Apoptotic Pathways Linked to Endocrine System as Potential Therapeutic Targets for Benign Prostatic Hyperplasia
Abstract
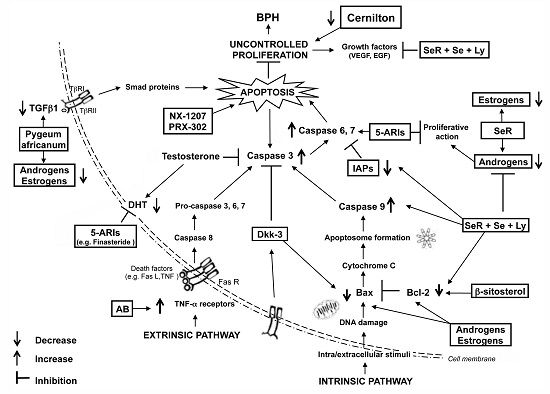
:1. Introduction
2. Endocrine Control of Prostatic Growth
3. Molecular Pathways of the Apoptosis Machinery and Benign Prostatic Hyperplasia (BPH)
4. Treatments of BPH
5. α1-Blockers
6. Finasteride
7. Phytotherapic Supplements
8. NX-1207
9. PRX-302
10. Conclusions
Author Contributions
Conflict of Interest
Abbreviations
References
- Kirby, R.S. The natural history of benign prostatic hyperplasia: What have we learned in the last decade? Urology 2000, 56, 3–6. [Google Scholar] [CrossRef]
- Emberton, M.; Fitzpatrick, J.M.; Garcia-Losa, M.; Qizilbash, N.; Djavan, B. Progression of benign prostatic hyperplasia: Systematic review of the placebo arms of clinical trials. Br. J. Urol. 2008, 102, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Speakman, M.; Kirby, R.; Doyle, S.; Ioannou, C. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH)—Focus on the UK. Br. J. Urol. 2014, 115, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Saigal, C.S.; Joyce, G. Economic costs of benign prostatic hyperplasia in the private sector. J. Urol. 2005, 173, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Vuichoud, C.; Loughlin, K.R. Benign prostatic hyperplasia: Epidemiology, economics and evaluation. Can. J. Urol. 2015, 22, 1–6. [Google Scholar] [PubMed]
- Parsons, J.K.; Wilt, T.J.; Wang, P.Y.; Barrett-Connor, E.; Bauer, D.C.; Marshall, L.M. Osteoporotic Fractures in Men Research Group. Progression of lower urinary tract symptoms among older men: A community based study. J. Urol. 2010, 183, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Schauer, I.G.; Rowley, D.R. The functional role of reactive stroma in benign prostatic hyperplasia. Differentiation 2011, 8, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Van der Heul-Nieuwenhuijsen, L.; Hendriksen, P.J.; van der Kwast, T.H.; Jenster, G. Gene expression profiling of the human prostate zones. Br. J. Urol. 2006, 98, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G.; McConnell, J.D. Benign prostatic hyperplasia: Etiology, pathophysiology, epidemiology, and natural history. In Campbell-Walsh Urology: Expert Consult Premium, 10th ed.; Wein, A.J., Kavoussi, L.R., Novick, A.C., Partin, A.W., Peters, C.A., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2010; Volume 3, pp. 2570–2610. [Google Scholar]
- Fibbi, B.; Penna, G.; Morelli, A.; Adorini, L.; Maggi, M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int. J. Androl. 2010, 33, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, G.; Madersbacher, S.; Berger, P. Benign prostatic hyperplasia: Age-related tissue-remodeling. Exp. Gerontol. 2005, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Iacopino, F.; Angelucci, C.; Lama, G.; Zelano, G.; La Torre, G.; D’Addessi, A.; Giovannini, C.; Bertaccini, A.; Macaluso, M.P.; Martorana, G.; et al. Apoptosis-related gene expression in benign prostatic hyperplasia and prostate carcinoma. Anticancer Res. 2006, 26, 1849–1854. [Google Scholar] [PubMed]
- Chughtai, B.; Lee, R.; Te, A.; Kaplan, S. Role of inflammation in benign prostatic hyperplasia. Rev. Urol. 2011, 13, 147–150. [Google Scholar] [PubMed]
- Cunha, G.R.; Ricke, W.A. A historical perspective on the role of stroma in the pathogenesis of benign prostatic hyperplasia. Differentiation 2011, 82, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, N.; Bhagwat, D. Benign prostatic hyperplasia: An overview of existing treatment. Indian J. Pharmacol. 2011, 43, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Bruckheimer, E.M.; Kyprianou, N. Apoptosis in prostate carcinogenesis. A growth regulator and a therapeutic target. Cell Tissue Res. 2000, 301, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, C.; Iacopino, F.; Lama, G.; Zelano, G.; Gianesini, G.; Sica, G.; Bono, A.V. Reverse transcriptase-PCR analysis of apoptosis-regulating gene expression in human benign prostatic hyperplasia. Anticancer Res. 2005, 25, 3937–3942. [Google Scholar] [PubMed]
