibition and nitric oxide scavenging activities of the products. Molecules 2013, 18, 8845–8857. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Cross-coupling reactions of organoboranes: An easy way to construct C–C bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S.; Lahiri, K.; Kashinath, D. Recent applications of the Suzuki–Miyaura cross-coupling reaction in organic synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar] [CrossRef]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Non-conventional methodologies for transition-metal catalysed carbon–carbon coupling: A critical overview. Part 2: The Suzuki reaction. Tetrahedron 2008, 64, 3047–3101. [Google Scholar] [CrossRef]
- Noreen, M.; Rasool, N.; Gull, Y.; Zahoor, A.F.; Yaqoob, A.; Kousar, S.; Zubair, M.; Bukhari, I.H.; Rana, U.A. A facile synthesis of new 5-aryl-thiophenes bearing sulfonamide moiety via Pd(0)-catalyzed Suzuki–Miyaura cross coupling reactions and 5-bromothiophene-2-acetamide: As potent urease inhibitor, antibacterial agent and hemolytically active compounds. J. Saudi Chem. Soc. 2014, in press. [Google Scholar] [CrossRef]
- Ali, S.; Rasool, N.; Ullah, A.; Nasim, F.-u.-H.; Yaqoob, A.; Zubair, M.; Rashid, U.; Riaz, M. Design and synthesis of arylthiophene-2-carbaldehydes via Suzuki-Miyaura reactions and their biological evaluation. Molecules 2013, 18, 14711–14725. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Rasool, N.; Noreen, M.; Gull, Y.; Zubair, M.; Ullah, A.; Rana, U.A. Palladium(0) catalyzed Suzuki cross-coupling reactions of 2,4-dibromothiophene: Selectivity, characterization and biological applications. J. Sulfur Chem. 2015, 36, 240–250. [Google Scholar] [CrossRef]
- Dong, C.-G.; Hu, Q.-S. Preferential oxidative addition in palladium(0)-catalyzed Suzuki cross-coupling reactions of dihaloarenes with arylboronic acids. J. Am. Chem. Soc. 2005, 127, 10006–10007. [Google Scholar] [CrossRef] [PubMed]
- Campaigne, E.; Foye, W.O. The synthesis of 2,5-diarylthiophenes. J. Org. Chem. 1952, 17, 1405–1412. [Google Scholar] [CrossRef]
- Robbins, D.W.; Hartwig, J.F. A C–H borylation approach to Suzuki–Miyaura coupling of typically unstable 2-heteroaryl and polyfluorophenyl boronates. Org. Lett. 2012, 14, 4266–4269. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.P.; Becker, M.R.; Knochel, P. Continuous flow magnesiation of functionalized heterocycles and acrylates with TMPMgCl⋅LiCl. Angew. Chem. Int. Ed. 2014, 53, 7933–7937. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.-T.; Liu, H.; Lin, Z.-J.; Jiang, J.; Shen, D.-S.; Liu, R.-Z.; Ke, Z.; Liu, F.-S. Aerobic and efficient direct arylation of five-membered heteroarenes and their benzocondensed derivatives with aryl bromides by bulky α-hydroxyimine palladium complexes. Organometallics 2015, 34, 4881–4894. [Google Scholar] [CrossRef]
