The Impact of Growth Hormone Therapy on the Apoptosis Assessment in CD34+ Hematopoietic Cells from Children with Growth Hormone Deficiency
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Clinical Parameters
2.2. GHR Is Expressed at the Protein Level in CD34+ Hematopoietic Cells from GHD Children
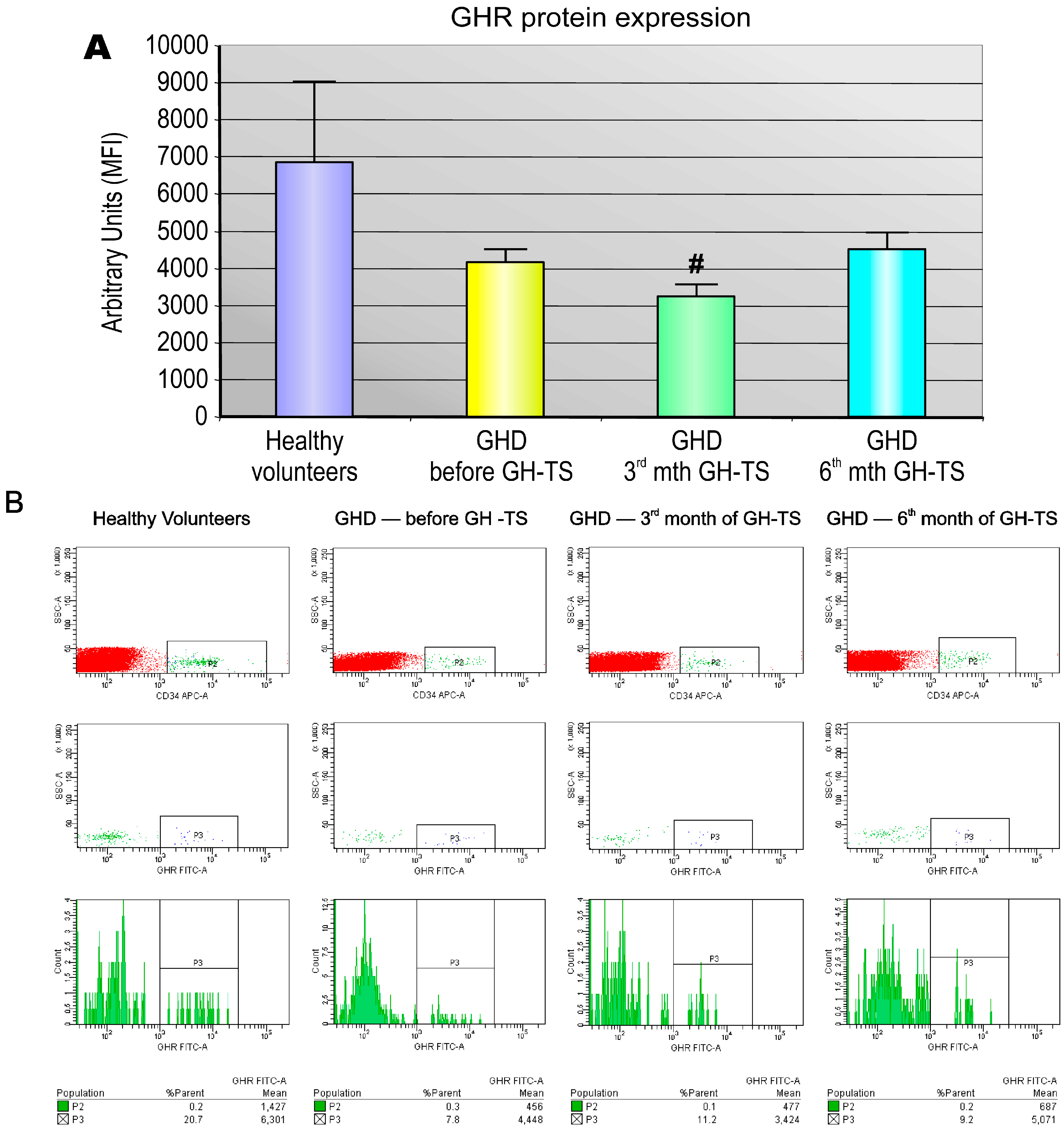
2.3. GHR Protein Expression in Individual CD34+ Hematopoietic Cells Is Decreased in GHD Children and Not Changing in the Course of GH Therapy
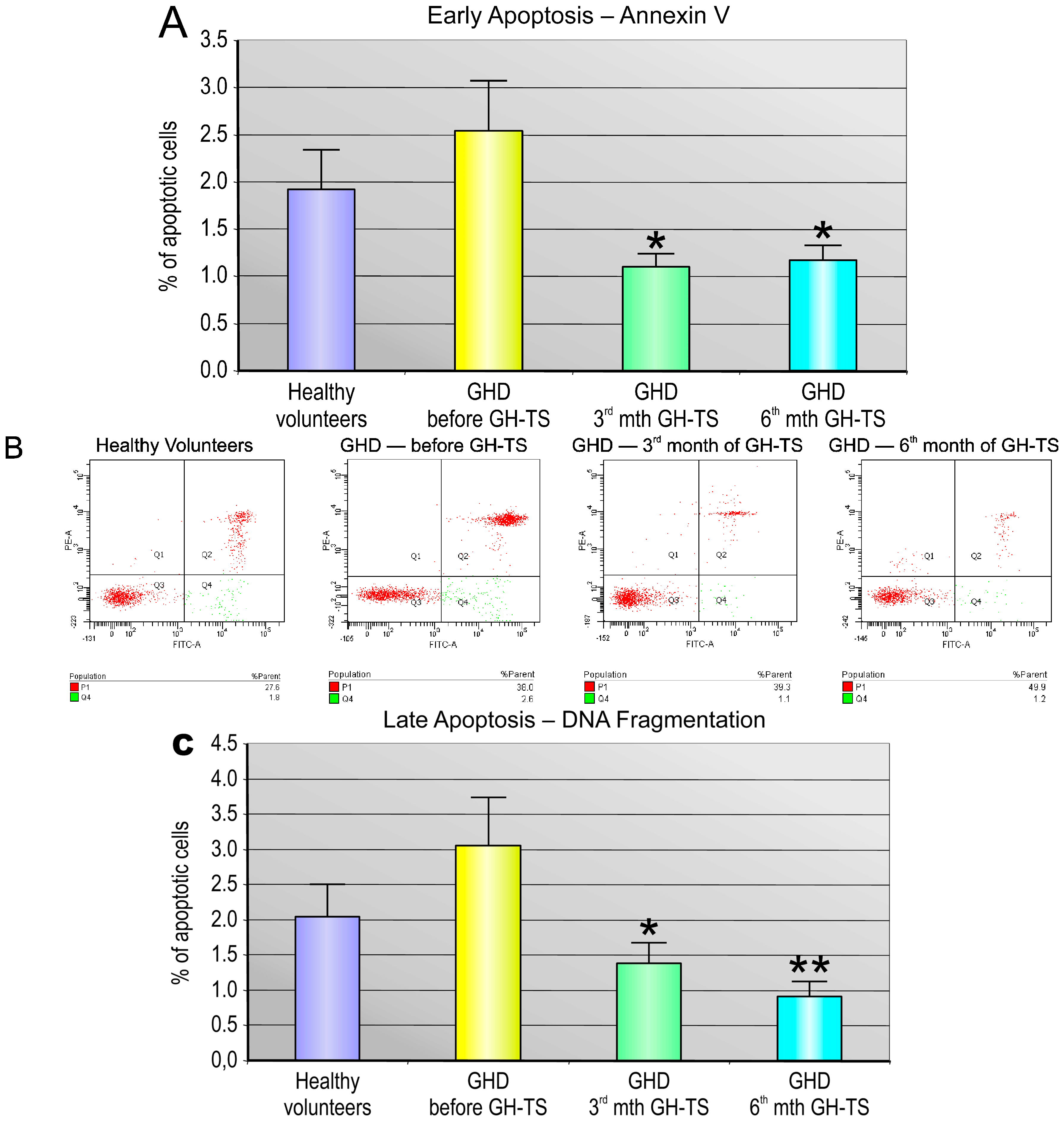
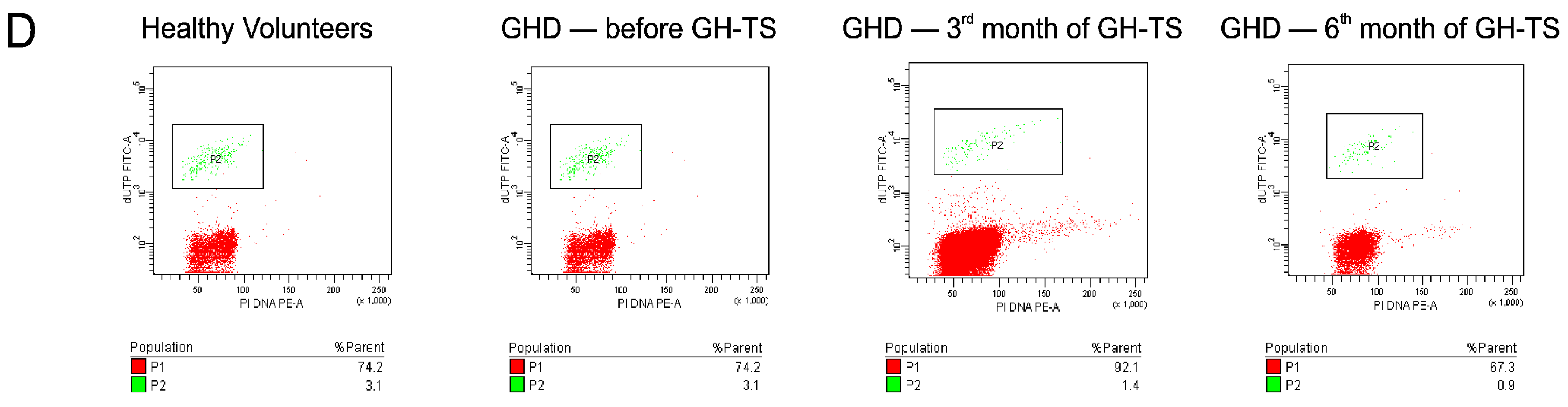
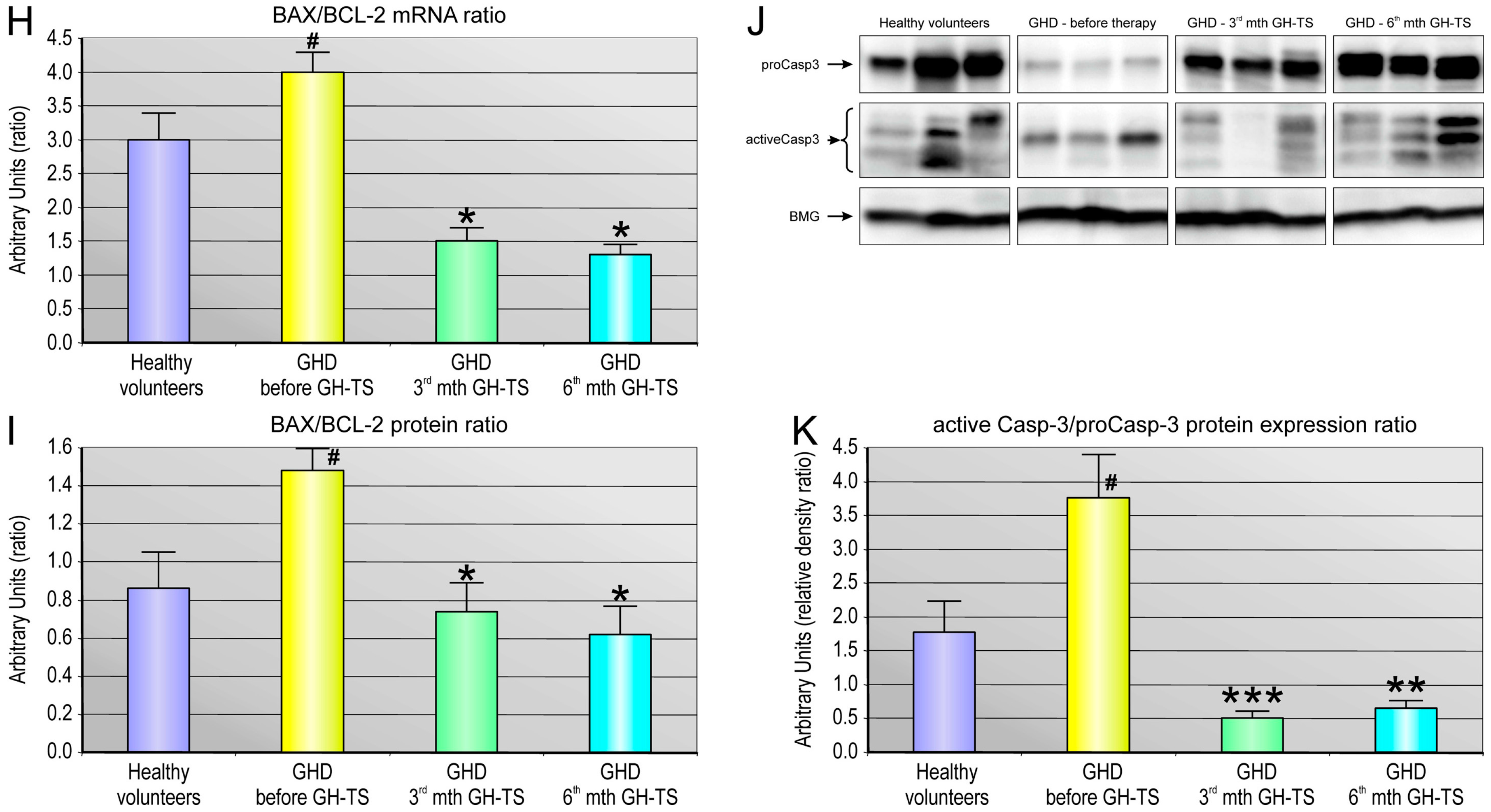
2.4. GH Therapy Inhibits Apoptosis in CD34+ Hematopoietic Cells from GHD Children
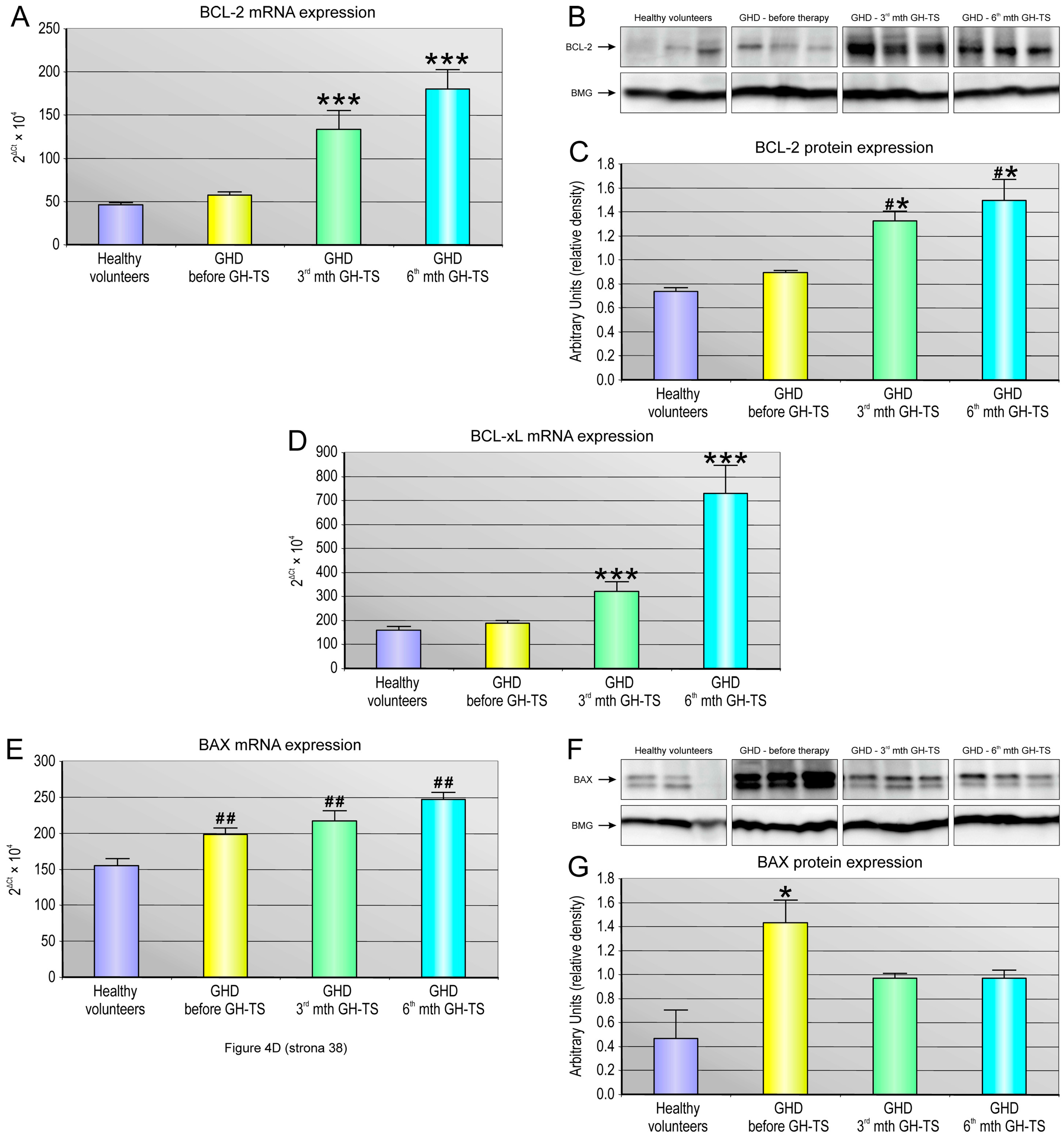
2.5. GH Therapy Modulates the Gene Expression of Selected Apoptosis-Regulating Proteins in CD34+ Hematopoietic Cells from GHD Children
2.6. Microarray Analysis of Apoptosis-Related Gene Expression Changes in CD34+ Hematopoietic Cells over 6 Months of GH Treatment
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Laboratory Measurements and Cell Isolation
4.3. Immunofluorescence Staining of PB-Derived CD34+ Cells
4.4. Flow Cytometry
4.5. Apoptosis Detection
4.6. RNA Isolation and Gene Expression Analysis
4.7. RNA Microarray Data Analysis
4.8. ELISA
4.9. Western Blot Analysis
4.10. Statistical Methods
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brooks, A.J.; Waters, M.J. The growth hormone receptor: Mechanism of activation and clinical implications. Nat. Rev. Endocrinol. 2010, 6, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, J.; Cheng, C.M.; Kopchick, J.J.; Bondy, C.A. Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth. J. Endocrinol. 2004, 180, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulos, A.; Ohanna, M.; Kedzia, C.; Menon, R.K.; Kopchick, J.J.; Kelly, P.A.; Pende, M. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc. Natl. Acad. Sci. USA 2006, 103, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- McLenachan, S.; Lum, M.G.; Waters, M.J.; Turnley, A.M. Growth hormone promotes proliferation of adult neurosphere cultures. Growth Horm. IGF Res. 2009, 19, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Gagnerault, M.C.; Postel-Vinay, M.C.; Dardenne, M. Expression of growth hormone receptors in murine lymphoid cells analyzed by flow cytometry. Endocrinology 1996, 137, 1719–1726. [Google Scholar] [PubMed]
- Jeay, S.; Sonenshein, G.E.; Postel-Vinay, M.C.; Baixeras, E. Growth hormone prevents apoptosis through activation of nuclear factor-kappaB in interleukin-3-dependent Ba/F3 cell line. Mol. Endocrinol. 2000, 14, 650–661. [Google Scholar] [PubMed]
- Matsuda, T.; Saito, H.; Inoue, T.; Fukatsu, K.; Han, I.; Furukawa, S.; Ikeda, S.; Muto, T. Growth hormone inhibits apoptosis and up-regulates reactive oxygen intermediates production by human polymorphonuclear neutrophils. JPEN J. Parenter. Enter. Nutr. 1998, 22, 368–374. [Google Scholar] [CrossRef]