- Vanden Berghe, T.; Kaiser, W.J.; Bertrand, M.J.; Vandenabeele, P. Molecular crosstalk between apoptosis, necroptosis, and survival signaling. Mol. Cell. Oncol. 2015, 2, e975093. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Berriguete, G.; Fraile, B.; de Bethencourt, F.R.; Prieto-Folgado, A.; Bartolome, N.; Nuñez, C.; Prati, B.; Martínez-Onsurbe, P.; Olmedilla, G.; Paniagua, R.; et al. Role of IAPs in prostate cancer progression: Immunohistochemical study in normal and pathological (benign hyperplastic, prostatic intraepithelial neoplasia and cancer) human prostate. BMC Cancer 2010, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Gomella, L.G.; Godwin, B.W. Apoptosis and benign prostatic hypertrophy. J. Urol. 1997, 158, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.; Peukert, K.; Karsunky, H.; Beuger, V.; Lutz, W.; Elsässer, H.P.; Möröy, T.; Eilers, M. Miz1 is required for early embryonic development during gastrulation. Mol. Cell. Biol. 2003, 23, 7648–7657. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.; Vaux, D.L. Alterations in the apoptotic machinery and their potential role in anticancer drug resistance. Oncogene 2003, 22, 7414–7430. [Google Scholar] [CrossRef] [PubMed]
- Poreba, M.; Strózyk, A.; Salvesen, G.S.; Drag, M. Caspase substrates and inhibitors. Cold Spring Harb. Perspect. Biol. 2013, 5, a008680. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Altavilla, D.; Marini, H.; Rinaldi, M.; Irrera, N.; Pizzino, G.; Bitto, A.; Arena, S.; Cimino, S.; Squadrito, F.; et al. Inhibitors of apoptosis proteins in experimental benign prostatic hyperplasia: Effects of Serenoa repens, selenium and lycopene. J. Biomed. Sci. 2014, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, V.; Sarswat, A.; Maikhuri, J.P.; Jain, A.; Jain, R.K.; Sharma, V.L.; Dalela, D.; Gupta, G. Selective estrogen receptor modulators regulate stromal proliferation in human benign prostatic hyperplasia by multiple beneficial mechanisms—Action of two new agents. Investig. New Drugs 2012, 30, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, T.M.; Ricke, W.A. Androgens and estrogens in benign prostatic hyperplasia: Past, present and future. Differentiation 2011, 82, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.; Rittmaster, R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 2003, 61, 2–7. [Google Scholar] [CrossRef]
- Kyprianou, N.; Isaacs, J.T. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology 1988, 122, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.T.; Isaacs, J.T. Mechanisms involved in the progression of androgen-independent prostate cancers: It is not only the cancer cell’s fault. Endocr. Relat. Cancer 2002, 9, 61–73. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Condorelli, R.A.; Russo, G.I.; Morgia, G.; Calogero, A.E. Endocrine control of benign prostatic hyperplasia. Andrology 2016, 4, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.T.; Yingling, M.; Vyas, A.; Atiemo, H.; Borkowski, A.; Jacobs, S.C.; Kyprianou, N. Finasteride targets prostate vascularity by inducing apoptosis and inhibiting cell adhesion of benign and malignant prostate cells. Prostate 2006, 66, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Arnold, J.T.; Isaacs, J.T. Conversion from a paracrine to an autocrine mechanism of androgen-stimulated growth during malignant transformation of prostatic epithelial cells. Cancer Res. 2001, 61, 5038–5044. [Google Scholar] [PubMed]
- Omezzine, A.; Mauduit, C.; Tabone, E.; Nabli, N.; Bouslama, A.; Benahmed, M. Caspase-3 and -6 expression and activation are targeted by hormone action in the rat ventral prostate during the apoptotic cell death process. Biol. Reprod. 2003, 69, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, P.J.; Dits, N.F.; Kokame, K.; Veldhoven, A.; van Weerden, W.M.; Bangma, C.H.; Trapman, J.; Jenster, G. Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res. 2006, 66, 5012–5020. [Google Scholar] [CrossRef] [PubMed]