- Noreen, M.; Rasool, N.; Gull, Y.; Zubair, M.; Mahmood, T.; Ayub, K.; Nasim, F.-U.-H.; Yaqoob, A.; Zia-Ul-Haq, M.; de Feo, V. Synthesis, density functional theory (DFT), urease inhibition and antimicrobial activities of 5-aryl thiophenes bearing sulphonylacetamide moieties. Molecules 2015, 20, 19914–19928. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.U.; Arfan, M.; Mahmood, T.; Liaqat, W.; Gilani, M.A.; Uddin, G.; Ludwig, R.; Zaman, K.; Choudhary, M.I.; Khattak, K.F. Isolation, spectroscopic and density functional theory studies of 7-(4-methoxyphenyl)-9H-furo[2,3-f]chromen-9-one: A new flavonoid from the bark of Millettia ovalifolia. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 146, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Liégault, B.; Petrov, I.; Gorelsky, S.I.; Fagnou, K. Modulating reactivity and diverting selectivity in palladium-catalyzed heteroaromatic direct arylation through the use of a chloride activating/blocking group. J. Org. Chem. 2010, 75, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Itami, K. Palladium/2,2′-bipyridyl/Ag2CO3 catalyst for C–H bond arylation of heteroarenes with haloarenes. Tetrahedron 2011, 67, 4425–4430. [Google Scholar] [CrossRef]
- René, O.; Fagnou, K. Room-temperature direct arylation of polyfluorinated arenes under biphasic conditions. Org. Lett. 2010, 12, 2116–2119. [Google Scholar] [CrossRef] [PubMed]
- Bayh, O.; Awad, H.; Mongin, F.; Hoarau, C.; Bischoff, L.; Trécourt, F.; Quéguiner, G.; Marsais, F.; Blanco, F.; Abarca, B. Deprotonation of benzoxazole and oxazole using lithium magnesates. J. Org. Chem. 2005, 70, 5190–5196. [Google Scholar] [CrossRef] [PubMed]
- Tùng, Đ.T.; Tuân, Đ.T.; Rasool, N.; Villinger, A.; Reinke, H.; Fischer, C.; Langer, P. Regioselective Palladium(0)-catalyzed cross-coupling reactions and metal-halide exchange reactions of tetrabromothiophene: Optimization, scope and limitations. Adv. Synth. Catal. 2009, 351, 1595–1609. [Google Scholar] [CrossRef]
- Bak, B.; Christensen, D.; Hansen-Nygaard, L.; Rastrup-Andersen, J. The structure of thiophene. J. Mol. Spectrosc. 1961, 7, 58–63. [Google Scholar] [CrossRef]
- Ahmed, M.N.; Yasin, K.A.; Ayub, K.; Mahmood, T.; Tahir, M.N.; Khan, B.A.; Hafeez, M.; Ahmed, M. Click one pot synthesis, spectral analyses, crystal structures, DFT studies and brine shrimp cytotoxicity assay of two newly synthesized 1,4,5-trisubstituted 1,2,3-triazoles. J. Mol. Struct. 2016, 1106, 430–439. [Google Scholar] [CrossRef]
- Arshad, M.N.; Mahmood, T.; Khan, A.F.; Zia-Ur-Rehman, M.; Asiri, A.M.; Khan, I.U.; Ayub, K.; Mukhtar, A.; Saeed, M.T. Synthesis, crystal structure and spectroscopic properties of 1,2-benzothiazine derivatives: An experimental and DFT study. Chin. J. Struct. Chem. 2015, 34, 15–25. [Google Scholar]