- Haeffner, A.; Déas, O.; Mollereau, B.; Estaquier, J.; Mignon, A.; Haeffner-Cavaillon, N.; Harpentier, B.; Senik, A.; Hirsch, F. Growth hormone prevents human monocytic cells from Fas-mediated apoptosis by up-regulating Bcl-2 expression. Eur. J. Immunol. 1999, 29, 334–344. [Google Scholar] [CrossRef]
- Mitsunaka, H.; Dobashi, H.; Sato, M.; Tanaka, T.; Kitanaka, A.; Yamaoka, G.; Tokuda, M.; Matoba, K.; Hiraishi, T.; Ishida, T. Growth hormone prevents Fas-induced apoptosis in lymphocytes through modulation of Bcl-2 and caspase-3. Neuroimmunomodulation 2001, 9, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Lempereur, L.; Brambilla, D.; Scoto, G.M.; D’Alcamo, M.; Goffin, V.; Crosta, L.; Palmucci, T.; Rampello, L.; Bernardini, R.; Cantarella, G. Growth hormone protects human lymphocytes from irradiation-induced cell death. Br. J. Pharmacol. 2003, 138, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Pulkki, K.J. Cytokines and cardiomyocyte death. Ann. Med. 1997, 29, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.E.; Weigent, D.A. The inhibition of apoptosis in EL4 lymphoma cells overexpressing growth hormone. Neuroimmunomodulation 2004, 11, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Decker, D.; Springer, W.; Tolba, R.; Lauschke, H.; Hirner, A.; von Ruecker, A. Perioperative treatment with human growth hormone down-regulates apoptosis and increases superoxide production in PMN from patients undergoing infrarenal abdominal aortic aneurysm repair. Growth Horm. IGF Res. 2005, 15, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Christ, E.R.; Cummings, M.H.; Westwood, N.B.; Sawyer, B.M.; Pearson, T.C.; Sönksen, P.H.; Russell-Jones, D.L. The importance of growth hormone in the regulation of erythropoiesis, red cell mass, and plasma volume in adults with growth hormone deficiency. J. Clin. Endocrinol. Metab. 1997, 82, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Valerio, G.; di Maio, S.; Salerno, M.; Argenziano, A.; Badolato, R.; Tenore, A. Assessment of red blood cell indices in growth-hormone-treated children. Horm. Res. Paediatr. 1997, 47, 62–66. [Google Scholar] [CrossRef]
- Miniero, R.; Altomare, F.; Rubino, M.; Matarazzo, P.; Montanari, C.; Petri, A.; Raiola, G.; Bona, G. Effect of recombinant human growth hormone (rhGH) on hemoglobin concentration in children with idiopathic growth hormone deficiency-related anemia. J. Pediatr. Hematol. Oncol. 2012, 34, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Sohmiya, M.; Kanazawa, I.; Kato, Y. Effect of recombinant human GH on circulating granulocyte colony-stimulating factor and neutrophils in patients with adult GH deficiency. Eur. J. Endocrinol. 2005, 152, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Monson, J.P. Long-term experience with GH replacement therapy: Efficacy and safety. Eur. J. Endocrinol. 2003, 148 (Suppl. 