- Hammarsten, J.; Damber, J.E.; Karlsson, M.; Knutson, T.; Ljunggren, O.; Ohlsson, C.; Peeker, R.; Smith, U.; Mellström, D. Insulin and free oestradiol are independent risk factors for benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2009, 12, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Kaneda, T.; Yokoyama, O. Association between lower urinary tract symptoms and serum levels of sex hormones in men. Urology 2008, 72, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Ellem, S.J.; Risbridger, G.P. The dual, opposing roles of estrogen in the prostate. Ann. N. Y. Acad. Sci. 2009, 1155, 174–186. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.J.; Hussain, S.; Balanathan, P.; Hedwards, S.L.; Niranjan, B.; Grant, M.; Chandrasiri, U.P.; Toivanen, R.; Wang, Y.; Taylor, R.A.; et al. Estrogen receptor-β activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNF α mediated. Proc. Natl. Acad. Sci. USA 2010, 107, 3123–3128. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, N.; Barros, R.P.; Warner, M.; Gustafsson, J.A. ERβ: Recent understanding of estrogen signaling. Trends Endocrinol. Metab. 2010, 21, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Megyesi, J.; Tarcsafalvi, A.; Seng, N.; Hodeify, R.; Price, P.M. Cdk2 phosphorylation of Bcl-xL after stress converts it to a pro-apoptotic protein mimicking Bax/Bak. Cell Death Discov. 2016, 2, 15066. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. BioMed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [PubMed]
- Khor, L.Y.; Moughan, J.; Al-Saleem, T.; Hammond, E.H.; Venkatesan, V.; Rosenthal, S.A.; Ritter, M.A.; Sandler, H.M.; Hanks, GE.; Shipley, W.U.; et al. Bcl-2 and Bax expression predict prostate cancer outcome in men treated with androgen deprivation and radiotherapy on radiation therapy oncology group protocol 92-02. Clin. Cancer Res. 2007, 13, 3585–3590. [Google Scholar] [CrossRef] [PubMed]
- Saker, Z.; Tsintsadze, O.; Jiqia, I.; Managadze, L.; Chkhotua, A. Importance of apoptpsis markers (MDM2, Bcl-2 and Bax) in benign prostatic hyperplasia and prostate cancer. Georgian Med. News 2015, 249, 7–14. [Google Scholar] [PubMed]
- Zellweger, T.; Ninck, C.; Bloch, M.; Mirlacher, M.; Koivisto, P.A.; Helin, H.J.; Mihatsch, M.J.; Gasser, T.C.; Bubendorf, L. Expression patterns of potential therapeutic targets in prostate cancer. Int. J. Cancer 2005, 113, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.P.; Banerjee, S.; Brown, T.R. Bcl-2 protein expression correlates with cell survival and androgen independence in rat prostatic lobes. Endocrinology 2002, 143, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y.; Feng, X.H. Smads: Transcriptional activators of TGF-β responses. Cell 1998, 95, 737–740. [Google Scholar] [CrossRef]
- Tuxhorn, J.A.; McAlhany, S.J.; Yang, F.; Dang, T.D.; Rowley, D.R. Inhibition of transforming growth factor-β activity decreases angiogenesis in a human prostate cancer-reactive stroma xenograft model. Cancer Res. 2002, 62, 6021–6025. [Google Scholar] [PubMed]
- Partin, J.V.; Anglin, I.E.; Kyprianou, N. Quinazoline-based α1-adrenoceptor antagonists induce prostate cancer cell apoptosis via TGF-β signaling and IkBα induction. Br. J. Cancer 2003, 88, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tuxhorn, J.A.; Ressler, S.J.; McAlhany, S.J.; Dang, T.D.; Rowley, D.R. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005, 65, 8887–8895. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, M.C.; Maxwell, C.A.; Barcellos-Hoff, M.H. The pleiotropic roles of transforming growth factor β in homeostasis and carcinogenesis of endocrine organs. Endocr. Relat. Cancer 2006, 13, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Justulin, L.A., Jr.; Acquaro, C.; Carvalho, R.F.; Silva, M.D.; Felisbino, S.L. Combined effect of the finasteride and doxazosin on rat ventral prostate morphology and physiology. Int. J. Androl. 2010, 33, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Peng, J.; Zheng, L.; Yu, W.; Jin, J. Transforming growth factor β1 mediates apoptotic activity of angiotensin II type I receptor blocker on prostate epithelium in vitro. Prostate 2010, 70, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Glassman, D.T.; Chon, J.K.; Borkowski, A.; Jacobs, S.C.; Kyprianou, N. Combined effect of terazosin and finasteride on apoptosis, cell proliferation, and transforming growth factor-b expression in benign prostatic hyperplasia. Prostate 2001, 46, 45–51. [Google Scholar] [CrossRef]
