The FKBP5 Gene Affects Alcohol Drinking in Knockout Mice and Is Implicated in Alcohol Drinking in Humans
Abstract
:1. Introduction
2. Results
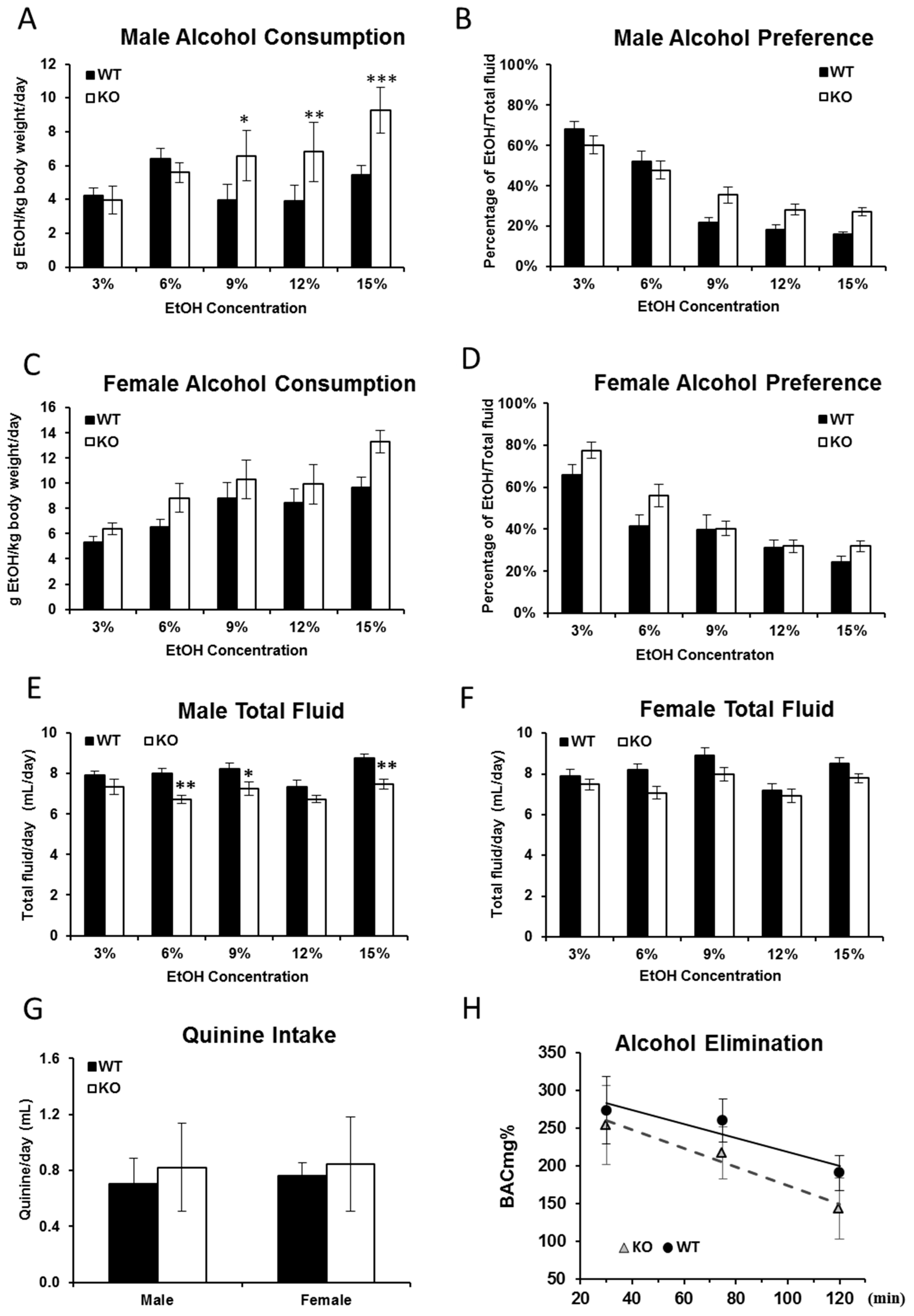
2.1. Alcohol Consumption Is Increased in Fkbp5−/− Mice
2.2. Blood Alcohol Concentration Is Higher in KO than WT Mice
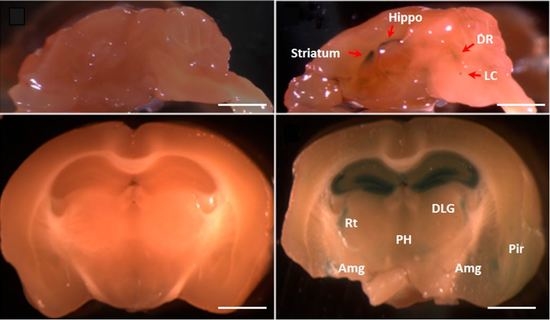
2.3. The Fkbp5 Gene Is Highly Expressed in Brain Regions Important for the Stress Response
2.4. Basal Corticosterone Is Not Different Between Fkbp5−/− and WT Mice
2.5. SNPs in FKBP5 Are Associated with Alcohol Drinking Behavior in Humans
3. Discussion
3.1. FKBP5 Is Associated with Psychiatric Disease Including Alcohol Use Disorder
3.2. No Basal Difference in CORT Level, but Fkbp5 Expressed in the Brain Regions Involving Stress Response
4. Materials and Methods
4.1. Animal and Human Subjects
4.2. Alcohol Drinking Tests
4.3. Alcohol Elimination Rate
4.4. Blood Alcohol Concentration after 3-h Limited Access
4.5. LacZ Staining
4.6. CORT Measurement
4.7. Human Sample Phenotypes
4.8. Human Sample Genotyping
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kessler, R.C.; Nelson, C.B.; McGonagle, K.A.; Edlund, M.J.; Frank, R.G.; Leaf, P.J. The epidemiology of co-occurring addictive and mental disorders: Implications for prevention and service utilization. Am. J. Orthopsychiatry 1996, 66, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.S.; Grant, B.F. The national epidemiologic survey on alcohol and related conditions (NESARC) Waves 1 and 2: Review and summary of findings. Soc. Psychiatry Psychiatr. Epidemiol. 2015, 50, 1609–1640. [Google Scholar] [CrossRef] [PubMed]
- Kellendonk, C.; Gass, P.; Kretz, O.; Schutz, G.; Tronche, F. Corticosteroid receptors in the brain: Gene targeting studies. Brain Res. Bull. 2002, 57, 73–83. [Google Scholar] [CrossRef]
- Roy, A.; Mittal, N.; Zhang, H.; Pandey, S.C. Modulation of cellular expression of glucocorticoid receptor and glucocorticoid response element-DNA binding in rat brain during alcohol drinking and withdrawal. J. Pharmacol. Exp. Ther. 2002, 301, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.C.; Schwandt, M.L.; Chester, J.A.; Kirchhoff, A.M.; Kao, C.F.; Liang, T.; Tapocik, J.D.; Ramchandani, V.A.; George, D.T.; Hodgkinson, C.A.; et al. FKBP5 moderates alcohol withdrawal severity: Human genetic association and functional validation in knockout mice. Neuropsychopharmacology 2014, 39, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Desrivieres, S.; Lourdusamy, A.; Muller, C.; Ducci, F.; Wong, C.P.; Kaakinen, M.; Pouta, A.; Hartikainen, A.L.; Isohanni, M.; Charoen, P.; et al. Glucocorticoid receptor (NR3C1) gene polymorphisms and onset of alcohol abuse in adolescents. Addict. Biol. 2011, 16, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Jose, B.S.; van Oers, H.A.; van de Mheen, H.D.; Garretsen, H.F.; Mackenbach, J.P. Stressors and alcohol consumption. Alcohol 2000, 35, 307–312. [Google Scholar] [CrossRef]