2), S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A.; Sun, L.Y.; Longo, V. Somatotropic signaling: Trade-offs between growth, reproductive development, and longevity. Physiol. Rev. 2013, 93, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Kiess, W.; Gallaher, B. Hormonal control of programmed cell death/apoptosis. Eur. J. Endocrinol. 1998, 138, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Grymuła, K.; Paczkowska, E.; Dziedziejko, V.; Baśkiewicz-Masiuk, M.; Kawa, M.; Baumert, B.; Celewicz, Z.; Gawrych, E.; Machaliński, B. The influence of 3,3′,5-triiodo-l-thyronine on human haematopoiesis. Cell Prolif. 2007, 40, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Kawa, M.P.; Grymula, K.; Paczkowska, E.; Baskiewicz-Masiuk, M.; Dabkowska, E.; Koziolek, M.; Tarnowski, M.; Kłos, P.; Dziedziejko, V.; Kucia, M.; et al. Clinical relevance of thyroid dysfunction in human haematopoiesis: Biochemical and molecular studies. Eur. J. Endocrinol. 2010, 162, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Kotzmann, H.; Riedl, M.; Clodi, M.; Barnas, U.; Kaider, A.; Luger, A. The influence of growth hormone substitution therapy on erythroid and myeloid progenitor cells and on peripheral blood cells in adult patients with growth hormone deficiency. Eur. J. Clin. Investig. 1996, 26, 1175–1181. [Google Scholar] [CrossRef]
- Vihervuori, E.; Sipilä, I.; Siimes, M.A. Increases in hemoglobin concentration and iron needs in response to growth hormone treatment. J. Pediatr. 1994, 125, 242–245. [Google Scholar] [CrossRef]
- Meazza, C.; Bonomelli, I.; Pagani, S.; Travaglino, P.; Laarej, K.; Cantoni, F.; Bozzola, M. Effect of human recombinant growth hormone therapy on circulating levels of EPO and G-CSF in short children. J. Pediatr. Endocrinol. Metab. 2009, 22, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Clark, R. The somatogenic hormones and insulin-like growth factor-1: Stimulators of lymphopoiesis and immune function. Endocr. Rev. 1997, 18, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Goodier, M.R.; Imami, N.; Moyle, G.; Gazzard, B.; Gotch, F. Loss of the CD56hiCD16− NK cell subset and NK cell interferon-gamma production during antiretroviral therapy for HIV-1: Partial recovery by human growth hormone. Clin. Exp. Immunol. 2003, 134, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, R.; Sills, I.N.; Green, L.; Barrett, P.; Labus, J.; Skuza, K.A.; Chartoff, A.; Goode, L.A.U.R.A.; Stene, M.A.R.K.; Petersen, B.H. Detection of human growth hormone receptors on IM-9 cells and peripheral blood mononuclear cell subsets by flow cytometry: Correlation with growth hormone-binding protein levels. J. Clin. Endocrinol. Metab. 1995, 80, 2612–2619. [Google Scholar] [PubMed]
- Bresson, J.L.; Jeay, S.; Gagnerault, M.C.; Kayser, C.; Beressi, N.; Wu, Z.; Kinet, S.; Dardenne, M.; Postel-Vinay, M.C. Growth hormone (GH) and prolactin receptors in human peripheral blood mononuclear cells: Relation with age and GH-binding protein. Endocrinology 1999, 140, 3203–3209. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Saito, T.; Yagyu, T.; Jiang, B.H.; Kitagawa, K.; Inagaki, C. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J. Clin. Endocrinol. Metab. 2001, 86, 4284–4291. [Google Scholar] [CrossRef] [PubMed]
