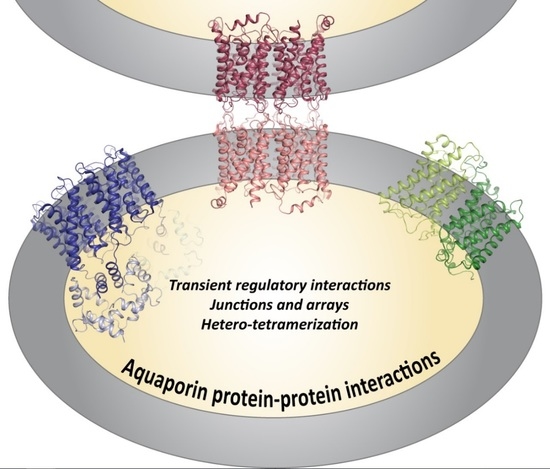
Aquaporin Protein-Protein Interactions
Abstract
:1. Introduction
2. Interactions between Aquaporin (AQP) Tetramers in Supramolecular Assemblies
2.1. AQP0
2.1.1. AQP0 Junction Formation Is Regulated by Proteolytic Cleavage
2.1.2. Junctional AQP0 Is Closed
2.2. AQP4
2.2.1. AQP4 Orthogonal Array Formation Is Isoform-Dependent
2.2.2. Does AQP4 Form Junctions?
3. AQP Hetero-Tetramerization
3.1. Plasma Membrane Intrinsic Protein (PIP) Hetero-Tetramerization Controls PIP1 Trafficking
3.2. PIP Hetero-Tetramerization and Substrate Conductance
3.3. Structural Aspects of PIP Hetero-Tetramerization
3.3.1. Loop A
3.3.2. Loop E
3.3.3. Transmembrane Region
4. Transient Interactions between AQPs and Regulatory Proteins
4.1. Gating of AQP0 by Calmodulin
Calmodulin Inhibits AQP0 Water Permeability through an Allosteric Mechanism
4.2. Trafficking of AQP2
4.2.1. Protein-Protein Interactions Control AQP2 Trafficking
4.2.2. AQP2 Phosphorylation Allosterically Controls its Interaction with LYST-interacting protein 5 (LIP5)
4.3. The AQP C-Terminal Helix—A Common Motif for Protein-Protein Interactions?
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AQP | Aquaporin |
| PIP | Plasma membrane intrinsic protein |
| CaM | Calmodulin |
| MIM | MIT interacting motif |
| MIT | Microtubule interaction and trafficking |
| MVB | Multivesicular body |
| LIP5 | LYST-interacting protein 5 |
References
- Braun, P.; Gingras, A.C. History of protein-protein interactions: From egg-white to complex networks. Proteomics 2012, 12, 1478–1498. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Garvin, J.L. Aquaporins as gas channels. Pflugers Arch. 2011, 462, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Kaldenhoff, R.; Kai, L.; Uehlein, N. Aquaporins and membrane diffusion of CO2 in living organisms. Biochim. Biophys. Acta 2014, 1840, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Garneau, A.P.; Carpentier, G.A.; Marcoux, A.A.; Frenette-Cotton, R.; Simard, C.F.; Remus-Borel, W.; Caron, L.; Jacob-Wagner, M.; Noel, M.; Powell, J.J.; et al. Aquaporins mediate silicon transport in humans. PLoS ONE 2015, 10, e0136149. [Google Scholar] [CrossRef] [PubMed]
- Byrt, C.S.; Zhao, M.; Kourghi, M.; Bose, J.; Henderson, S.W.; Qiu, J.; Gilliham, M.; Schultz, C.; Schwarz, M.; Ramesh, S.A.; et al. Non-selective cation channel activity of aquaporin AtPIP2;1 regulated by Ca2+ and pH. Plant Cell Environ. 2017, 40, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Ehring, G.R.; Zampighi, G.; Horwitz, J.; Bok, D.; Hall, J.E. Properties of channels reconstituted from the major intrinsic protein of lens fiber membranes. J. Gen. Physiol. 1990, 96, 631–664. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Hazama, A.; Kwon, T.H.; Nielsen, S.; Guggino, W.B.; Agre, P. Rapid gating and anion permeability of an intracellular aquaporin. Nature 1999, 402, 184–187. [Google Scholar] [PubMed]
- Zampighi, G.A.; Hall, J.E.; Kreman, M. Purified lens junctional protein forms channels in planar lipid films. Proc. Natl. Acad. Sci. USA 1985, 82, 8468–8472. [Google Scholar] [CrossRef] [PubMed]
- Boassa, D.; Stamer, W.D.; Yool, A.J. Ion channel function of aquaporin-1 natively expressed in choroid plexus. J. Neurosci. 2006, 26, 7811–7819. [Google Scholar] [CrossRef] [PubMed]
- Kreida, S.; Tornroth-Horsefield, S. Structural insights into aquaporin selectivity and regulation. Curr. Opin. Struct. Biol. 2015, 33, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Gonen, T.; Cheng, Y.; Kistler, J.; Walz, T. Aquaporin-0 membrane junctions form upon proteolytic cleavage. J. Mol. Biol. 2004, 342, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Rash, J.E.; Yasumura, T.; Hudson, C.S.; Agre, P.; Nielsen, S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc. Natl. Acad. Sci. USA 1998, 95, 11981–11986. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Verkman, A.S. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in xenopus oocytes. J. Biol. Chem. 1997, 272, 16140–16146. [Google Scholar] [CrossRef] [PubMed]