- Soulitzis, N.; Karyotis, I.; Delakas, D.; Spandidos, D.A. Expression analysis of peptide growth factors VEGF, FGF2, TGFB1, EGF and IGF1 in prostate cancer and benign prostatic hyperplasia. Int. J. Oncol. 2006, 29, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Romero, D.; Kypta, R. Dickkopf-3 function in the prostate: Implications for epithelial homeostasis and tumor progression. Bioarchitecture 2013, 3, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Zenzmaier, C.; Sampson, N.; Plas, E.; Berger, P. Dickkopf-related protein 3 promotes pathogenic stromal remodeling in benign prostatic hyperplasia and prostate cancer. Prostate 2013, 73, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, A.R.; Seo, E.M.; Nam, J.S. Genetic polymorphism in extracellular regulators of Wnt signaling pathway. BioMed Res. Int. 2015, 2015, 847529. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006, 25, 7469–7481. [Google Scholar] [CrossRef] [PubMed]
- Zenzmaier, C.; Untergasser, G.; Hermann, M.; Dirnhofer, S.; Sampson, N.; Berger, P. Dysregulation of Dkk-3 expression in benign and malignant prostatic tissue. Prostate 2008, 68, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Kitaoka, M.; Hamada, Y.; Walker, M.M.; Waxman, J.; Kypta, R.M. Regulation of prostate cell growth and morphogenesis by Dickkopf-3. Oncogene 2006, 25, 6528–6537. [Google Scholar] [CrossRef] [PubMed]
- Eckelman, B.P.; Salvesen, G.S.; Scott, F.L. Human inhibitor of apoptosis proteins: Why XIAP is the black sheep of the family. EMBO Rep. 2006, 7, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Mita, A.C.; Mita, M.M.; Nawrocki, S.T.; Giles, F.J. Survivin: Key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin. Cancer Res. 2008, 15, 5000–5005. [Google Scholar] [CrossRef] [PubMed]
- Lagacé, M.; Xuan, J.Y.; Young, S.S.; McRoberts, C.; Maier, J.; Rajcan-Separovic, E.; Korneluk, R.G. Genomic organization of the X-linked inhibitor of apoptosis and identification of a novel testis-specific transcript. Genomics 2001, 77, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Berthelet, J.; Dubrez, L. Regulation of apoptosis by inhibitors of apoptosis (IAPs). Cells 2013, 2, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Lotan, Y.; Saboorian, H.; Khoddami, S.M.; Roehrborn, C.G.; Slawin, K.M.; Ashfaq, R. Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer 2004, 100, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, A.D. Inhibitor of apoptosis proteins: Translating basic knowledge into clinical practice. Cancer Res. 2004, 64, 7183–7190. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.T.; Calhoun, E.; Jacobsen, S.J. Urologic diseases in America project: Benign prostatic hyperplasia. J. Urol. 2005, 173, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.V.; Jacobson, D.J.; McGree, M.E.; Roberts, R.O.; Lieber, M.M.; Jacobsen, S.J. A population based study of incidence and treatment of benign prostatic hyperplasia among residents of Olmsted County, Minnesota: 1987 to 1997. J. Urol. 2005, 173, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.C.; Curtis, L.H.; Schulman, K.A.; Albala, D.M. Geographic variations in the use of medical and surgical therapies for benign prostatic hyperplasia. J. Urol. 2006, 175, 1023–1027. [Google Scholar] [CrossRef]
- McVary, K.T. A review of combination therapy in patients with benign prostatic hyperplasia. Clin. Ther. 2007, 29, 387–398. [Google Scholar] [CrossRef]
- Hollingsworth, J.M.; Wei, J.T. Economic impact of surgical intervention in the treatment of benign prostatic hyperplasia. Rev. Urol. 2006, 8, S9–S15. [Google Scholar] [PubMed]
- Alcaraz, A.; Carballido-Rodríguez, J.; Unda-Urzaiz, M.; Medina-López, R.; Ruiz-Cerdá, J.L.; Rodríguez-Rubio, F.; García-Rojo, D.; Brenes-Bermúdez, F.J.; Cózar-Olmo, J.M.; Baena-González, V.; et al. Quality of life in patients with lower urinary tract symptoms associated with BPH: Change over time in real-life practice according to treatment-the QUALIPROST study. Int. Urol. Nephrol. 2016, 48, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Norby, B.; Nordling, J.; Mortensen, S. Lower urinary tract symptoms in the danish population: A population-based study of symptom prevalence, health-care seeking behavior and prevalence of treatment in elderly males and females. Eur. Urol. 2005, 47, 817–823. [Google Scholar] [CrossRef] [PubMed]