- Cool, S.M.; Grünert, M.; Jackson, R.; Li, H.; Nurcombe, V.; Waters, M.J. Role of growth hormone receptor signaling in osteogenesis from murine bone marrow progenitor cells. Biochem. Biophys. Res. Commun. 2005, 338, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.A.; Rakoczy, S.G.; Brown-Borg, H.M. Long-living Ames dwarf mouse hepatocytes readily undergo apoptosis. Exp. Gerontol. 2003, 38, 997–1008. [Google Scholar] [CrossRef]
- Baixeras, E.; Jeay, S.; Kelly, P.A.; Postel-Vinay, M.C. The proliferative and antiapoptotic actions of growth hormone and insulin-like growth factor-1 are mediated through distinct signaling pathways in the Pro-B Ba/F3 cell line. Endocrinology 2001, 142, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Segard, H.B.; Moulin, S.; Boumard, S.; Augier de Crémiers, C.; Kelly, P.A.; Finidori, J. Autocrine growth hormone production prevents apoptosis and inhibits differentiation in C2C12 myoblasts. Cell Signal. 2003, 15, 615–623. [Google Scholar] [CrossRef]
- Mylonas, P.G.; Matsouka, P.T.; Papandoniou, E.V.; Vagianos, C.; Kalfarentzos, F.; Alexandrides, T.K. Growth hormone and insulin-like growth factor I protect intestinal cells from radiation induced apoptosis. Mol. Cell. Endocrinol. 2000, 160, 115–122. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Makarevich, A.V. GH regulates secretory activity and apoptosis in cultured bovine granulosa cells through the activation of the cAMP/protein kinase A system. J. Endocrinol. 1999, 163, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Argetsinger, L.S.; Campbell, G.S.; Yang, X.; Witthuhn, B.A.; Silvennoinen, O.; Ihle, J.N.; Carter-Su, C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 1993, 74, 237–244. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, T.; Chen, Y.; Mertani, H.C.; Lee, K.O.; Lobie, P.E. Human growth hormone-regulated HOXA1 is a human mammary epithelial oncogene. J. Biol. Chem. 2003, 278, 7580–7590. [Google Scholar] [CrossRef] [PubMed]
- Kölle, S.; Stojkovic, M.; Boie, G.; Wolf, E.; Sinowatz, F. Growth hormone inhibits apoptosis in in vitro produced bovine embryos. Mol. Reprod. Dev. 2002, 61, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Costoya, J.A.; Finidori, J.; Moutoussamy, S.; Seãris, R.; Devesa, J.; Arce, V.M. Activation of growth hormone receptor delivers an antiapoptotic signal: Evidence for a role of Akt in this pathway. Endocrinology 1999, 140, 5937–5943. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.J.; Parker, E.; Harvey, S. Retinal ganglion cell survival in development: Mechanisms of retinal growth hormone action. Exp. Eye Res. 2006, 83, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Bogazzi, F.; Ultimieri, F.; Raggi, F.; Russo, D.; Vanacore, R.; Guida, C.; Brogioni, S.; Cosci, C.; Gasperi, M.; Bartalena, L.; et al. Growth hormone inhibits apoptosis in human colonic cancer cell lines: Antagonistic effects of peroxisome proliferator activated receptor-gamma ligands. Endocrinology 2004, 145, 3353–3362. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.; Tajouri, L.; Gray, B. The Effect of Growth Hormone Administration on the Regulation of Mitochondrial Apoptosis in-Vivo. Int. J. Mol. Sci. 2015, 16, 12753–12772. [Google Scholar] [CrossRef] [PubMed]