- Neely, J.D.; Christensen, B.M.; Nielsen, S.; Agre, P. Heterotetrameric composition of aquaporin-4 water channels. Biochemistry 1999, 38, 11156–11163. [Google Scholar] [CrossRef] [PubMed]
- Hiroaki, Y.; Tani, K.; Kamegawa, A.; Gyobu, N.; Nishikawa, K.; Suzuki, H.; Walz, T.; Sasaki, S.; Mitsuoka, K.; Kimura, K.; et al. Implications of the aquaporin-4 structure on array formation and cell adhesion. J. Mol. Biol. 2006, 355, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Alcala, J.; Lieska, N.; Maisel, H. Protein composition of bovine lens cortical fiber cell membranes. Exp. Eye. Res. 1975, 21, 581–595. [Google Scholar] [CrossRef]
- Varadaraj, K.; Kushmerick, C.; Baldo, G.J.; Bassnett, S.; Shiels, A.; Mathias, R.T. The role of mip in lens fiber cell membrane transport. J. Membr. Biol. 1999, 170, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Chandy, G.; Zampighi, G.A.; Kreman, M.; Hall, J.E. Comparison of the water transporting properties of MIP and AQP1. J. Membr. Biol. 1997, 159, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kushmerick, C.; Rice, S.J.; Baldo, G.J.; Haspel, H.C.; Mathias, R.T. Ion, water and neutral solute transport in xenopus oocytes expressing frog lens MIP. Exp. Eye. Res. 1995, 61, 351–362. [Google Scholar] [CrossRef]
- Kushmerick, C.; Varadaraj, K.; Mathias, R.T. Effects of lens major intrinsic protein on glycerol permeability and metabolism. J. Membr. Biol. 1998, 161, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Mulders, S.M.; Preston, G.M.; Deen, P.M.; Guggino, W.B.; van Os, C.H.; Agre, P. Water channel properties of major intrinsic protein of lens. J. Biol. Chem. 1995, 270, 9010–9016. [Google Scholar] [CrossRef] [PubMed]
- Nemeth-Cahalan, K.L.; Hall, J.E. pH and calcium regulate the water permeability of aquaporin 0. J. Biol. Chem. 2000, 275, 6777–6782. [Google Scholar] [CrossRef] [PubMed]
- Zampighi, G.A.; Simon, S.A.; Hall, J.E. The specialized junctions of the lens. Int. Rev. Cytol. 1992, 136, 185–225. [Google Scholar] [PubMed]
- Donaldson, P.; Kistler, J.; Mathias, R.T. Molecular solutions to mammalian lens transparency. News Physiol. Sci. 2001, 16, 118–123. [Google Scholar] [PubMed]
- Mathias, R.T.; Rae, J.L.; Baldo, G.J. Physiological properties of the normal lens. Physiol. Rev. 1997, 77, 21–50. [Google Scholar] [PubMed]
- Bateman, J.B.; Johannes, M.; Flodman, P.; Geyer, D.D.; Clancy, K.P.; Heinzmann, C.; Kojis, T.; Berry, R.; Sparkes, R.S.; Spence, M.A. A new locus for autosomal dominant cataract on chromosome 12q13. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2665–2670. [Google Scholar]
- Berry, V.; Francis, P.; Kaushal, S.; Moore, A.; Bhattacharya, S. Missense mutations in MIP underlie autosomal dominant ‘polymorphic’ and lamellar cataracts linked to 12q. Nat. Genet. 2000, 25, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.; Berry, V.; Bhattacharya, S.; Moore, A. Congenital progressive polymorphic cataract caused by a mutation in the major intrinsic protein of the lens, MIP (AQP0). Br. J. Ophthalmol. 2000, 84, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Geyer, D.D.; Spence, M.A.; Johannes, M.; Flodman, P.; Clancy, K.P.; Berry, R.; Sparkes, R.S.; Jonsen, M.D.; Isenberg, S.J.; Bateman, J.B. Novel single-base deletional mutation in major intrinsic protein (MIP) in autosomal dominant cataract. Am. J. Ophthalmol. 2006, 141, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.S.; Gandhi, J.; Mustehsan, M.H.; Eren, S.; Varadaraj, K. Functional characterization of an AQP0 missense mutation, R33C, that causes dominant congenital lens cataract, reveals impaired cell-to-cell adhesion. Exp. Eye. Res. 2013, 116, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.J.; McIntosh, T.J.; Robertson, J.D. Distribution of gap junctions and square array junctions in the mammalian lens. Investig. Ophthalmol. Vis. Sci. 1989, 30, 975–989. [Google Scholar]
