The Drosophila TRPA1 Channel and Neuronal Circuits Controlling Rhythmic Behaviours and Sleep in Response to Environmental Temperature
Abstract
:1. Introduction
2. TRPA1 in Temperature Related Behaviours
3. TRPA1 in Rhythmic Behaviours
3.1. TRPA1 and Circadian Clock Entrainment
3.2. TRPA1 and "Siesta" under Physiological Warm Temperatures
3.3. TRPA1 and "A peak" under Noxious Hot Temperatures
3.4. TRPA1 Has Opposing Effects on Afternoon Activity
3.5. TRPA1 and Temperature Preference Rhythm
4. TRPA1 Circuit and the Regulation of Siesta Sleep
- AC neurons: AC neurons are a small set of warm-activated thermosensors. They are often called internal thermosensors since they are the only cells in the adult brain known to be autonomously thermosensitive (Recently, cell autonomous thermosensors that express TRPA1 were found in the larval brain [11], the BLP neurons. The existence and function of these neurons in the adult brain has not yet been characterised), without input from peripheral temperature sensors. AC neurons express TRPA1, which is required for their function in temperature preference behaviour [19,26]. In addition, the AC neurons integrate temperature information from peripheral sensors located in the antennae [56].
- A subset of non-clock dorsal neurons: Under natural and semi-natural conditions that simulate hot summer days, flies show an A peak that is TRPA1 dependent [39,40]. Interestingly, it has been reported that the A peak is dependent on TRPA1 expression in a small subset of neurons [39] that are located in the dorsal brain [57] but may also include the AC neurons [23]. We will call these neurons “non-clock dorsal neurons”, not to confuse them with the DN clock neuronal groups. After we conducted our studies, personal communication with Charalambos Kyriacou revealed that, contrary to what was reported in [39], knock-down of trpA1 in this small subset of dorsal neurons (using trpA148951-gal4) did not interfere with the A peak. This was due to a labelling error that had occurred between the line trpA148951-gal4 and another trpA1GAL4 (knock-in) line originally created and used by Kim et al. [3]. In fact, the effect on A peak reported in this study [39] was due to knock-down of trpA1 in a combination of AC and other non-clock neurons, mediated by the latter trpA1GAL4 (knock-in) driver. This is consistent with later findings by Das et al. [40] that the A peak is regulated by TRPA1 in CRY- trpA1SH-gal4-expressing cells.