- Hockenbery, D.; Nuñez, G.; Milliman, C.; Schreiber, R.D.; Korsmeyer, S.J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990, 348, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Bogazzi, F.; Russo, D.; Raggi, F.; Ultimieri, F.; Urbani, C.; Gasperi, M.; Bartalena, L.; Martino, E. Transgenic mice overexpressing growth hormone (GH) have reduced or increased cardiac apoptosis through activation of multiple GH-dependent or -independent cell death pathways. Endocrinology 2008, 149, 5758–5769. [Google Scholar] [CrossRef] [PubMed]
- Bogazzi, F.; Ultimieri, F.; Raggi, F.; Russo, D.; Lombardi, M.; Cosci, C.; Brogioni, S.; Gasperi, M.; Bartalena, L.; Martino, E. Reduced colonic apoptosis in mice overexpressing bovine growth hormone occurs through changes in several kinase pathways. Growth Horm. IGF Res. 2009, 19, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Dalla Libera, L.; Ravara, B.; Volterrani, M.; Gobbo, V.; Della Barbera, M.; Angelini, A.; Betto, D.D.; Germinario, E.; Vescovo, G. Beneficial effects of GH/IGF-1 on skeletal muscle atrophy and function in experimental heart failure. Am. J. Physiol. Cell Physiol. 2004, 286, C138–C144. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Lee, E.; Kim, J.W.; Kwon, B.S.; Jung, M.K.; Jee, Y.H.; Kim, J.; Bae, S.R.; Chang, Y.P. Protective effect of growth hormone on neuronal apoptosis after hypoxia-ischemia in the neonatal rat brain. Neurosci. Lett. 2004, 354, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Andiran, N.; Yordam, N. TNF-alpha levels in children with growth hormone deficiency and the effect of long-term growth hormone replacement therapy. Growth Horm. IGF Res. 2007, 17, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Serri, O.; St-Jacques, P.; Sartippour, M.; Renier, G. Alterations of monocyte function in patients with growth hormone (GH) deficiency: Effect of substitutive GH therapy. J. Clin. Endocrinol. Metab. 1999, 84, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Parissis, J.T.; Adamopoulos, S.; Karatzas, D.; Paraskevaidis, J.; Livanis, E.; Kremastinos, D. Growth hormone-induced reduction of soluble apoptosis mediators is associated with reverse cardiac remodelling and improvement of exercise capacity in patients with idiopathic dilated cardiomyopathy. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Feldt-Rasmussen, B.; Lange, M.; Sulowicz, W.; Gafter, U.; Lai, K.N.; Wiedemann, J.; El Nahas, M. Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J. Am. Soc. Nephrol. 2007, 18, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Palmero, I.; Holder, A.; Sinclair, A.J.; Dickson, C.; Peters, G. Cyclins D1 and D2 are differentially expressed in human B-lymphoid cell lines. Oncogene 1993, 8, 1049–1054. [Google Scholar] [PubMed]