- Koob, G.F.; Ahmed, S.H.; Boutrel, B.; Chen, S.A.; Kenny, P.J.; Markou, A.; O’Dell, L.E.; Parsons, L.H.; Sanna, P.P. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci. Biobehav. Rev. 2004, 27, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Le Moal, M. Drug abuse: Hedonic homeostatic dysregulation. Science 1997, 278, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Fahlke, C.; Lorenz, J.G.; Long, J.; Champoux, M.; Suomi, S.J.; Higley, J.D. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol. Clin. Exp. Res. 2000, 24, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Errico, A.L.; King, A.C.; Lovallo, W.R.; Parsons, O.A. Cortisol dysregulation and cognitive impairment in abstinent male alcoholics. Alcohol. Clin. Exp. Res. 2002, 26, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Rouge-Pont, F.; Deroche, V.; Le Moal, M.; Piazza, P.V. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur. J. Neurosci. 1998, 10, 3903–3907. [Google Scholar] [CrossRef] [PubMed]
- Adinoff, B.; Iranmanesh, A.; Veldhuis, J.; Fisher, L. Disturbances of the stress response: The role of the HPA axis during alcohol withdrawal and abstinence. Alcohol. Health Res. World 1998, 22, 67–72. [Google Scholar] [PubMed]
- Nader, K.; Schafe, G.E.; Le Doux, J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 2000, 406, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, L.F.; Barbier, E.; Schlosburg, J.E.; Misra, K.K.; Whitfield, T.W.; Logrip, M.L.; Rivier, C.; Repunte-Canonigo, V.; Zorrilla, E.P.; Sanna, P.P.; et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J. Neurosci. 2012, 32, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Koenig, H.N.; Olive, M.F. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology 2004, 29, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Wochnik, G.M.; Ruegg, J.; Abel, G.A.; Schmidt, U.; Holsboer, F.; Rein, T. Fk506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005, 280, 4609–4616. [Google Scholar] [CrossRef] [PubMed]
- Schiene, C.; Fischer, G. Enzymes that catalyse the restructuring of proteins. Curr. Opin. Struct. Biol. 2000, 10, 40–45. [Google Scholar] [CrossRef]
- Binder, E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009, 34 (Suppl. 1), S186–S195. [Google Scholar] [CrossRef] [PubMed]
- Ising, M.; Depping, A.M.; Siebertz, A.; Lucae, S.; Unschuld, P.G.; Kloiber, S.; Horstmann, S.; Uhr, M.; Muller-Myhsok, B.; Holsboer, F. Polymorphisms in the fkbp5 gene region modulate recovery from psychosocial stress in healthy controls. Eur. J. Neurosci. 2008, 28, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Tatro, E.T.; Everall, I.P.; Kaul, M.; Achim, C.L. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins Fkbp51 and Fkbp52: Implications for major depressive disorder. Brain Res. 2009, 1286, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Periyasamy, S.; Wolf, I.M.; Hinds, T.D.; Yong, W.; Shou, W.; Sanchez, E.R. Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins. Biochemistry 2008, 47, 10471–10480. [Google Scholar] [CrossRef] [PubMed]