- Clock neurons: Siesta behaviour under LD cycles is at least partly mediated by temperature sensitive splicing of per [36], which directly affects arousal state [37]. In addition, it has been shown that temperature effects on the siesta are clock regulated [28]. The authors showed that PER expression in a subset of clock neurons was sufficient to drive siesta behaviour in response to temperature changes. Together, this raises the possibility that clock neurons (by definition, the neurons that express PER) regulate siesta sleep.
- A combination of the above.
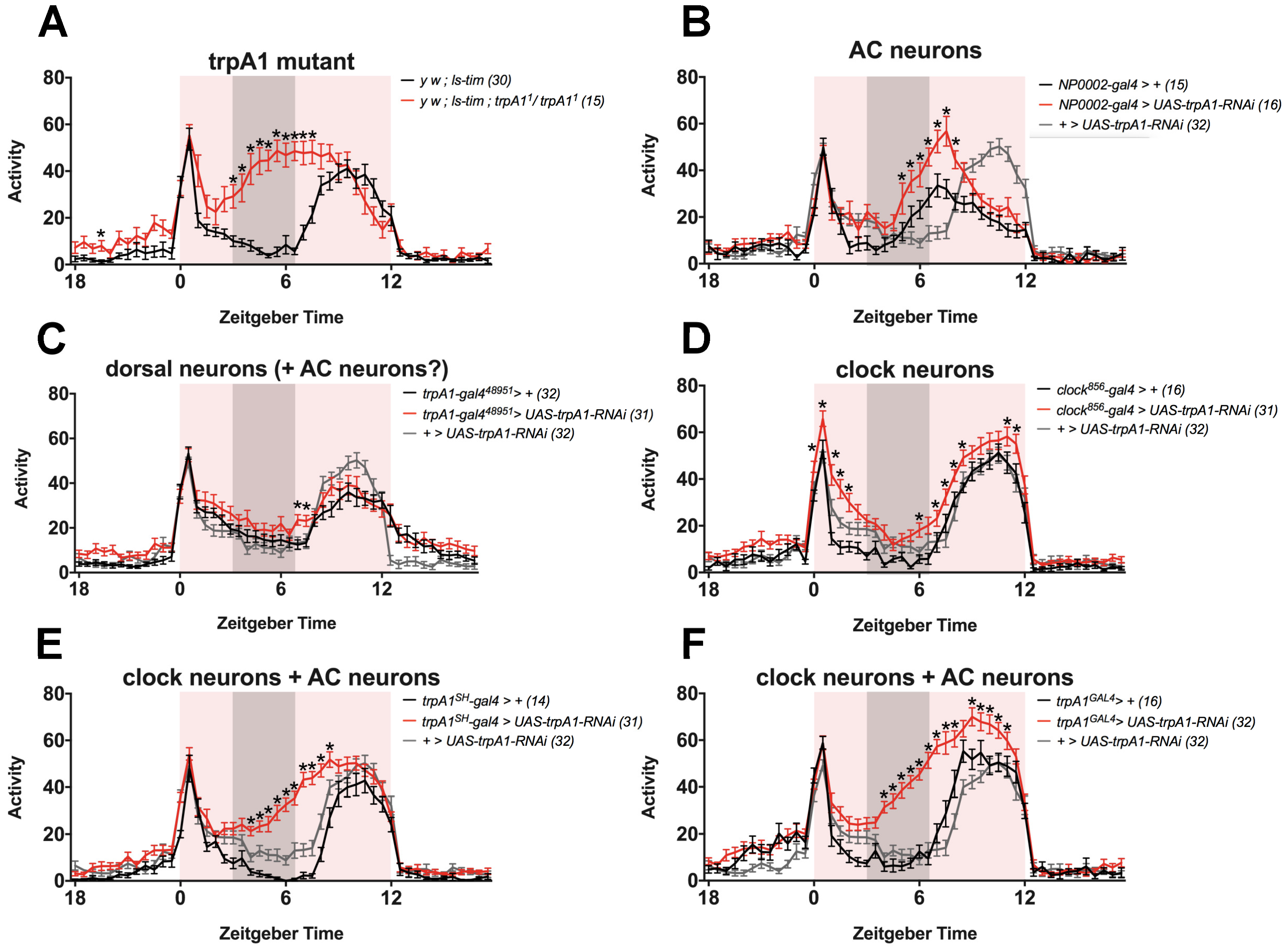
- TRPA1 in AC neurons (NP0002-gal4), ’dorsal neurons’ (trpA148951-gal4) or clock neurons (clock856-gal4) may contribute to regulation of siesta, and knock-down solely in these tissues is not sufficient to reproduce the phenotype, presumably because TRPA1 is still functional in other cells. It is a possibility that TRPA1 regulates siesta in a combination of these neuronal groups, and that knock-down of trpA1 only reproduces the siesta phenotype successfully when all (or the majority of) these cells are targeted by RNAi. TRPA1 is known to exert its function in AC neurons in the regulation of temperature preference behaviour [19]. Although we observe that TRPA1 is not required in AC neurons for normal siesta under TC (Figure 3B, using NP0002-gal4), their known role as temperature sensors and the prominent expression of trpA1SH-gal4 within them, makes it enticing to speculate that AC neurons are indeed part of the circuit that regulates siesta sleep. However, since expression of the NP0002-gal4 driver has been reported to be very weak [26], we cannot be sure that TRPA1 was sufficiently knocked-down when using this driver. These results should therefore be treated with caution and it remains possible that exclusive TRPA1 expression in AC neurons contributes to the regulation of siesta sleep.