- Siebert, R.; Willers, C.P.; Opalka, B. Role of the cyclin-dependent kinase 4 and 6 inhibitor gene family p15, p16, p18 and p19 in leukemia and lymphoma. Leuk. Lymphoma 1996, 23, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Chung, I.J.; Krantz, S.B. In human immature BFU-E tumor necrosis factor-alpha not only downregulates CDK6 but also directly produces apoptosis which is prevented by stem cell factor. Exp. Hematol. 2004, 32, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Jeay, S.; Sonenshein, G.E.; Postel-Vinay, M.C.; Kelly, P.A.; Baixeras, E. Growth hormone can act as a cytokine controlling survival and proliferation of immune cells: New insights into signaling pathways. Mol. Cell. Endocrinol. 2002, 188, 1–7. [Google Scholar] [CrossRef]
- Growth Hormone Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: Summary statement of the GH Research Society. J. Clin. Endocrinol. Metab. 2000, 85, 3990–3993. [Google Scholar]

| Epidemiological and Clinical Characteristics | GHD Patients | Controls |
|---|---|---|
| Number of subjects/group (n) | 40 | 60 |
| Gender (male/female) (n) | 26/12 | 43/17 |
| mean ± SD | mean ± SD | |
| Mean age (years) | 11 ± 4.2 | 13 ± 3.3 |
| Mean BMI (units) | 18.65 ± 3.5 | 19.17 ± 3.1 |
| Plasma GH concentration (ng/mL) in different conditions: | ||
| 1. Mean nocturnal GH release | 5.6 ± 2.7 | NA |
| 2. The oral clonidine provocative test | 5.7 ± 2.4 | NA |
| 3. The L-Dopa provocative test | 4.5 ± 2.2 | NA |
| Plasma IGF-1 concentration (ng/mL) | 125 ± 49.6 # | 162.3 ± 58.6 |
| Plasma IGF-BP-3 concentration (µg/mL) | 4.0 ± 1.6 | 4.15 ± 0.86 |
| Sexual Maturity Rating system according to Tanner’s scale | ||
| 1. Tanner’s stage 1 (%) | 60 | 60 |
| 2. Tanner’s stage 2 (%) | 25 | 30 |
| 3. Tanner’s stage 3 (%) | 15 | 10 |
| No. | Gene Symbol | Gene Description | Entrez GeneID | Fold Change |
|---|---|---|---|---|
| 1 | TNF | tumor necrosis factor | 7124 | −3.5 * |
| 2 | TNFAIP2 | tumor necrosis factor, alpha-induced protein 2 | 7127 | −3.0 |
| 3 | TNFRSF1B | tumor necrosis factor receptor superfamily, member 1B | 7133 | −3.0 |
| 4 | SMNDC1 | survival motor neuron domain containing 1 | 10285 | −2.8 |
| 5 | CD27 | CD27 molecule | 939 | −2.4 |
| 6 | BCL6 | B-cell CLL/lymphoma 6 | 604 | −2.4 |
| 7 | NFKBIZ | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, zeta | 64332 | −2.4 |
| 8 | IL6R | interleukin 6 receptor | 3570 | −2.3 |
| 9 | LITAF | lipopolysaccharide-induced TNF factor | 9516 | −2.3 |
| 10 | TNFRSF10C | tumor necrosis factor receptor superfamily, member 10c | 8794 | −2.2 |
| 11 | TNFSF8 | tumor necrosis factor superfamily, member 8 | 944 | −2.1 |
| 12 | FOSB | FBJ murine osteosarcoma viral oncogene homolog B | 2354 | −2.0 |
| 13 | FNTA | farnesyltransferase, CAAX box, alpha | 2339 | −2.0 |