- Scharf, S.H.; Liebl, C.; Binder, E.B.; Schmidt, M.V.; Muller, M.B. Expression and regulation of the fkbp5 gene in the adult mouse brain. PLoS ONE 2011, 6, e16883. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.V.; Marinescu, D.; Hartmann, J.; Wang, X.D.; Labermaier, C.; Scharf, S.H.; Liebl, C.; Uhr, M.; Holsboer, F.; Muller, M.B.; et al. Differences in Fkbp51 regulation following chronic social defeat stress correlate with individual stress sensitivity: Influence of paroxetine treatment. Neuropsychopharmacology 2012, 37, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Wagner, K.V.; Liebl, C.; Scharf, S.H.; Wang, X.D.; Wolf, M.; Hausch, F.; Rein, T.; Schmidt, U.; Touma, C.; et al. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology 2012, 62, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, M.; Budziszewska, B.; Jaworska-Feil, L.; Basta-Kaim, A.; Kubera, M.; Leskiewicz, M.; Regulska, M.; Lason, W. The effect of antidepressant drugs on the HPA axis activity, glucocorticoid receptor level and Fkbp51 concentration in prenatally stressed rats. Psychoneuroendocrinology 2009, 34, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Kerns, R.T.; Ravindranathan, A.; Hassan, S.; Cage, M.P.; York, T.; Sikela, J.M.; Williams, R.W.; Miles, M.F. Ethanol-responsive brain region expression networks: Implications for behavioral responses to acute ethanol in DBA/2J versus C57B1/6J mice. J. Neurosci. 2005, 25, 2255–2266. [Google Scholar] [CrossRef] [PubMed]
- Treadwell, J.A.; Singh, S.M. Microarray analysis of mouse brain gene expression following acute ethanol treatment. Neurochem. Res. 2004, 29, 357–369. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.A.; Nestler, E.J.; Zachariou, V. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J. Neurosci. 2005, 25, 6005–6015. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, H.; Hendriks-Stegeman, B.I.; van der Burg, B.; van Buul-Offers, S.C.; Jansen, M. Glucocorticoid-induced increase in lymphocytic Fkbp51 messenger ribonucleic acid expression: A potential marker for glucocorticoid sensitivity, potency, and bioavailability. J. Clin. Endocrinol. Metab. 2003, 88, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.; Tamashiro, K.L.; Yang, X.; Purcell, R.H.; Harvey, A.; Willour, V.L.; Huo, Y.; Rongione, M.; Wand, G.S.; Potash, J.B. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of fkbp5 in mice. Endocrinology 2010, 151, 4332–4343. [Google Scholar] [CrossRef] [PubMed]