- TRPA1 is not required in AC neurons (NP0002-gal4), ’dorsal neurons’ (trpA148951-gal4) or clock neurons (clock856-gal4) to induce siesta, and the trpA1SH-gal4-expressing cells that are responsible for siesta regulation are not overlapping with any of these cells. Based on published expression patterns, this is indeed a possibility [19,23,57,61]. For example, trpA1SH-gal4 expression is reported in four to six cells above the superior arch, and two cells just below it [23], which are not AC or clock neurons [23] and are also not stained by trpA148951-gal4 ([57] and Figure S3A in [23]) (Figure 2). In addition, trpA1SH-gal4 is expressed in peripheral neurons in the ventral nerve cord (VNC) [61]. trpA148951-gal4 is expressed in the same region [57], but it has not been characterised if these cells overlap with trpA1SH-gal4 expression. To the best of our knowledge, no peripheral expression studies have been performed with drivers NP0002-gal4 and clock856-gal4. It therefore remains an open question if the peripheral expression of trpA1SH-gal4 is specific to this driver or not. Since trpA1GAL4 is expressed widely throughout the brain, it is also conceivable that trpA1GAL4-expressing cells exist that are not overlapping with AC-, ’dorsal’- or clock-drivers. Taken together, it remains a possibility that trpA1SH-gal4- and trpA1GAL4-expressing neurons, which are not AC, ’dorsal’ or clock neurons, are the neurons that regulate siesta behaviour.
- TRPA1 is actually expressed in clock-cells, but the antibody does not detect it: Although the TRPA1 antibody is very specific, in theory, it is possible that the antibody is not sensitive enough to detect small amounts of TRPA1 protein. If this is true, it remains possible that TRPA1 is expressed in clock neurons, consistent with the observed expression of trpA1SH-gal4 and trpA1GAL4 in clock neurons.
- Unidentical expression patterns of gal4 and gal80 lines result in false conclusions about the role of TRPA1 in clock cells: Das et al. [23] used a gal4/gal80 intersectional strategy to investigate if TRPA1 is functioning in clock and/or non-clock trpA1SH-gal4-expressing cells. This strategy assumes that the expression patterns of the gal4 and gal80 lines are identical. However, it is unknown if the cry-gal4 and cry-gal80 expression patterns completely overlap, especially in the periphery. If they don’t target exactly the same cells, this leaves the possibility that trpA1SH-gal4+,cry-gal80+,cry-gal4- cells exist, in which TRPA1 will not be knocked-down with either cry-gal4 or trpA1SH-gal4,cry-gal80. Das et al. [23] suggest this as a possible explanation for the lack of a siesta phenotype in both these genotypes, compared to the observed siesta phenotype with trpA1SH-gal4. If this is indeed true, this would mean that TRPA1 functions in non-clock and CRY- trpA1SH-gal4-expressing cells (that are targeted by cry-gal80 but not cry-gal4). To be complete, since trpA1SH-gal4 is expressed in the peripheral nervous system [61], these cells don’t have to be located in the brain but can also be peripheral.
5. Clock Circuit and the Regulation of Siesta Sleep
6. Clock Circuit and Sensory Integration
7. Discussion
8. Methods Accompanying Figure 3
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| A peak | Afternoon activity peak |
| AC neurons | Anterior cell neurons |
| BLP neurons | Brain lateral posterior neurons |
| cho | Chordotonal organs |
| DDTC | Temperature cycles in constant darkness |
| DPP | Dorsal posterior protocerebrum |
| E peak | Evening activity peak |
| LD | Light dark cycles |
| LPF | Local field potential |
| M peak | Morning activity peak |
| md neurons | Multidendritic neurons |
| PER | PERIOD |
| PLC | Phospholipase C |
| PMW | Prolonged morning wakefulness |
| SP | Sex peptide |
| SPR | Sex peptide receptor |
| TC | Temperature cycles |
| TIM | TIMELESS |
| TPR | Temperature preference rhythm |
| TRP channel | Transient receptor potential channel |
| VNC | Ventral nerve cord |
References