| 14 | DAP | death-associated protein | 1611 | −2.0 |
| 15 | GADD45B | growth arrest and DNA-damage-inducible, beta | 4616 | −2.0 |
| 16 | TMBIM1 | transmembrane BAX inhibitor motif containing 1 | 64114 | −2.0 |
| 17 | FOXO3 | forkhead box O3 | 2309 | −2.0 |
| 18 | ATM | ataxia telangiectasia mutated | 472 | −2.0 |
| 19 | BID | BH3 interacting domain death agonist | 637 | −2.0 |
| 20 | DEDD2 | death effector domain containing 2 | 162989 | −2.0 |
| 21 | TRADD | TNFRSF1A-associated via death domain | 8717 | −2.0 |
| 22 | MYD88 | myeloid differentiation primary response gene (88) | 4615 | −2.0 |
| 23 | PYCARD | PYD and CARD domain containing | 29108 | −2.0 |
| 24 | TNFRSF1A | tumor necrosis factor receptor superfamily, member 1A | 7132 | −2.0 |
| 25 | TNFRSF14 | tumor necrosis factor receptor superfamily, member 14 | 8764 | −2.0 |
| 26 | TNFSF10 | tumor necrosis factor superfamily, member 10 | 8743 | 2.0 # |
| 27 | TNFSF13B | tumor necrosis factor superfamily, member 13b | 10673 | 2.0 |
| 28 | CASP1 | caspase 1 | 834 | 2.0 |
| 29 | CASP2 | caspase 2 | 835 | 2.0 |
| 30 | CASP4 | caspase 4 | 837 | 2.0 |
| 31 | DAPK1 | death-associated protein kinase 1 | 1612 | 2.0 |
| 32 | XAF1 | XIAP associated factor 1 | 54739 | 2.9 |
| 33 | TNFAIP6 | tumor necrosis factor, alpha-induced protein 6 | 7130 | 4.8 |
| No. | Gene Symbol | Gene Description | Entrez GeneID | Fold Change |
|---|---|---|---|---|
| 1 | CDK6 | cyclin-dependent kinase 6 | 1021 | 5.7 # |
| 2 | NPM1 | nucleophosmin | 4869 | 4.0 |
| 3 | TNFAIP3 | tumor necrosis factor, alpha-induced protein 3 | 7128 | 2.5 |
| 4 | BCL2A1 | BCL2-related protein A1 | 597 | 2.1 |
| 5 | CCND2 | cyclin D2 | 894 | 2.0 |
| 6 | BCL3 | B-cell CLL/lymphoma 3 | 602 | 2.0 |
| 7 | JTB | jumping translocation breakpoint | 10899 | 2.0 |
| 8 | MCL1 | myeloid cell leukemia sequence 1 (BCL2-related) | 4170 | 2.0 |
| 9 | PROK2 | prokineticin 2 | 60675 | 2.0 |
| 10 | PRDX5 | peroxiredoxin 5 | 25824 | 2.0 |
| 11 | DAD1 | defender against cell death 1 | 1603 | 2.0 |
| 12 | CFLAR | CASP8 and FADD-like apoptosis regulator | 8837 | 2.0 |
| 13 | ATF6 | activating transcription factor 6 | 22926 | 2.0 |
| 14 | CASP2 | caspase 2 | 835 | 2.0 |
| No. | Gene Symbol | Gene Description | Entrez GeneID | Fold Change |
|---|---|---|---|---|
| 1 | BCL2A1 | BCL2-related protein A1 | 597 | −2.4 * |
| 2 | TNFAIP3 | tumor necrosis factor, alpha-induced protein 3 | 7128 | −2.4 |
| 3 | BCL3 | B-cell CLL/lymphoma 3 | 602 | −2.1 |
| 4 | ATF6 | activating transcription factor 6 | 22926 | −2.1 |
| 5 | CFLAR | CASP8 and FADD-like apoptosis regulator | 8837 | −2.1 |
| 6 | MCL1 | myeloid cell leukemia sequence 1 (BCL2-related) | 4170 | −2.0 |
| 7 | PROK2 | prokineticin 2 | 60675 | −2.0 |
| 8 | PRDX5 | peroxiredoxin 5 | 25824 | −2.0 |
| 9 | JTB | jumping translocation breakpoint | 10899 | −2.0 |
| No. | Gene Symbol | Gene Description | Entrez GeneID | Fold Change |
|---|---|---|---|---|
| 1 | FNTA | farnesyltransferase, CAAX box, alpha | 2339 | 2.0 # |