- Gonen, T.; Cheng, Y.; Sliz, P.; Hiroaki, Y.; Fujiyoshi, Y.; Harrison, S.C.; Walz, T. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature 2005, 438, 633–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonen, T.; Sliz, P.; Kistler, J.; Cheng, Y.; Walz, T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature 2004, 429, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Harries, W.E.; Akhavan, D.; Miercke, L.J.; Khademi, S.; Stroud, R.M. The channel architecture of aquaporin 0 at a 2.2-Å resolution. Proc. Natl. Acad. Sci. USA 2004, 101, 14045–14050. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Zhai, H.; Li, D.; Zhao, L.; Li, C.; Huang, S.; Ma, X. A novel mutation in major intrinsic protein of the lens gene (MIP) underlies autosomal dominant cataract in a chinese family. Mol. Vis. 2007, 13, 1651–1656. [Google Scholar] [PubMed]
- Ho, J.D.; Yeh, R.; Sandstrom, A.; Chorny, I.; Harries, W.E.; Robbins, R.A.; Miercke, L.J.; Stroud, R.M. Crystal structure of human aquaporin 4 at 1.8 Å and its mechanism of conductance. Proc. Natl. Acad. Sci. USA 2009, 106, 7437–7442. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Han, B.G.; Lee, J.K.; Walian, P.; Jap, B.K. Structural basis of water-specific transport through the AQP1 water channel. Nature 2001, 414, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.E.; Little, M.; Nowak, M.W.; Garland, D.L.; Crouch, R.K.; Schey, K.L. Water permeability of C-terminally truncated aquaporin 0 (AQP0 1-243) observed in the aging human lens. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4820–4828. [Google Scholar] [CrossRef]
- Frigeri, A.; Gropper, M.A.; Turck, C.W.; Verkman, A.S. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc. Natl. Acad. Sci. USA 1995, 92, 4328–4331. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Ma, T.; Skach, W.; Matthay, M.A.; Verkman, A.S. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J. Biol. Chem. 1994, 269, 5497–5500. [Google Scholar] [PubMed]
- Jung, J.S.; Bhat, R.V.; Preston, G.M.; Guggino, W.B.; Baraban, J.M.; Agre, P. Molecular characterization of an aquaporin cDNA from brain: Candidate osmoreceptor and regulator of water balance. Proc. Natl. Acad. Sci. USA 1994, 91, 13052–13056. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.; Varrin-Doyer, M.; Zamvil, S.S.; Verkman, A.S. Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation. FASEB J. 2011, 25, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Padmawar, P.; Yao, X.; Bloch, O.; Manley, G.T.; Verkman, A.S. K+ waves in brain cortex visualized using a long-wavelength k+-sensing fluorescent indicator. Nat. Methods 2005, 2, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Manley, G.T.; Krishna, S.; Verkman, A.S. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004, 18, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Watanabe, H.; Yan, D.; Manley, G.T.; Verkman, A.S. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J. Cell. Sci. 2005, 118, 5691–5698. [Google Scholar] [CrossRef] [PubMed]
- Lennon, V.A.; Kryzer, T.J.; Pittock, S.J.; Verkman, A.S.; Hinson, S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005, 202, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Graber, D.J.; Levy, M.; Kerr, D.; Wade, W.F. Neuromyelitis optica pathogenesis and aquaporin 4. J. Neuroinflamm. 2008, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Fenton, R.A.; Moeller, H.B.; Zelenina, M.; Snaebjornsson, M.T.; Holen, T.; MacAulay, N. Differential water permeability and regulation of three aquaporin 4 isoforms. Cell. Mol. Life Sci. 2010, 67, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, C.; Bouley, R.; Huang, Y.; Fang, P.; Pastor-Soler, N.; Brown, D.; Van Hoek, A.N. Membrane organization and function of M1 and M23 isoforms of aquaporin-4 in epithelial cells. Am. J. Physiol. Renal Physiol. 2004, 287, F501–F511. [Google Scholar] [CrossRef] [PubMed]