- McVary, K.T.; Roehrborn, C.G.; Avins, A.L.; Barry, M.J.; Bruskewitz, R.C.; Donnell, R.F.; Foster, H.E., Jr.; Gonzalez, C.M.; Kaplan, S.A.; Penson, D.F.; et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J. Urol. 2011, 185, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Oelke, M.; Bachmann, A.; Descazeaud, A.; Emberton, M.; Gravas, S.; Michel, M.C.; N’dow, J.; Nordling, J.; de la Rosette, J.J. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur. Urol. 2013, 64, 118–140. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Ficarra, V.; Sebastianelli, A.; Corona, G.; Serni, S.; Shariat, S.F.; Maggi, M.; Zattoni, F.; Carini, M.; Novara, G. Impact of medical treatments for male lower urinary tract symptoms due to Benign Prostatic Hyperplasia on ejaculatory function: A systematic review and meta-analysis. J. Sex. Med. 2014, 11, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.C. Is there a scientific basis for the therapeutic effects of Serenoa repens in benign prostatic hyperplasia? Mechanisms of action. J. Urol. 2004, 172, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, C.N.S.T.; Kirby, R. Benign prostatic hyperplasia and male lower urinary tract symptoms (LUTS). BMJ. Clin. Evid. 2011, 2011, 1801. [Google Scholar] [PubMed]
- Tacklind, J.; MacDonald, R.; Rutks, I.; Stanke, J.U.; Wilt, T.J. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2012, 12, CD001423. [Google Scholar] [PubMed]
- Keehn, A.; Lowe, F.C. Complementary and alternative medications for benign prostatic hyperplasia. Can. J. Urol. 2015, 22, 18–23. [Google Scholar] [PubMed]
- Kim, T.H.; Lim, H.J.; Kim, M.S.; Lee, M.S. Dietary supplements for benign prostatic hyperplasia: An overview of systematic review. Maturitas 2012, 73, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Kunit, T.; Lusuardi, L. An evidence-based review of NX1207 and its potential in the treatment of benign prostatic hyperplasia. Res. Rep. Urol. 2014, 6, 67–70. [Google Scholar] [PubMed]
- Hennenberg, M.; Stief, C.G.; Gratzke, C. Prostatic α1-adrenoceptors: New concepts of function, regulation, and intracellular signaling. Neurourol. Urodyn. 2014, 33, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.; Hartanto, V.; Lepor, H. The response to α blockade in benign prostatic hyperplasia is related to the percent area density of prostate smooth muscle. Prostate 1992, 21, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Chłosta, P.; Drewa, T.; Kaplan, S. α-Blockade, apoptosis, and prostate shrinkage: How are they related? Growth 2013, 6, 189–194. [Google Scholar]
- Kyprianou, N. Doxazosin and terazosin suppress prostate growth by inducing apoptosis: Clinical significance. J. Urol. 2003, 169, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Tahmatzopoulos, A.; Lagrange, C.A.; Zeng, L.; Mitchell, B.L.; Conner, W.T.; Kyprianou, N. Effect of terazosin on tissue vascularity and apoptosis in transitional cell carcinoma of bladder. Urology 2005, 65, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Garrison, J.B.; Kyprianou, N. Doxazosin induces apoptosis of benign and malignant prostate cells via a death receptor-mediated pathway. Cancer Res. 2006, 66, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Drewa, T.; Wolski, Z.; Misterek, B.; Debski, R.; Styczynski, J. The influence of α1–antagonist on the expression pattern of TNF receptor family in primary culture of prostate epithelial cells from BPH patients. Prostate Cancer Prostatic Dis. 2008, 11, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Furuya, Y.; Fuse, H.; Masai, M. Serum soluble Fas level for detection and staging of prostate cancer. Anticancer Res. 2001, 21, 3595–3598. [Google Scholar] [PubMed]
- Paick, J.S.; Cho, M.C.; Song, S.H.; Kim, S.W.; Ku, J.H. Impacts of the quinazoline-based α1-antagonist, terazosin, and of the sulfonamide derivative, tamsulosin, on serum prostate-specific antigen and prostate volume. J. Korean Med. Sci. 2008, 23, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.R. α Adrenoceptor antagonists in the year 2000: Is there anything new? Curr. Opin. Urol. 2001, 11, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Emberton, M.; Lukacs, B.; Matzkin, H.; Alcaraz, A.; Elhilali, M.; Vallancien, G. Response to daily 10 mg alfuzosin predicts acute urinary retention and benign prostatic hyperplasia related surgery in men with lower urinary tract symptoms. J. Urol. 2006, 176, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Lepor, H. Medical treatment of benign prostatic hyperplasia. Rev. Urol. 2011, 13, 20–33. [Google Scholar] [PubMed]