- Fowler, M.A.; Montell, C. Drosophila TRP channels and animal behavior. Life Sci. 2013, 92, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, S.H.; Ronderos, D.S.; Lee, Y.; Akitake, B.; Woodward, O.M.; Guggino, W.B.; Smith, D.P.; Montell, C. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr. Biol. 2010, 20, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, Y.; Akitake, B.; Woodward, O.M.; Guggino, W.B.; Montell, C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl. Acad. Sci. USA 2010, 107, 8440–8445. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Pulver, S.R.; Panzano, V.C.; Chang, E.C.; Griffith, L.C.; Theobald, D.L.; Garrity, P.A. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 2010, 464, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Guntur, A.R.; Gu, P.; Takle, K.; Chen, J.; Xiang, Y.; Yang, C.H. Drosophila TRPA1 isoforms detect UV light via photochemical production of H2O2. Proc. Natl. Acad. Sci. USA 2015, 112, E5753–E5761. [Google Scholar] [CrossRef] [PubMed]
- Guntur, A.R.; Gou, B.; Gu, P.; He, R.; Stern, U.; Xiang, Y.; Yang, C.H. H2O2-Sensitive Isoforms of Drosophila melanogaster TRPA1 Act in Bitter-Sensing Gustatory Neurons to Promote Avoidance of UV During Egg-Laying. Genetics 2017, 205, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Bellemer, A.; Yan, H.; Ken, H.; Jessica, R.; Hwang, R.Y.; Pitt, G.S.; Tracey, W.D. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP Channel. Cell Rep. 2012, 1, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Yuan, Q.; Vogt, N.; Looger, L.L.; Jan, L.Y.; Jan, Y.N. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 2010, 468, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, V.; Story, G.M.; Peier, A.M.; Petrus, M.J.; Lee, V.M.; Hwang, S.W.; Patapoutian, A.; Jegla, T. Opposite thermosensor in fruitfly and mouse. Nature 2003, 423, 822–823. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Panzano, V.C.; Chang, E.C.; Ni, L.; Dainis, A.M.; Jenkins, A.M.; Regna, K.; Muskavitch, M.A.T.; Garrity, P.A. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature 2011, 481, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shen, W.L.; Montell, C. TRPA1 mediates sensation of the rate of temperature change in Drosophila larvae. Nat. Neurosci. 2017, 20, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Shim, H.S.; Wang, X.; Montell, C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci. 2008, 11, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, N.K.; Albert, J.T. Sensory transduction: Confusing the senses. Curr. Biol. 2013, 23, R22–R23. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Montell, C. Forcing open TRP channels: Mechanical gating as a unifying activation mechanism. Biochem. Biophys. Res. Commun. 2015, 460, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Bellemer, A. Thermotaxis, circadian rhythms, and TRP channels in Drosophila. Temperature (Austin) 2015, 2, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, B.; Garrity, P.A. Temperature sensation in Drosophila. Curr. Opin. Neurobiol. 2015, 34, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, M.; Brennan, K.M.; Tayler, T.D.; Phelps, P.O.; Patapoutian, A.; Garrity, P.A. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005, 19, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Sokabe, T.; Chen, H.C.; Luo, J.; Montell, C. A Switch in Thermal Preference in Drosophila Larvae Depends on Multiple Rhodopsins. Cell Rep. 2016, 17, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Hamada, F.N.; Rosenzweig, M.; Kang, K.; Pulver, S.R.; Ghezzi, A.; Jegla, T.J.; Garrity, P.A. An internal thermal sensor controlling temperature preference in Drosophila. Nature 2008, 454, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Head, L.M.; Ling, J.; Tang, X.; Liu, Y.; Hardin, P.E.; Emery, P.; Hamada, F.N. Circadian rhythm of temperature preference and its neural control in Drosophila. Curr. Biol. 2012, 22, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Neely, G.G.; Keene, A.C.; Duchek, P.; Chang, E.C.; Wang, Q.P.; Aksoy, Y.A.; Rosenzweig, M.; Costigan, M.; Woolf, C.J.; Garrity, P.A.; et al. TrpA1 regulates thermal nociception in Drosophila. PLoS ONE 2011, 6, e24343. [Google Scholar] [CrossRef] [PubMed]