- Amiry-Moghaddam, M.; Frydenlund, D.S.; Ottersen, O.P. Anchoring of aquaporin-4 in brain: Molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience 2004, 129, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.M.; Lam, C.; Rossi, A.; Gupta, T.; Bennett, J.L.; Verkman, A.S. Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays. J. Biol. Chem. 2011, 286, 16516–16524. [Google Scholar] [CrossRef] [PubMed]
- Phuan, P.W.; Ratelade, J.; Rossi, A.; Tradtrantip, L.; Verkman, A.S. Complement-dependent cytotoxicity in neuromyelitis optica requires aquaporin-4 protein assembly in orthogonal arrays. J. Biol. Chem. 2012, 287, 13829–13839. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Lee, M.D.; Smith, B.L.; Jung, J.S.; Agre, P.; Verdijk, M.A.; Merkx, G.; Rijss, J.P.; Deen, P.M. The human AQP4 gene: Definition of the locus encoding two water channel polypeptides in brain. Proc. Natl. Acad. Sci. USA 1996, 93, 10908–10912. [Google Scholar] [CrossRef] [PubMed]
- Rash, J.E.; Davidson, K.G.; Yasumura, T.; Furman, C.S. Freeze-fracture and immunogold analysis of aquaporin-4 (AQP4) square arrays, with models of AQP4 lattice assembly. Neuroscience 2004, 129, 915–934. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.M.; Bennett, J.L.; Verkman, A.S. Live cell analysis of aquaporin-4 M1/M23 interactions and regulated orthogonal array assembly in glial cells. J. Biol. Chem. 2009, 284, 35850–35860. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Moritz, T.J.; Ratelade, J.; Verkman, A.S. Super-resolution imaging of aquaporin-4 orthogonal arrays of particles in cell membranes. J. Cell. Sci. 2012, 125, 4405–4412. [Google Scholar] [CrossRef] [PubMed]
- Tani, K.; Mitsuma, T.; Hiroaki, Y.; Kamegawa, A.; Nishikawa, K.; Tanimura, Y.; Fujiyoshi, Y. Mechanism of aquaporin-4’s fast and highly selective water conduction and proton exclusion. J. Mol. Biol. 2009, 389, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.M.; Verkman, A.S. Determinants of aquaporin-4 assembly in orthogonal arrays revealed by live-cell single-molecule fluorescence imaging. J. Cell. Sci. 2009, 122, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Nishikawa, K.; Hiroaki, Y.; Fujiyoshi, Y. Formation of aquaporin-4 arrays is inhibited by palmitoylation of N-terminal cysteine residues. Biochim. Biophys. Acta 2008, 1778, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.; Fujiyoshi, Y.; Gonen, T.; Walz, T. Junction-forming aquaporins. Curr. Opin. Struct. Biol. 2008, 18, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Verkman, A.S. Evidence against involvement of aquaporin-4 in cell-cell adhesion. J. Mol. Biol. 2008, 382, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.P. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [PubMed]
- Sohara, E.; Rai, T.; Yang, S.S.; Uchida, K.; Nitta, K.; Horita, S.; Ohno, M.; Harada, A.; Sasaki, S.; Uchida, S. Pathogenesis and treatment of autosomal-dominant nephrogenic diabetes insipidus caused by an aquaporin 2 mutation. Proc. Natl. Acad. Sci. USA 2006, 103, 14217–14222. [Google Scholar] [CrossRef] [PubMed]
- Harvengt, P.; Vlerick, A.; Fuks, B.; Wattiez, R.; Ruysschaert, J.M.; Homble, F. Lentil seed aquaporins form a hetero-oligomer which is phosphorylated by a Mg2+-dependent and Ca2+-regulated kinase. Biochem. J. 2000, 352, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Jozefkowicz, C.; Berny, M.C.; Chaumont, F.; Alleva, K. Heteromerization of plant aquaporins. In Plant Aquaporins; Chaumont, F., Tyerman, S.D., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 29–46. [Google Scholar]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new endscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Biela, A.; Grote, K.; Otto, B.; Hoth, S.; Hedrich, R.; Kaldenhoff, R. The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J. 1999, 18, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Barrieu, F.; Jung, R.; Chrispeels, M.J. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 2000, 122, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Daniels, M.J.; Mirkov, T.E.; Chrispeels, M.J. The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol. 1994, 106, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Karlsson, M.; Shukla, V.K.; Chrispeels, M.J.; Larsson, C.; Kjellbom, P. Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 1998, 10, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Marin-Olivier, M.; Chevalier, T.; Fobis-Loisy, I.; Dumas, C.; Gaude, T. Aquaporin PIP genes are not expressed in the stigma papillae in Brassica oleracea. Plant J. 2000, 24, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Moshelion, M.; Becker, D.; Biela, A.; Uehlein, N.; Hedrich, R.; Otto, B.; Levi, H.; Moran, N.; Kaldenhoff, R. Plasma membrane aquaporins in the motor cells of samanea saman: Diurnal and circadian regulation. Plant Cell 2002, 14, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Weig, A.; Deswarte, C.; Chrispeels, M.J. The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiol. 1997, 114, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Katsuhara, M.; Kelly, W.B.; Michalowski, C.B.; Bohnert, H.J. A family of transcripts encoding water channel proteins: Tissue-specific expression in the common ice plant. Plant Cell 1995, 7, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Fetter, K.; van Wilder, V.; Moshelion, M.; Chaumont, F. Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 2004, 16, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Zelazny, E.; Borst, J.W.; Muylaert, M.; Batoko, H.; Hemminga, M.A.; Chaumont, F. Fret imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc. Natl. Acad. Sci. USA 2007, 104, 12359–12364. [Google Scholar] [CrossRef] [PubMed]
- Alleva, K.; Marquez, M.; Villarreal, N.; Mut, P.; Bustamante, C.; Bellati, J.; Martinez, G.; Civello, M.; Amodeo, G. Cloning, functional characterization, and co-expression studies of a novel aquaporin (FaPIP2;1) of strawberry fruit. J. Exp. Bot. 2010, 61, 3935–3945. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.; Cavez, D.; Miled, N.; Chaumont, F.; Masmoudi, K. Identification and characterization of two plasma membrane aquaporins in durum wheat (Triticum turgidum L. Subsp. durum) and their role in abiotic stress tolerance. Plant Physiol. Biochem. 2011, 49, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Bellati, J.; Alleva, K.; Soto, G.; Vitali, V.; Jozefkowicz, C.; Amodeo, G. Intracellular pH sensing is altered by plasma membrane PIP aquaporin co-expression. Plant Mol. Biol. 2010, 74, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Cavez, D.; Besserer, A.; Berny, M.C.; Gilis, D.; Rooman, M.; Chaumont, F. A conserved cysteine residue is involved in disulfide bond formation between plant plasma membrane aquaporin monomers. Biochem. J. 2012, 445, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Mahdieh, M.; Mostajeran, A.; Horie, T.; Katsuhara, M. Drought stress alters water relations and expression of PIP-type aquaporin genes in Nicotiana tabacum plants. Plant Cell Physiol. 2008, 49, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Uehlein, N.; Sdorra, S.; Fischer, M.; Ayaz, M.; Belastegui-Macadam, X.; Heckwolf, M.; Lachnit, M.; Pede, N.; Priem, N.; et al. Aquaporin tetramer composition modifies the function of tobacco aquaporins. J. Biol. Chem. 2010, 285, 31253–31260. [Google Scholar] [CrossRef] [PubMed]