- Arshad, M.N.; Asiri, A.M.; Alamry, K.A.; Mahmood, T.; Gilani, M.A.; Ayub, K.; Birinji, A.S. Synthesis, crystal structure, spectroscopic and density functional theory (DFT) study of N-[3-anthracen-9-yl-1-(4-bromo-phenyl)-allylidene]-N-benzenesulfonohydrazine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 142, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Honig, B.; Nicholls, A. Classical electrostatics in biology and chemistry. Science 1995, 268, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor. Chem. Acc. 2002, 108, 134–142. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Ali, E.A.; Mokhtar, S.M.; Eweis, M. Synthesis, characterization polymerization and antibacterial properties of novel thiophene substituted acrylamide. React. Funct. Polym. 2011, 71, 1187–1194. [Google Scholar] [CrossRef]
- Bondock, S.; Fadaly, W.; Metwally, M.A. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur. J. Med. Chem. 2010, 45, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Refat, H.M.; Fadda, A. Synthesis and antimicrobial activity of some novel hydrazide, benzochromenone, dihydropyridine, pyrrole, thiazole and thiophene derivatives. Eur. J. Med. Chem. 2013, 70, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Mitra, I.; Saha, A.; Roy, K. Pharmacophore mapping of arylamino-substituted benzo[b]thiophenes as free radical scavengers. J. Mol. Model. 2010, 16, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Harinath, Y.; Reddy, D.H.K.; Kumar, B.N.; Apparao, C.; Seshaiah, K. Synthesis, spectral characterization and antioxidant activity studies of a bidentate Schiff base, 5-methyl thiophene-2-carboxaldehyde-carbohydrazone and its Cd(II), Cu(II), Ni(II) and Zn(II) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 101, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Meotti, F.C.; Silva, D.O.; dos Santos, A.R.; Zeni, G.; Rocha, J.B.T.; Nogueira, C.W. Thiophenes and furans derivatives: A new class of potential pharmacological agents. Environ. Toxicol. Pharmacol. 2003, 15, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.-J.R.; Ferreira, I.C.; Calhelha, R.C.; Estevinho, L.M. Synthesis and antioxidant activity evaluation of new 7-aryl or 7-heteroarylamino-2,3-dimethylbenzo[b]thiophenes obtained by Buchwald–Hartwig C–N cross-coupling. Bioorg. Med. Chem. 2007, 15, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.A.; Berghot, M.A.; Amer, F.A.; Badawy, D.S.; Bayoumy, N.M. Synthesis and antioxidant and antitumor activity of novel pyridine, chromene, thiophene and thiazole derivatives. Arch. Pharm. 2012, 345, 378–385. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro; Agilent Technologies: Oxfordshire, UK, 2012.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2007, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.J. X-Seed—A software tool for supramolecular crystallography. J. Supramol. Chem. 2001, 1, 189–191. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Fox Gaussian 09, Revision C. 01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Roy, D.; Todd, K.; John, M. Gauss View; Version 5; Semichem, Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–Correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Garratt, D. Synthetic Organic Compounds. In The Quantitative Analysis of Drugs; Springer: New York, NY, USA, 1964; pp. 700–715. [Google Scholar]