- Babcock, D.T.; Shi, S.; Jo, J.; Shaw, M.; Gutstein, H.B.; Galko, M.J. Hedgehog signaling regulates nociceptive sensitization. Curr. Biol. 2011, 21, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Holmes, T.C.; Sheeba, V. dTRPA1 in Non-circadian Neurons Modulates Temperature-dependent Rhythmic Activity in Drosophila melanogaster. J. Biol. Rhythm. 2016, 31, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Lamaze, A.; Öztürk-Çolak, A.; Fischer, R.; Peschel, N.; Koh, K.; Jepson, J.E.C. Regulation of sleep plasticity by a thermo-sensitive circuit in Drosophila. Sci. Rep. 2017, 7, 40304. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Yoon, S.E.; Zhang, Q.; Chae, H.S.; Daubnerová, I.; Shafer, O.T.; Choe, J.; Kim, Y.J. A homeostatic sleep-stabilizing pathway in Drosophila composed of the sex peptide receptor and its ligand, the myoinhibitory peptide. PLoS Biol. 2014, 12, e1001974. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Roessingh, S.; Hayley, S.E.; Chu, M.L.; Tanaka, N.K.; Wolfgang, W.; Song, S.; Stanewsky, R.; Hamada, F.N. The role of PDF neurons in setting preferred temperature before dawn in Drosophila. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yu, J.; Jung, H.J.; Abruzzi, K.C.; Luo, W.; Griffith, L.C.; Rosbash, M. Circadian neuron feedback controls the Drosophila sleep-activity profile. Nature 2016, 536, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Parisky, K.M.; Agosto Rivera, J.L.; Donelson, N.C.; Kotecha, S.; Griffith, L.C. Reorganization of Sleep by Temperature in Drosophila Requires Light, the Homeostat, and the Circadian Clock. Curr. Biol. 2016, 26, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Montell, C. Drosophila TRPA1 functions in temperature control of circadian rhythm in pacemaker neurons. J. Neurosci. 2013, 33, 6716–6725. [Google Scholar] [CrossRef] [PubMed]
- Roessingh, S.; Wolfgang, W.; Stanewsky, R. Loss of Drosophila melanogaster TRPA1 Function Affects “Siesta” Behavior but Not Synchronization to Temperature Cycles. J. Biol. Rhythm. 2015, 30, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Sehadova, H.; Glaser, F.T.; Gentile, C.; Simoni, A.; Giesecke, A.; Albert, J.T.; Stanewsky, R. Temperature entrainment of Drosophila’s circadian clock involves the gene nocte and signaling from peripheral sensory tissues to the brain. Neuron 2009, 64, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Buhl, E.; Xu, M.; Croset, V.; Rees, J.S.; Lilley, K.S.; Benton, R.; Hodge, J.J.L.; Stanewsky, R. Drosophila Ionotropic Receptor 25a mediates circadian clock resetting by temperature. Nature 2015, 527, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, W.; Simoni, A.; Gentile, C.; Stanewsky, R. The Pyrexia transient receptor potential channel mediates circadian clock synchronization to low temperature cycles in Drosophila melanogaster. Proc. Biol. Sci. 2013, 280, 20130959. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.A.; Hamblen-Coyle, M.J.; Dushay, M.S.; Hall, J.C. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J. Biol. Rhythm. 1993, 8, 67–94. [Google Scholar] [CrossRef] [PubMed]
- Helfrich-Förster, C. Differential control of morning and evening components in the activity rhythm of Drosophila melanogaster–sex-specific differences suggest a different quality of activity. J. Biol. Rhythm. 2000, 15, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Majercak, J.; Sidote, D.; Hardin, P.E.; Edery, I. How a circadian clock adapts to seasonal decreases in temperature and day length. Neuron 1999, 24, 219–230. [Google Scholar] [CrossRef]
- Cao, W.; Edery, I. A novel pathway for sensory-mediated arousal involves splicing of an intron in the period clock gene. Sleep 2015, 38, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Low, K.H.; Chen, W.F.; Yildirim, E.; Edery, I. Natural variation in the Drosophila melanogaster clock gene period modulates splicing of its 3’-terminal intron and mid-day siesta. PLoS ONE 2012, 7, e49536. [Google Scholar] [CrossRef] [PubMed]