- Vandeleur, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C.; Chrispeels, M.J.; Brown, P.H. Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol. 2000, 124, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Uehlein, N.; Lovisolo, C.; Siefritz, F.; Kaldenhoff, R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 2003, 425, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, A.S.; Bienert, G.P.; Chaumont, F. A new LxxxA motif in the transmembrane Helix3 of maize aquaporins belonging to the plasma membrane intrinsic protein PIP2 group is required for their trafficking to the plasma membrane. Plant Physiol. 2014, 166, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Sorieul, M.; Santoni, V.; Maurel, C.; Luu, D.T. Mechanisms and effects of retention of over-expressed aquaporin AtPIP2;1 in the endoplasmic reticulum. Traffic 2011, 12, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Zelazny, E.; Miecielica, U.; Borst, J.W.; Hemminga, M.A.; Chaumont, F. An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. Plant J. 2009, 57, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Berny, M.C.; Gilis, D.; Rooman, M.; Chaumont, F. Single mutations in the transmembrane domains of maize plasma membrane aquaporins affect the activity of monomers within a heterotetramer. Mol. Plant 2016, 9, 986–1003. [Google Scholar] [CrossRef] [PubMed]
- Jozefkowicz, C.; Sigaut, L.; Scochera, F.; Soto, G.; Ayub, N.; Pietrasanta, L.I.; Amodeo, G.; Gonzalez Flecha, F.L.; Alleva, K. PIP water transport and its pH dependence are regulated by tetramer stoichiometry. Biophys. J. 2016, 110, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Yaneff, A.; Sigaut, L.; Marquez, M.; Alleva, K.; Pietrasanta, L.I.; Amodeo, G. Heteromerization of PIP aquaporins affects their intrinsic permeability. Proc. Natl. Acad. Sci. USA 2014, 111, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Yaneff, A.; Vitali, V.; Amodeo, G. PIP1 aquaporins: Intrinsic water channels or PIP2 aquaporin modulators? FEBS Lett. 2015, 589, 3508–3515. [Google Scholar] [CrossRef] [PubMed]
- Tornroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural mechanism of plant aquaporin gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Tournaire-Roux, C.; Sutka, M.; Javot, H.; Gout, E.; Gerbeau, P.; Luu, D.T.; Bligny, R.; Maurel, C. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 2003, 425, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Preston, G.M.; Smith, B.L.; Guggino, W.B.; Agre, P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J. Biol. Chem. 1994, 269, 14648–14654. [Google Scholar] [PubMed]
- Mathai, J.C.; Agre, P. Hourglass pore-forming domains restrict aquaporin-1 tetramer assembly. Biochemistry 1999, 38, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Moshelion, M.; Daniels, M.J. Regulation of plant aquaporin activity. Biol. Cell 2005, 97, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Jarva, M.; Ekvall, M.; Uzdavinys, P.; Nyblom, M.; Tornroth-Horsefield, S. Mercury increases water permeability of a plant aquaporin through a non-cysteine-related mechanism. Biochem. J. 2013, 454, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjohamn, J.; Hedfalk, K. Unraveling aquaporin interaction partners. Biochim. Biophys. Acta 2014, 1840, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Rabaud, N.E.; Song, L.; Wang, Y.; Agre, P.; Yasui, M.; Carbrey, J.M. Aquaporin 6 binds calmodulin in a calcium-dependent manner. Biochem. Biophys. Res. Commun. 2009, 383, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Reichow, S.L.; Clemens, D.M.; Freites, J.A.; Nemeth-Cahalan, K.L.; Heyden, M.; Tobias, D.J.; Hall, J.E.; Gonen, T. Allosteric mechanism of water-channel gating by Ca2+-calmodulin. Nat. Struct. Mol. Biol. 2013, 20, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Jesus, T.T.; Bernardino, R.L.; Martins, A.D.; Sa, R.; Sousa, M.; Alves, M.G.; Oliveira, P.F. Aquaporin-4 as a molecular partner of cystic fibrosis transmembrane conductance regulator in rat sertoli cells. Biochem. Biophys. Res. Commun. 2014, 446, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Jesus, T.T.; Bernardino, R.L.; Martins, A.D.; Sa, R.; Sousa, M.; Alves, M.G.; Oliveira, P.F. Aquaporin-9 is expressed in rat sertoli cells and interacts with the cystic fibrosis transmembrane conductance regulator. IUBMB Life 2014, 66, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Neely, J.D.; Amiry-Moghaddam, M.; Ottersen, O.P.; Froehner, S.C.; Agre, P.; Adams, M.E. Syntrophin-dependent expression and localization of aquaporin-4 water channel protein. Proc. Natl. Acad. Sci. USA 2001, 98, 14108–14113. [Google Scholar] [CrossRef] [PubMed]