- McQuillin, A.; Rizig, M.; Gurling, H.M. A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mrna to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharmacogenet. Genom. 2007, 17, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.B.; Bradley, R.G.; Liu, W.; Epstein, M.P.; Deveau, T.C.; Mercer, K.B.; Tang, Y.; Gillespie, C.F.; Heim, C.M.; Nemeroff, C.B.; et al. Association of fkbp5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. J. Am. Med. Assoc. 2008, 299, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Pacek, L.R.; Storr, C.L.; Mojtabai, R.; Green, K.M.; La Flair, L.N.; Alvanzo, A.A.; Cullen, B.A.; Crum, R.M. Comorbid alcohol dependence and anxiety disorders: A national survey. J. Dual. Diagn. 2013, 9, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Izidio, G.S.; Ramos, A. Positive association between ethanol consumption and anxiety-related behaviors in two selected rat lines. Alcohol 2007, 41, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Barrenha, G.D.; Chester, J.A. Genetic correlation between innate alcohol preference and fear-potentiated startle in selected mouse lines. Alcohol. Clin. Exp. Res. 2007, 31, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.B.; Salyakina, D.; Lichtner, P.; Wochnik, G.M.; Ising, M.; Putz, B.; Papiol, S.; Seaman, S.; Lucae, S.; Kohli, M.A.; et al. Polymorphisms in fkbp5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 2004, 36, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Lekman, M.; Laje, G.; Charney, D.; Rush, A.J.; Wilson, A.F.; Sorant, A.J.; Lipsky, R.; Wisniewski, S.R.; Manji, H.; McMahon, F.J.; et al. The FKBP5-gene in depression and treatment response—An association study in the sequenced treatment alternatives to relieve depression (STAR*D) cohort. Biol. Psychiatry 2008, 63, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Koenen, K.C.; Saxe, G.; Purcell, S.; Smoller, J.W.; Bartholomew, D.; Miller, A.; Hall, E.; Kaplow, J.; Bosquet, M.; Moulton, S.; et al. Polymorphisms in fkbp5 are associated with peritraumatic dissociation in medically injured children. Mol. Psychiatry 2005, 10, 1058–1059. [Google Scholar] [CrossRef] [PubMed]
- Willour, V.L.; Chen, H.; Toolan, J.; Belmonte, P.; Cutler, D.J.; Goes, F.S.; Zandi, P.P.; Lee, R.S.; MacKinnon, D.F.; Mondimore, F.M.; et al. Family-based association of fkbp5 in bipolar disorder. Mol. Psychiatry 2009, 14, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Kranzler, H.R.; Poling, J.; Stein, M.B.; Anton, R.F.; Farrer, L.A.; Gelernter, J. Interaction of fkbp5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology 2010, 35, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Levran, O.; Peles, E.; Randesi, M.; Li, Y.; Rotrosen, J.; Ott, J.; Adelson, M.; Kreek, M.J. Stress-related genes and heroin addiction: A role for a functional fkbp5 haplotype. Psychoneuroendocrinology 2014, 45, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kirchheiner, J.; Lorch, R.; Lebedeva, E.; Seeringer, A.; Roots, I.; Sasse, J.; Brockmoller, J. Genetic variants in fkbp5 affecting response to antidepressant drug treatment. Pharmacogenomics 2008, 9, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Cai, G.; Golier, J.A.; Sarapas, C.; Galea, S.; Ising, M.; Rein, T.; Schmeidler, J.; Muller-Myhsok, B.; Holsboer, F.; et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the world trade center attacks. Biol. Psychiatry 2009, 66, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.; Yang, Z.; Periyasamy, S.; Chen, H.; Yucel, S.; Li, W.; Lin, L.Y.; Wolf, I.M.; Cohn, M.J.; Baskin, L.S.; et al. Essential role for co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J. Biol. Chem. 2007, 282, 5026–5036. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.A.; Kelly, S.D.; Greenlee, M.M.; Eaton, D.C.; Duke, B.J.; Bourke, C.H.; Neigh, G.N. Estradiol stimulates an anti-translocation expression pattern of glucocorticoid co-regulators in a hippocampal cell model. Physiol. Behav. 2013, 122, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Tatro, E.T.; Everall, I.P.; Masliah, E.; Hult, B.J.; Lucero, G.; Chana, G.; Soontornniyomkij, V.; Achim, C.L. Differential expression of immunophilins fkbp51 and fkbp52 in the frontal cortex of hiv-infected patients with major depressive disorder. J. Neuroimmune Pharmacol. 2009, 4, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, K.A.; Moon, I.; Eckloff, B.W.; Fridley, B.L.; Jenkins, G.D.; Batzler, A.; Biernacka, J.M.; Abo, R.; Brisbin, A.; Ji, Y.; et al. Fkbp5 genetic variation: Association with selective serotonin reuptake inhibitor treatment outcomes in major depressive disorder. Pharmacogenet. Genom. 2013, 23, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Hori, H.; Ota, M.; Hattori, K.; Teraishi, T.; Sasayama, D.; Yamamoto, N.; Higuchi, T.; Kunugi, H. Effect of the common functional fkbp5 variant (rs1360780) on the hypothalamic-pituitary-adrenal axis and peripheral blood gene expression. Psychoneuroendocrinology 2014, 42, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Hubler, T.R.; Scammell, J.G. Intronic hormone response elements mediate regulation of fkbp5 by progestins and glucocorticoids. Cell Stress Chaperones. 2004, 9, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Schumann, G.; Rujescu, D.; Kissling, C.; Soyka, M.; Dahmen, N.; Preuss, U.W.; Wieman, S.; Depner, M.; Wellek, S.; Lascorz, J.; et al. Analysis of genetic variations of protein tyrosine kinase fyn and their association with alcohol dependence in two independent cohorts. Biol. Psychiatry 2003, 54, 1422–1426. [Google Scholar] [CrossRef]
- Prasad, C.; Prasad, A. A relationship between increased voluntary alcohol preference and basal hypercorticosteronemia associated with an attenuated rise in corticosterone output during stress. Alcohol 1995, 12, 59–63. [Google Scholar] [CrossRef]