- Green, E.W.; O’Callaghan, E.K.; Hansen, C.N.; Bastianello, S.; Bhutani, S.; Vanin, S.; Armstrong, J.D.; Costa, R.; Kyriacou, C.P. Drosophila circadian rhythms in seminatural environments: Summer afternoon component is not an artifact and requires TrpA1 channels. Proc. Natl. Acad. Sci. USA 2015, 112, 8702–8707. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Holmes, T.C.; Sheeba, V. dTRPA1 Modulates Afternoon Peak of Activity of Fruit Flies Drosophila melanogaster. PLoS ONE 2015, 10, e0134213. [Google Scholar] [CrossRef] [PubMed]
- Vanin, S.; Bhutani, S.; Montelli, S.; Menegazzi, P.; Green, E.W.; Pegoraro, M.; Sandrelli, F.; Costa, R.; Kyriacou, C.P. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature 2012, 484, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Menegazzi, P.; Yoshii, T.; Helfrich-Förster, C. Laboratory versus nature: The two sides of the Drosophila circadian clock. J. Biol. Rhythm. 2012, 27, 433–442. [Google Scholar] [CrossRef] [PubMed]
- De, J.; Varma, V.; Saha, S.; Sheeba, V.; Sharma, V.K. Significance of activity peaks in fruit flies, Drosophila melanogaster, under seminatural conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 8984–8989. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Sheeba, V. Temperature Input for Rhythmic Behaviours in Flies: The Role of Temperature-Sensitive Ion Channels; Springer (India) Pvt. Ltd.: New Delhi, India, 2017. [Google Scholar]
- De, J.; Varma, V.; Sharma, V.K. Adult emergence rhythm of fruit flies Drosophila melanogaster under seminatural conditions. J. Biol. Rhythm. 2012, 27, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, J.C.; Finn, S.M.; Panckeri, K.A.; Chavkin, J.; Williams, J.A.; Sehgal, A.; Pack, A.I. Rest in Drosophila is a sleep-like state. Neuron 2000, 25, 129–138. [Google Scholar] [CrossRef]
- Shaw, P.J.; Cirelli, C.; Greenspan, R.J.; Tononi, G. Correlates of sleep and waking in Drosophila melanogaster. Science 2000, 287, 1834–1837. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nat. Rev. Neurosci. 2009, 10, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Dubowy, C.; Sehgal, A. Circadian Rhythms and Sleep in Drosophila melanogaster. Genetics 2017, 205, 1373–1397. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar] [PubMed]
- Daan, S.; Beersma, D.G.; Borbély, A.A. Timing of human sleep: Recovery process gated by a circadian pacemaker. Am. J. Physiol. 1984, 246, R161–R183. [Google Scholar] [PubMed]
- Borbély, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, H.; Lark, A.; Kitamoto, T. Factors that Differentially Affect Daytime and Nighttime Sleep in Drosophila melanogaster. Front. Neurol. 2012, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Van Alphen, B.; Yap, M.H.W.; Kirszenblat, L.; Kottler, B.; van Swinderen, B. A dynamic deep sleep stage in Drosophila. J. Neurosci. 2013, 33, 6917–6927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, R.; Hill, S.L.; Holladay, C.; Biesiadecki, M.; Tononi, G.; Cirelli, C. Sleep homeostasis in Drosophila melanogaster. Sleep 2004, 27, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Platt, M.D.; Lagnese, C.M.; Leslie, J.R.; Hamada, F.N. Temperature integration at the AC thermosensory neurons in Drosophila. J. Neurosci. 2013, 33, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Jenett, A.; Rubin, G.M.; Ngo, T.T.B.; Shepherd, D.; Murphy, C.; Dionne, H.; Pfeiffer, B.D.; Cavallaro, A.; Hall, D.; Jeter, J.; et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012, 2, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.G.; Garrity, P.A.; Boyden, E.S. Optogenetics and thermogenetics: Technologies for controlling the activity of targeted cells within intact neural circuits. Curr. Opin. Neurobiol. 