| Compound | 2d | 2f |
|---|---|---|
| Empirical formula | C10H5Cl2SF | C10H5SCl3 |
| Formula weight | 247.10 | 263.55 |
| Temperature/K | 293(2) | 293(2) |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/c | P21/n |
| a/Å | 3.8805(3) | 11.3539(3) |
| b/Å | 21.8785(12) | 6.9384(2) |
| c/Å | 11.9541(8) | 13.1916(4) |
| α/° | 90 | 90 |
| β/° | 98.688(8) | 90.807(3) |
| γ/° | 90 | 90 |
| Volume/Å3 | 1003.25(12) | 1039.10(5) |
| Z | 4 | 4 |
| ρcalcg/cm3 | 1.636 | 1.685 |
| μ/mm−1 | 7.516 | 9.467 |
| F(000) | 496.0 | 528.0 |
| Crystal size/mm3 | 0.26 × 0.06 × 0.06 | 0.33 × 0.18 × 0.15 |
| Radiation | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) |
| 2θ range for data collection/° | 8.082 to 162.368 | 10.208 to 152.446 |
| Index ranges | −4 ≤ h ≤ 4, −27 ≤ k ≤ 25, −12 ≤ l ≤ 14 | −11 ≤ h ≤ 14, −8 ≤ k ≤ 8, −16 ≤ l ≤ 16 |
| Reflections collected | 6585 | 10801 |
| Independent reflections | 2064 (Rint = 0.0292, Rsigma = 0.0228) | 2170 (Rint = 0.0324, Rsigma = 0.0183) |
| Data/restraints/parameters | 2064/0/127 | 2170/0/127 |
| Goodness-of-fit on F2 | 0.981 | 1.015 |
| Final R indexes (I ≥ 2σ (I)) | R1 = 0.0463, wR2 = 0.1180 | R1 = 0.0303, wR2 = 0.0795 |
| Final R indexes (all data) | R1 = 0.0643, wR2 = 0.1466 | R1 = 0.0340, wR2 = 0.0830 |
| Largest diff. peak/hole/e Å−3 | 0.37/−0.41 | 0.27/−0.30 |
| (2d) | X-ray | Calc. (B3LYP) | (2f) | X-ray | Calc. (B3LYP) |
|---|---|---|---|---|---|
| C1–C2 | 1.400 (4) | 1.406 | C1–C2 | 1.399 (2) | 1.405 |
| C1–C6 | 1.391 (5) | 1.406 | C1–C6 | 1.401 (2) | 1.404 |
| C1–C7 | 1.474 (4) | 1.467 | C1–C7 | 1.464 (2) | 1.466 |
| C2–C3 | 1.375 (5) | 1.39 | C2–C3 | 1.374 (2) | 1.389 |
| C3–C4 | 1.373 (5) | 1.396 | C3–C4 | 1.387 (3) | 1.396 |
| C3–Cl1 | 1.727 (4) | 1.746 | C3–Cl1 | 1.728 (18) | 1.747 |
| C4–C5 | 1.363 (6) | 1.389 | C4–C5 | 1.386 (3) | 1.401 |
| C5–C6 | 1.371 (5) | 1.391 | C4–Cl2 | 1.726 (18) | 1.745 |
| C7–C8 | 1.355 (5) | 1.374 | C5–C6 | 1.374 (3) | 1.392 |
| C7–S1 | 1.725 (3) | 1.757 | C7–C8 | 1.369 (2) | 1.375 |
| C8–C9 | 1.421 (5) | 1.424 | C7–S1 | 1.734 (18) | 1.757 |
| C9–C10 | 1.342 (6) | 1.366 | C8–C9 | 1.415 (3) | 1.423 |
| C10–S1 | 1.707 (4) | 1.742 | C9–C10 | 1.346 (3) | 1.366 |
| C10–Cl2 | 1.715 (4) | 1.73 | C10–S1 | 1.714 (18) | 1.742 |
| C4–F1 | 1.357 (4) | 1.34 | C10–Cl3 | 1.714 (2) | 1.73 |
| Bond (2d) | X-ray | Calc. (B3LYP) | Bond (2f) | X-ray | Calc. (B3LYP) |
|---|---|---|---|---|---|
| C2–C1–C6 | 118.0 (3) | 118.4 | C2–C1–C6 | 117.7 (16) | 118.1 |
| C2–C1–C7 | 120.3 (3) | 119.7 | C2–C1–C7 | 121.1 (16) | 121.4 |
| C6–C1–C7 | 121.7 (3) | 121.8 | C6–C1–C7 | 121.0 (16) | 120.4 |
| C3–C2–C1 | 120.3 (3) | 120.7 | C3–C2–C1 | 121.1 (17) | 121.1 |
| C2–C3–C4 | 119.7 (3) | 119.7 | C2–C3–C4 | 120.1 (17) | 120 |
| C2–C3–Cl1 | 120.9 (3) | 120.5 | C2–C3–Cl1 | 118.8 (15) | 118.5 |
| C4–C3–Cl1 | 119.5 (3) | 119.6 | C4–C3–Cl1 | 120.9 (14) | 121.4 |
| C5–C4–C3 | 121.3 (3) | 120.4 | C3–C4–C12 | 121.1 (15) | 121.7 |
| C6–C5–C4 | 119.4 (4) | 119.6 | C5–C4–C3 | 119.5 (17) | 119.1 |