- O’Leary, J.C., 3rd; Dharia, S.; Blair, L.J.; Brady, S.; Johnson, A.G.; Peters, M.; Cheung-Flynn, J.; Cox, M.B.; de Erausquin, G.; Weeber, E.J.; et al. A new anti-depressive strategy for the elderly: Ablation of fkbp5/fkbp51. PLoS ONE 2011, 6, e24840. [Google Scholar]
- Soontornniyomkij, V.; Risbrough, V.B.; Young, J.W.; Wallace, C.K.; Soontornniyomkij, B.; Jeste, D.V.; Achim, C.L. Short-term recognition memory impairment is associated with decreased expression of fk506 binding protein 51 in the aged mouse brain. Age (Dordr.) 2010, 32, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Jinwal, U.K.; Koren, J., 3rd; Borysov, S.I.; Schmid, A.B.; Abisambra, J.F.; Blair, L.J.; Johnson, A.G.; Jones, J.R.; Shults, C.L.; O’Leary, J.C., 3rd; et al. The hsp90 cochaperone, fkbp51, increases tau stability and polymerizes microtubules. J. Neurosci. 2010, 30, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Bonkale, W.L.; Turecki, G.; Austin, M.C. Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synapse 2006, 60, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Bardo, M.T.; Bhatnagar, R.K.; Gebhart, G.F. Chronic naltrexone increases opiate binding in brain and produces supersensitivity to morphine in the locus coeruleus of the rat. Brain Res. 1983, 289, 223–234. [Google Scholar] [CrossRef]
- Grahame, N.J.; Li, T.K.; Lumeng, L. Selective breeding for high and low alcohol preference in mice. Behav. Genet. 1999, 29, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.; Brown, P. Blood collection. In Current Protocols in Immunology; Coligan, J., Bierer, B., Margulies, D., Shevach, E., Strober, W., Coico, R., Eds.; John Wiley and Sons: New York, NY, USA, 2006. [Google Scholar]
- Tojo, H.; Takami, K.; Kaisho, Y.; Nakata, M.; Abe, T.; Shiho, O.; Igarashi, K. Analysis of neurotrophin-3 expression using the lacz reporter gene suggests its local mode of neurotrophic activity. Neuroscience 1996, 71, 221–230. [Google Scholar] [CrossRef]
- Chen, H.; Yong, W.; Hinds, T.D.; Yang, Z.; Zhou, Y.; Sanchez, E.R.; Shou, W. Fkbp52 regulates androgen receptor transactivation activity and male urethra morphogenesis. J. Biol. Chem. 2010, 285, 27776–27784. [Google Scholar] [CrossRef] [PubMed]
- McArthur, T.; Ohtoshi, A. A brain-specific homeobox gene, Bsx, is essential for proper postnatal growth and nursing. Mol. Cell. Biol. 2007, 27, 5120–5127. [Google Scholar] [CrossRef] [PubMed]
- Chester, J.A.; Kirchhoff, A.M.; Barrenha, G.D. Relation between corticosterone and fear-related behavior in mice selectively bred for high or low alcohol preference. Addict. Biol. 2014, 19, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Maisto, S.A.; Sobell, M.B.; Cooper, A.M.; Sobell, L.C. Test-retest reliability of retrospective self-reports in three populations of alcohol abusers. J. Behav. Assess. 1979, 1, 315–326. [Google Scholar] [CrossRef]