2012, 22, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Ito, K.; Sado, Y.; Taniguchi, M.; Akimoto, A.; Takeuchi, H.; Aigaki, T.; Matsuzaki, F.; Nakagoshi, H.; Tanimura, T.; et al. GETDB, a database compiling expression patterns and molecular locations of a collection of Gal4 enhancer traps. Genesis 2002, 34, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Gummadova, J.O.; Coutts, G.A.; Glossop, N.R.J. Analysis of the Drosophila Clock promoter reveals heterogeneity in expression between subgroups of central oscillator cells and identifies a novel enhancer region. J. Biol. Rhythm. 2009, 24, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.W.; Chiang, A.S. Anatomical characterization of thermosensory AC neurons in the adult Drosophila brain. J. Neurogenet. 2011, 25, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Hermann-Luibl, C.; Kistenpfennig, C.; Schmid, B.; Tomioka, K.; Helfrich-Förster, C. Cryptochrome-dependent and -independent circadian entrainment circuits in Drosophila. J. Neurosci. 2015, 35, 6131–6141. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Contribution of Drosophila TRPA1-expressing neurons to circadian locomotor activity patterns. PLoS ONE 2013, 8, e85189. [Google Scholar] [CrossRef] [PubMed]
- Andretic, R.; Shaw, P.J. Essentials of sleep recordings in Drosophila: Moving beyond sleep time. Methods Enzymol. 2005, 393, 759–772. [Google Scholar] [PubMed]
- Isaac, R.E.; Li, C.; Leedale, A.E.; Shirras, A.D. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc. Biol. Sci. 2010, 277, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.T.J.; O’Neill, T.L.; Audsley, N.; Isaac, R.E. The sexually dimorphic behaviour of adult Drosophila suzukii: Elevated female locomotor activity and loss of siesta is a post-mating response. J. Exp. Biol. 2015, 218, 3855–3861. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.Q.; Zhou, R.; Czech, B.; Liu, L.P.; Holderbaum, L.; Yang-Zhou, D.; Shim, H.S.; Tao, R.; Handler, D.; Karpowicz, P.; et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 2011, 8, 405–407. [Google Scholar] [CrossRef] [PubMed]


| Driver | Nature of Gal4 | Expression Pattern |
|---|---|---|
| NP0002-gal4 | gal4 enhancer trap line [59] | Anterior Cell (AC) neurons [26] |
| trpA148951-gal4 | short (putative) enhancer fragment of the trpA1 promoter driving gal4 [57] | non-clock dorsal neurons [23,57] |
| occasionally AC neurons [23] | ||
| clock856-gal4 | clock promoter fragment driving gal4 [60] | all clock neurons, but not photoreceptors R1-R8 like tim-gal4 [60] |
| trpA1SH-gal4 | trpA1 promoter fragment driving gal4 [19] | AC neurons [19,23,61] |
| limited brain expression [23,61] | ||
| clock neurons: 1 s-LNv [23], 5th s-LNv [23,62], 2-3 LNd [23,62], 0-1 DN1a [23,62]. | ||
| in contrast, [26] don’t find expression in clock neurons. | ||
| trpA1GAL4 | GAL4 knock-in into the trpA1 gene, deleting 185 bp spanning start codon [3] | AC neurons [63] |
| wider brain expression [63] | ||
| clock neurons: 5th s-LNv, 3 LNd, 2-3 DN1, 1 DN2, 1 DN3, 3 LPN [29,63]. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roessingh, S.; Stanewsky, R. The Drosophila TRPA1 Channel and Neuronal Circuits Controlling Rhythmic Behaviours and Sleep in Response to Environmental Temperature. Int. J. Mol. Sci. 2017, 18, 2028. https://doi.org/10.3390/ijms18102028
Roessingh S, Stanewsky R. The Drosophila TRPA1 Channel and Neuronal Circuits Controlling Rhythmic Behaviours and Sleep in Response to Environmental Temperature. International Journal of Molecular Sciences. 2017; 18(10):2028. https://doi.org/10.3390/ijms18102028
Chicago/Turabian StyleRoessingh, Sanne, and Ralf Stanewsky. 2017. "The Drosophila TRPA1 Channel and Neuronal Circuits Controlling Rhythmic Behaviours and Sleep in Response to Environmental Temperature" International Journal of Molecular Sciences 18, no. 10: 2028. https://doi.org/10.3390/ijms18102028
APA StyleRoessingh, S., & Stanewsky, R. (2017). The Drosophila TRPA1 Channel and Neuronal Circuits Controlling Rhythmic Behaviours and Sleep in Response to Environmental Temperature. International Journal of Molecular Sciences, 18(10), 2028. https://doi.org/10.3390/ijms18102028