| F1–C4–C3 | 119.1 (4) | 119.9 | C5–C4–C12 | 119.2 (15) | 119 |
| C5–C4–F1 | 119.6 (4) | 119.5 | C6–C5–C4 | 120.3 (18) | 120.6 |
| C5–C6–C1 | 121.2 (3) | 120.9 | C5–C6–C1 | 120.9 (17) | 120.8 |
| C1–C7–S1 | 120.6 (2) | 121.1 | C1–C7–S1 | 120.4 (13) | 121.3 |
| C8–C7–C1 | 129.2 (3) | 128.5 | C8–C7–C1 | 129.4 (17) | 128.3 |
| C8–C7–S1 | 110.3 (3) | 110.3 | C8–C7–S1 | 110.0 (14) | 110.3 |
| C7–C8–C9 | 113.7 (4) | 114.1 | C7–C8–C9 | 113.9 (17) | 114.1 |
| C10–C9–C8 | 111.8 (4) | 111.9 | C10–C9–C8 | 111.6 (17) | 111.9 |
| C9–C10–S1 | 112.5 (3) | 112.4 | C9–C10–S1 | 112.9 (15) | 112.4 |
| C10–S1–C7 | 91.74 (17) | 91.1 | C9–C10–Cl3 | 127.7 (15) | 127 |
| S1–C10–C12 | 120.4 (2) | 120.5 | Cl3–C10–S1 | 119.2 (12) | 120.4 |
| C9–C10–C12 | 127.1 (3) | 127 | C10–S1–C7 | 91.34 (9) | 91 |
| Entry No. | HOMO (eV) | LUMO (eV) | HOMO-LUMO (ΔE) eV |
|---|---|---|---|
| 2a | −5.71 | −1.14 | 4.57 |
| 2b | −5.49 | −1.02 | 4.47 |
| 2c | −5.96 | −1.46 | 4.50 |
| 2d | −6.07 | −1.48 | 4.59 |
| 2e | −5.72 | −1.13 | 4.59 |
| 2f | −6.13 | −1.66 | 4.47 |
| 3a | −5.24 | −1.21 | 4.03 |
| 3b | −4.97 | −1.01 | 3.96 |
| 3c | −5.68 | −1.70 | 3.98 |
| 3d | −5.84 | −1.76 | 4.08 |
| 3e | −5.16 | −1.14 | 4.02 |
| 3f | −5.96 | −1.99 | 3.97 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasool, N.; Kanwal, A.; Rasheed, T.; Ain, Q.; Mahmood, T.; Ayub, K.; Zubair, M.; Khan, K.M.; Arshad, M.N.; M. Asiri, A.; et al. One Pot Selective Arylation of 2-Bromo-5-Chloro Thiophene; Molecular Structure Investigation via Density Functional Theory (DFT), X-ray Analysis, and Their Biological Activities. Int. J. Mol. Sci. 2016, 17, 912. https://doi.org/10.3390/ijms17070912
Rasool N, Kanwal A, Rasheed T, Ain Q, Mahmood T, Ayub K, Zubair M, Khan KM, Arshad MN, M. Asiri A, et al. One Pot Selective Arylation of 2-Bromo-5-Chloro Thiophene; Molecular Structure Investigation via Density Functional Theory (DFT), X-ray Analysis, and Their Biological Activities. International Journal of Molecular Sciences. 2016; 17(7):912. https://doi.org/10.3390/ijms17070912
Chicago/Turabian StyleRasool, Nasir, Aqsa Kanwal, Tehmina Rasheed, Quratulain Ain, Tariq Mahmood, Khurshid Ayub, Muhammad Zubair, Khalid Mohammed Khan, Muhammad Nadeem Arshad, Abdullah M. Asiri, and et al. 2016. "One Pot Selective Arylation of 2-Bromo-5-Chloro Thiophene; Molecular Structure Investigation via Density Functional Theory (DFT), X-ray Analysis, and Their Biological Activities" International Journal of Molecular Sciences 17, no. 7: 912. https://doi.org/10.3390/ijms17070912
APA StyleRasool, N., Kanwal, A., Rasheed, T., Ain, Q., Mahmood, T., Ayub, K., Zubair, M., Khan, K. M., Arshad, M. N., M. Asiri, A., Zia-Ul-Haq, M., & Jaafar, H. Z. E. (2016). One Pot Selective Arylation of 2-Bromo-5-Chloro Thiophene; Molecular Structure Investigation via Density Functional Theory (DFT), X-ray Analysis, and Their Biological Activities. International Journal of Molecular Sciences, 17(7), 912. https://doi.org/10.3390/ijms17070912