| 2 | NFKBIZ | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, zeta | 64332 | −2.0 * |
| 3 | GADD45B | growth arrest and DNA-damage-inducible, beta | 4616 | −2.0 |
| 4 | TNF | tumor necrosis factor | 7124 | −2.0 |
| 5 | TNFRSF10C | tumor necrosis factor receptor superfamily, member 10c | 8794 | −2.0 |
| 6 | TNFRSF1A | tumor necrosis factor receptor superfamily, member 1A | 7132 | −2.0 |
| 7 | LITAF | lipopolysaccharide-induced TNF factor | 9516 | −2.0 |
| 8 | CASP4 | caspase 4 | 837 | −2.0 |
| 9 | CASP8 | caspase 8 | 841 | −2.0 |
| 10 | TNFAIP6 | tumor necrosis factor, alpha-induced protein 6 | 7130 | −2.3 |
| 11 | SMNDC1 | survival motor neuron domain containing 1 | 10285 | −4.1 |
| No. | Gene Name | Gene Symbol | Primer Direction | Primer Sequence |
|---|---|---|---|---|
| 1 | B-cell CLL/lymphoma 2 | BCL-2 | Sense | GCC GGT TCA GGT ACT CAG TCA T |
| Antisense | CAT GTG TGT GGA GAG CGT CAA | |||
| 2 | B-cell lymphoma-extra large | BCL-XL | Sense | CTC AGC GCT TGC TTT AC |
| Antisense | CGC ACA GCA GCA GTT TGG | |||
| 3 | BCL2-associated X protein | BAX | Sense | GTT GCG GTC AGA AAA CAT GTC |
| Antisense | GCC GCC GTG GAC ACA | |||
| 4 | Beta-2-microglobulin | BMG | Sense | AAT GCG GCA TCT TCA AAC CT |
| Antisense | TGA CTT TGT CAC AGC CCA AGA TA |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawa, M.P.; Stecewicz, I.; Piecyk, K.; Paczkowska, E.; Rogińska, D.; Sobuś, A.; Łuczkowska, K.; Pius-Sadowska, E.; Gawrych, E.; Petriczko, E.; et al. The Impact of Growth Hormone Therapy on the Apoptosis Assessment in CD34+ Hematopoietic Cells from Children with Growth Hormone Deficiency. Int. J. Mol. Sci. 2017, 18, 111. https://doi.org/10.3390/ijms18010111
Kawa MP, Stecewicz I, Piecyk K, Paczkowska E, Rogińska D, Sobuś A, Łuczkowska K, Pius-Sadowska E, Gawrych E, Petriczko E, et al. The Impact of Growth Hormone Therapy on the Apoptosis Assessment in CD34+ Hematopoietic Cells from Children with Growth Hormone Deficiency. International Journal of Molecular Sciences. 2017; 18(1):111. https://doi.org/10.3390/ijms18010111
Chicago/Turabian StyleKawa, Miłosz Piotr, Iwona Stecewicz, Katarzyna Piecyk, Edyta Paczkowska, Dorota Rogińska, Anna Sobuś, Karolina Łuczkowska, Ewa Pius-Sadowska, Elżbieta Gawrych, Elżbieta Petriczko, and et al. 2017. "The Impact of Growth Hormone Therapy on the Apoptosis Assessment in CD34+ Hematopoietic Cells from Children with Growth Hormone Deficiency" International Journal of Molecular Sciences 18, no. 1: 111. https://doi.org/10.3390/ijms18010111
APA StyleKawa, M. P., Stecewicz, I., Piecyk, K., Paczkowska, E., Rogińska, D., Sobuś, A., Łuczkowska, K., Pius-Sadowska, E., Gawrych, E., Petriczko, E., Walczak, M., & Machaliński, B. (2017). The Impact of Growth Hormone Therapy on the Apoptosis Assessment in CD34+ Hematopoietic Cells from Children with Growth Hormone Deficiency. International Journal of Molecular Sciences, 18(1), 111. https://doi.org/10.3390/ijms18010111