- Lepor, H.; Kazzazi, A.; Djavan, B. α-Blockers for benign prostatic hyperplasia: The new era. Curr. Opin. Urol. 2012, 22, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Timme, T.L.; Park, S.H.; Wu, X.; Wyllie, M.G.; Thompson, T.C. Transforming growth factor β 1 transduced mouse prostate reconstitutions: II. Induction of apoptosis by doxazosin. Prostate 1997, 33, 157–163. [Google Scholar] [CrossRef]
- Benning, C.M.; Kyprianou, N. Quinazoline-derived α1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an α1-adrenoceptor-independent action. Cancer Res. 2002, 62, 597–602. [Google Scholar] [PubMed]
- Bozec, A.; Ruffion, A.; Decaussin, M.; Andre, J.; Devonec, M.; Benahmed, M.; Mauduit, C. Activation of caspases-3, -6, and -9 during finasteride treatment of benign prostatic hyperplasia. J. Clin. Endocrinol. Metab. 2005, 90, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Rittmaster, R.S. 5α-Reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Djavan, B.; Waldert, M.; Ghawidel, C.; Marberger, M. Benign prostatic hyperplasia progression and its impact on treatment. Curr. Opin. Urol. 2004, 14, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Azzouni, F.; Mohler, J. Role of 5α-reductase inhibitors in benign prostatic diseases. Prostate Cancer Prostatic Dis. 2012, 15, 222–230. [Google Scholar] [CrossRef] [PubMed]
- The Finasteride Study Group. Finasteride (MK-906) in the treatment of benign prostatic hyperplasia. Prostate 1993, 22, 291–299. [Google Scholar]
- McConnell, J.D.; Bruskewitz, R.; Walsh, P.; Andriole, G.; Lieber, M.; Holtgrewe, H.L.; Albertsen, P.; Roehrborn, C.G.; Nickel, J.C.; Wang, D.Z.; et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N. Engl. J. Med. 1998, 338, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.; Imperato-McGinley, J.; McConnell, J.; Matsumoto, A.M.; Bracken, B.; Roy, J.; Sullivan, M.; Pappas, F.; Cook, T.; Daurio, C.; et al. Long-term (7- to 8-year) experience with finasteride in men with benign prostatic hyperplasia. Urology 2002, 60, 1040–1044. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Holtgrewe, H.L.; Bruskewitz, R.; Saltzman, B.; Mobley, D.; Narayan, P.; Lund, R.H.; Weiner, S.; Wells, G.; Cook, T.J.; et al. Comparison of the efficacy and safety of finasteride in older versus younger men with benign prostatic hyperplasia. Urology 2001, 57, 1073–1077. [Google Scholar] [CrossRef]
- Hochberg, D.A.; Basillote, J.B.; Armenakas, N.A. Decreased suburethral prostatic microvessel density in finasteride treated prostates: A possible mechanism for reduced bleeding in benign prostatic hyperplasia. J. Urol. 2002, 167, 1731–1733. [Google Scholar] [CrossRef]
- Kearney, M.C.; Bingham, J.B.; Bergland, R.; Meade-D’Alisera, P.; Puchner, P.J. Clinical predictions in the use of finasteride for control of gross hematuria due to benign prostatic hyperplasia. J. Urol. 2002, 167, 2489–2491. [Google Scholar] [CrossRef]
- Perimenis, P.; Gyftopoulos, K.; Markou, S.; Barbalias, G. Effects of finasteride and cyproterone acetate on hematuria associated with benign prostatic hyperplasia: A prospective, randomized, controlled study. Urology 2002, 59, 373–377. [Google Scholar] [CrossRef]
- MacDonald, R.; Ishani, A.; Rutks, I.; Wilt, T.J. A systematic review of cernilton for the treatment of benign prostatic hyperplasia. BJU Int. 2000, 85, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.; Ishani, A.; MacDonald, R.; Stark, G.; Mulrow, C.; Lau, J. β-Sitosterols for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2000, 2, CD001043. [Google Scholar]
- Berges, R.R.; Kassen, A.; Senge, T. Treatment of symptomatic benign prostatic hyperplasia with β-sitosterol: An 18-month follow-up. BJU Int. 2000, 85, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.; Ishani, A.; Mac Donald, R.; Rutks, I.; Stark, G. Pygeum africanum for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2002, 1, CD001044. [Google Scholar]
- Altavilla, D.; Bitto, A.; Polito, F.; Irrera, N.; Marini, H.; Arena, S.; Favilla, V.; Squadrito, F.; Morgia, G.; Minutoli, L. The combination of Serenoa repens, selenium and lycopene is more effective than Serenoa repens alone to prevent hormone dependent prostatic growth. J. Urol. 2011, 186, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Tasyriq, M.; Najmuldeen, I.A.; In, L.L.; Mohamad, K.; Awang, K.; Hasima, N. 7α-Hydroxy-β-sitosterol from Chisocheton tomentosus induces apoptosis via dysregulation of cellular Bax/Bcl-2 ratio and cell cycle arrest by downregulating ERK1/2 activation. Evid. Based Complement. Altern. Med. 2012, 2012, 765316. [Google Scholar] [CrossRef] [PubMed]