- Sobell, L.C.; Sobell, M.B. Time-Line Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption, in Measuring Alcohol Consumption; Humana Press: Totowa, NJ, USA, 1992; pp. 73–98. [Google Scholar]
- Bucholz, K.K.; Cadoret, R.; Cloninger, C.R.; Dinwiddie, S.H.; Hesselbrock, V.M.; Nurnberger, J.I.; Reich, T.; Schmidt, I.; Schuckit, M.A. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. J. Stud. Alcohol 1994, 55, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Hesselbrock, M.; Easton, C.; Bucholz, K.K.; Schuckit, M.; Hesselbrock, V. A validity study of the SSAGA—A comparison with the scan. Addiction 1999, 94, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- APA. Diagnostic and statistical manual of mental disorders (DSM-IV); APA: Washington, DC, USA, 1994. [Google Scholar]
- Truett, G.E.; Heeger, P.; Mynatt, R.L.; Truett, A.A.; Walker, J.A.; Warman, M.L. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 2000, 29, 52–54. [Google Scholar] [PubMed]
- Hendershot, C.S.; Neighbors, C.; George, W.H.; McCarthy, D.M.; Wall, T.L.; Liang, T.; Larimer, M.E. ALDH2, ADH1B and alcohol expectancies: Integrating genetic and learning perspectives. Psychol. Addict. Behav. 2009, 23, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Luczak, S.E.; Glatt, S.J.; Wall, T.L. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol. Bull. 2006, 132, 607–621. [Google Scholar] [CrossRef] [PubMed]



| SNP | FKBP5 Intron (rs1360780) | FKBP5 3’-UTR (rs3800373) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype Distribution | HWE | Genotype Distribution | HWE | |||||||
| Ethnicity | CC | CT | TT | X2 | p | TT | TG | GG | X2 | p |
| Chinese | 190 (53%) | 141 (39%) | 29 (8%) | 0.2 | 0.69 | 192 (53%) | 138 (38%) | 29 (8%) | 0.4 | 0.55 |
| Korean | 195 (57%) | 131 (38%) | 17 (5%) | 0.7 | 0.40 | 205 (60%) | 123 (36%) | 15 (4%) | 0.4 | 0.52 |
| Caucasian | 226 (50%) | 189 (42%) | 34 (8%) | 0.4 | 0.52 | 240 (46%) | 175 (33%) | 33 (6%) | 0.0 | 0.89 |
| SNP | FKBP5 Intron (rs1360780) | FKBP51 UTR (rs3800373) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | CC | CT/TT | – | – | – | TT | TG/GG | – | – | – |
| Statistic | M (SD) | M (SD) | F | p | Change in R2 | M (SD) | M (SD) | F | p | Change in R2 |
| Chinese | n = 180 | n = 160 | – | – | – | n = 183 | n = 157 | – | – | – |
| Quantity | 3.8 (6.02) | 2.9 (1.52) | 2.13 | 0.034 | 0.013 | 3.3 (2.11) | 2.9 (1.53) | 1.39 | 0.164 | 0.005 |
| Frequency | 10.9 (11.31) | 9.7 (9.61) | 0.69 | 0.491 | 0.001 | 10.7 (11.30) | 9.8 (9.64) | 0.34 | 0.731 | 0.001 |
| Binges | 3.3 (5.20) | 2.8 (4.70) | 1.96 | 0.051 | 0.010 | 3.2 (5.17) | 2.8 (4.74) | 1.60 | 0.110 | 0.007 |
| Max drinks | 9.1 (8.54) | 6.3 (5.21) | 3.69 | <0.001 | 0.037 | 8.7 (8.46) | 6.4 (5.21) | 2.84 | 0.005 | 0.022 |