- Quiles, M.T.; Arbós, M.A.; Fraga, A.; de Torres, I.M.; Reventós, J.; Morote, J. Antiproliferative and apoptotic effects of the herbal agent Pygeum africanum on cultured prostate stromal cells from patients with benign prostatic hyperplasia (BPH). Prostate 2010, 70, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.; Schneider, H.; Ludwig, M.; Schnitker, J.; Brähler, E.; Weidner, W. A pollen extract (Cernilton) in patients with inflammatory chronic prostatitis–chronic pelvic pain syndrome: A multicentre, randomised, prospective, double-blind, placebo-controlled phase 3 study. Eur. Urol. 2009, 56, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, K.; Nandachi, N.; Satoh, S.; Honma, M.; Namikata, S.; Ishii, M.; Yasumoto, R.; Nishisaka, N.; Masuda, C.; Kishimoto, T. Effects of cernitin pollen extract (Cernilton) on inflammatory cytokines in sex-hormone induced nonbacterial prostatitis rats. Hinyokika Kiyo Acta Urol. Jpn. 2001, 47, 459–465. [Google Scholar]
- Bonvissuto, G.; Minutoli, L.; Morgia, G.; Bitto, A.; Polito, F.; Irrera, N.; Marini, H.; Squadrito, F.; Altavilla, D. Effect of Serenoa repens, lycopene, and selenium on proinflammatory phenotype activation: An in vitro and in vivo comparison study. Urology 2011, 77, e9–e16. [Google Scholar] [CrossRef] [PubMed]
- Sirab, N.; Robert, G.; Fasolo, V. Lipidosterolic extract of Serenoa repens modulates the expression of inflammation related-genes in benign prostatic hyperplasia epithelial and stromal cells. Int. J. Mol. Sci. 2013, 14, 14301–14320. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Bitto, A.; Squadrito, F.; Marini, H.; Irrera, N.; Morgia, G.; Passantino, A.; Altavilla, D. Serenoa repens, lycopene and selenium: A triple therapeutic approach to manage benign prostatic hyperplasia. Curr. Med. Chem. 2013, 20, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Morgia, G.; Cimino, S.; Favilla, V.; Russo, G.I.; Squadrito, F.; Mucciardi, G.; Masieri, L.; Minutoli, L.; Grosso, G.; Castelli, T. Effects of Serenoa repens, selenium and lycopene (Profluss®) on chronic inflammation associated with benign prostatic hyperplasia: Results of “FLOG” (flogosis and Profluss in prostatic and genital disease), a multicentre Italian study. Int. Braz. J. Urol. 2013, 39, 214–221. [Google Scholar] [PubMed]
- Dedhia, R.C.; McVary, K.T. Phytotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia. J. Urol. 2008, 179, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Marcoccia, D.; Georgiev, M.I.; Alipieva, K.I.; Lorenzetti, S. Inhibition of the DHT-induced PSA secretion by Verbascum xanthophoeniceum and Serenoa repens extracts in human LNCaP prostate epithelial cells. J. Ethnopharmacol. 2014, 155, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Magri, V.; Trinchieri, A.; Perletti, G.; Marras, E. Activity of Serenoa repens, lycopene and selenium on prostatic disease: Evidences and hypotheses. Arch. Ital. Urol. Androl. 2008, 80, 65–78. [Google Scholar] [PubMed]
- Allkanjari, O.; Vitalone, A. What do we know about phytotherapy of benign prostatic hyperplasia? Life Sci. 2015, 126, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Weisser, H.; Tunn, S.; Behnke, B.; Krieg, M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5α-reductase activity in human benign prostatic hyperplasia. Prostate 1996, 28, 300–306. [Google Scholar] [CrossRef]
- Russo, A.; Capogrosso, P.; La Croce, G.; Ventimiglia, E.; Boeri, L.; Briganti, A.; Damiano, R.; Montorsi, F.; Salonia, A. Serenoa repens, selenium and lycopene to manage lower urinary tract symptoms suggestive for benign prostatic hyperplasia. Expert Opin. Drug Saf. 2016, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Magistro, G.; Stief, C.G.; Gratzke, C. New intraprostatic injectables and prostatic urethral lift for male LUTS. Nat. Rev. Urol. 2015, 12, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.M.; Pimentel, M.A.; Gilling, P.J. Evolving and investigational therapies for benign prostatic hyperplasia. Can. J. Urol. 2015, 22, 82–87. [Google Scholar] [PubMed]
- Nymox Pharmaceutical Corporation Data on File. Available online: www.nymox.com (accessed on 10 August 2016).
- Shore, N. NX-1207: A novel investigational drug for the treatment of benign prostatic hyperplasia. Expert Opin. Investig. Drugs 2010, 19, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.; Cowan, B. The potential for NX-1207 in benign prostatic hyperplasia: An update for clinicians. Ther. Adv. Chronic Dis. 2011, 2, 377–383. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Clinical Evaluation of NX-1207 for the Treatment of Benign Prostatic Hyperplasia (BPH) NX02–0017. 2014. Identifier: NCT00918983. Available online: http://clinicaltrials.gov/ct2/show/NCT00918983 (accessed on 4 January 2016).
- ClinicalTrials.gov. Clinical Evaluation of NX-1207 for the Treatment of Benign Prostatic Hyperplasia (BPH) NX02–0018. 2014. Identifier: NCT00945490. Available online: http://clinicaltrials.gov/ct2/show/NCT00945490 (accessed on 4 January 2016).
- Elhilali, M.M.; Pommerville, P.; Yocum, R.C.; Merchant, R.; Roehrborn, C.G.; Denmeade, S.R. Prospective, randomized, double-blind, vehicle controlled, multicenter phase IIb clinical trial of the pore forming protein PRX302 for targeted treatment of symptomatic benign prostatic hyperplasia. J. Urol. 2013, 189, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Denmeade, S.R.; Egerdie, B.; Steinhoff, G.; Merchant, R.; Abi-Habib, R.; Pommerville, P. Phase 1 and 2 studies demonstrate the safety and efficacy of intraprostatic injection of PRX302 for the targeted treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur. Urol. 2011, 59, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Golubtsova, E.N.; Tomilov, A.A.; Veliev, E.I. Potentials for the combined therapy of urination disorders in men: The choice of optimal scheme of treatment. Urologiia 2013, 5, 96–98, 100–101. [Google Scholar] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minutoli, L.; Rinaldi, M.; Marini, H.; Irrera, N.; Crea, G.; Lorenzini, C.; Puzzolo, D.; Valenti, A.; Pisani, A.; Adamo, E.B.; et al. Apoptotic Pathways Linked to Endocrine System as Potential Therapeutic Targets for Benign Prostatic Hyperplasia. Int. J. Mol. Sci. 2016, 17, 1311. https://doi.org/10.3390/ijms17081311
Minutoli L, Rinaldi M, Marini H, Irrera N, Crea G, Lorenzini C, Puzzolo D, Valenti A, Pisani A, Adamo EB, et al. Apoptotic Pathways Linked to Endocrine System as Potential Therapeutic Targets for Benign Prostatic Hyperplasia. International Journal of Molecular Sciences. 2016; 17(8):1311. https://doi.org/10.3390/ijms17081311
Chicago/Turabian StyleMinutoli, Letteria, Mariagrazia Rinaldi, Herbert Marini, Natasha Irrera, Giovanni Crea, Cesare Lorenzini, Domenico Puzzolo, Andrea Valenti, Antonina Pisani, Elena B. Adamo, and et al. 2016. "Apoptotic Pathways Linked to Endocrine System as Potential Therapeutic Targets for Benign Prostatic Hyperplasia" International Journal of Molecular Sciences 17, no. 8: 1311. https://doi.org/10.3390/ijms17081311
APA StyleMinutoli, L., Rinaldi, M., Marini, H., Irrera, N., Crea, G., Lorenzini, C., Puzzolo, D., Valenti, A., Pisani, A., Adamo, E. B., Altavilla, D., Squadrito, F., & Micali, A. (2016). Apoptotic Pathways Linked to Endocrine System as Potential Therapeutic Targets for Benign Prostatic Hyperplasia. International Journal of Molecular Sciences, 17(8), 1311. https://doi.org/10.3390/ijms17081311