| AUD symptoms | 1.4 (1.00) | 1.2 (0.65) | 2.33 | 0.021 | 0.015 | 1.4 (0.97) | 1.2 (0.65) | 1.99 | 0.048 | 0.012 |
| Korean | n = 188 | n = 146 | – | – | – | n = 198 | n = 136 | – | – | – |
| Quantity | 4.2 (2.48) | 3.8 (2.21) | 1.18 | 0.238 | 0.004 | 4.1 (2.44) | 3.9 (2.20) | 0.33 | 0.741 | 0.001 |
| Frequency | 15.1 (14.89) | 12.2 (11.99) | 1.63 | 0.105 | 0.008 | 14.8 (15.01) | 12.5 (11.97) | 0.89 | 0.374 | 0.002 |
| Binges | 6.1 (8.17) | 5.2 (7.88) | 1.37 | 0.172 | 0.006 | 5.8 (7.99) | 5.4 (8.03) | 0.58 | 0.560 | 0.001 |
| Max drinks | 12.1 (9.89) | 10.4 (7.32) | 1.57 | 0.118 | 0.007 | 11.7 (9.73) | 10.7 (7.20) | 0.52 | 0.603 | 0.000 |
| AUD symptoms | 2.1 (1.77) | 1.7 (1.29) | 2.40 | 0.017 | 0.017 | 2.0 (1.72) | 1.7 (1.31) | 1.95 | 0.052 | 0.011 |
| Caucasian | n = 217 | n = 220 | – | – | – | n = 203 | n = 235 | – | – | – |
| Quantity | 3.1 (1.86) | 3.3 (2.24) | 0.21 | 0.65 | 0.000 | 3.2 (1.98) | 3.2 (2.20) | 0.00 | 0.98 | 0.000 |
| Frequency | 24.5 (18.80) | 24.2 (17.19) | 0.04 | 0.84 | 0.000 | 24.2 (18.40) | 24.1 (17.42) | 0.08 | 0.77 | 0.000 |
| Binges | 8.2 (12.16) | 8.0 (10.51) | 0.20 | 0.65 | 0.000 | 8.2 (12.28) | 8.0 (10.63) | 0.05 | 0.82 | 0.000 |
| Max drinks | 13.9 (10.13) | 14.2 (11.83) | 0.23 | 0.63 | 0.001 | 14.0 (10.35) | 13.9 (11.42) | 0.06 | 0.81 | 0.000 |
| AUD symptoms | 1.0 (1.71) | 1.0 (1.61) | 0.10 | 0.76 | 0.000 | 1.0 (1.75) | 1.0 (1.58) | 0.63 | 0.43 | 0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, B.; Luczak, S.E.; Wall, T.L.; Kirchhoff, A.M.; Xu, Y.; Eng, M.Y.; Stewart, R.B.; Shou, W.; Boehm, S.L.; Chester, J.A.; et al. The FKBP5 Gene Affects Alcohol Drinking in Knockout Mice and Is Implicated in Alcohol Drinking in Humans. Int. J. Mol. Sci. 2016, 17, 1271. https://doi.org/10.3390/ijms17081271
Qiu B, Luczak SE, Wall TL, Kirchhoff AM, Xu Y, Eng MY, Stewart RB, Shou W, Boehm SL, Chester JA, et al. The FKBP5 Gene Affects Alcohol Drinking in Knockout Mice and Is Implicated in Alcohol Drinking in Humans. International Journal of Molecular Sciences. 2016; 17(8):1271. https://doi.org/10.3390/ijms17081271
Chicago/Turabian StyleQiu, Bin, Susan E. Luczak, Tamara L. Wall, Aaron M. Kirchhoff, Yuxue Xu, Mimy Y. Eng, Robert B. Stewart, Weinian Shou, Stephen L. Boehm, Julia A. Chester, and et al. 2016. "The FKBP5 Gene Affects Alcohol Drinking in Knockout Mice and Is Implicated in Alcohol Drinking in Humans" International Journal of Molecular Sciences 17, no. 8: 1271. https://doi.org/10.3390/ijms17081271
APA StyleQiu, B., Luczak, S. E., Wall, T. L., Kirchhoff, A. M., Xu, Y., Eng, M. Y., Stewart, R. B., Shou, W., Boehm, S. L., Chester, J. A., Yong, W., & Liang, T. (2016). The FKBP5 Gene Affects Alcohol Drinking in Knockout Mice and Is Implicated in Alcohol Drinking in Humans. International Journal of Molecular Sciences, 17(8), 1271. https://doi.org/10.3390/ijms17081271