- Stokum, J.A.; Kwon, M.S.; Woo, S.K.; Tsymbalyuk, O.; Vennekens, R.; Gerzanich, V.; Simard, J.M. SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia 2017. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Tsuzaka, K.; Takeuchi, T.; Sasaki, Y.; Tsubota, K. Altered distribution of aquaporin 5 and its C-terminal binding protein in the lacrimal glands of a mouse model for Sjögren’s syndrome. Curr. Eye Res. 2008, 33, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Krintel, C.; Hernebring, M.; Haataja, T.J.; de Mare, S.; Wasserstrom, S.; Kosinska-Eriksson, U.; Palmgren, M.; Holm, C.; Stenkula, K.G.; et al. Perilipin 1 binds to aquaporin 7 in human adipocytes and controls its mobility via protein kinase a mediated phosphorylation. Metabolism 2016, 65, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jin, W.W.; Tsuji, K.; Chen, Y.; Nomura, N.; Su, L.; Yui, N.; Arthur, J.; Cotecchia, S.; Paunescu, T.G.; et al. Ezrin directly interacts with AQP2 and promotes its endocytosis. J. Cell. Sci. 2017, 130, 2914–2925. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Schey, K.L. Aquaporin-0 interacts with the ferm domain of ezrin/radixin/moesin proteins in the ocular lens. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5079–5087. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.A.; Sun, T.X.; Matsuzaki, T.; Yi, X.H.; Eswara, J.; Bouley, R.; McKee, M.; Brown, D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J. Biol. Chem. 2007, 282, 28721–28732. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Lim, J.S.; Jung, H.J.; Kim, E.; Han, K.H.; Kwon, T.H. The role of 70-kDa heat shock protein in dDAVP-induced AQP2 trafficking in kidney collecting duct cells. Am. J. Physiol. Renal Physiol. 2013, 304, F958–F971. [Google Scholar] [CrossRef] [PubMed]
- Trimpert, C.; Wesche, D.; de Groot, T.; Rodriguez, M.M.P.; Wong, V.; van den Berg, D.T.M.; Cheval, L.; Ariza, C.A.; Doucet, A.; Stagljar, I.; et al. NDFIP allows NEDD4/NEDD4L-induced AQP2 ubiquitination and degradation. PLoS ONE 2017, 12, e0183774. [Google Scholar] [CrossRef] [PubMed]
- Moeller, H.B.; Praetorius, J.; Rutzler, M.R.; Fenton, R.A. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc. Natl. Acad. Sci. USA 2010, 107, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Eriksson, U.K.; de Mattia, F.; Oberg, F.; Hedfalk, K.; Neutze, R.; de Grip, W.J.; Deen, P.M.; Tornroth-Horsefield, S. X-ray structure of human aquaporin 2 and its implications for nephrogenic diabetes insipidus and trafficking. Proc. Natl. Acad. Sci. USA 2014, 111, 6305–6310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005, 46, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Soveral, G.; Nielsen, S.; Casini, A. (Eds.) Regulation of eukaryotic aquaporins. In Aquaporins in Health and Disease: New Molecular Targets for Drug Discovery; CRC Press: Boca Raton, FL, USA, 2015; pp. 53–76. [Google Scholar]
- Kortenoeven, M.L.; Fenton, R.A. Renal aquaporins and water balance disorders. Biochim. Biophys. Acta 2014, 1840, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Van Balkom, B.W.; Savelkoul, P.J.; Markovich, D.; Hofman, E.; Nielsen, S.; van der Sluijs, P.; Deen, P.M. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J. Biol. Chem. 2002, 277, 41473–41479. [Google Scholar] [CrossRef] [PubMed]
- Fenton, R.A.; Moeller, H.B.; Hoffert, J.D.; Yu, M.J.; Nielsen, S.; Knepper, M.A. Acute regulation of aquaporin-2 phosphorylation at ser-264 by vasopressin. Proc. Natl. Acad. Sci. USA 2008, 105, 3134–3139. [Google Scholar] [CrossRef] [PubMed]
- Hoffert, J.D.; Fenton, R.A.; Moeller, H.B.; Simons, B.; Tchapyjnikov, D.; McDill, B.W.; Yu, M.J.; Pisitkun, T.; Chen, F.; Knepper, M.A. Vasopressin-stimulated increase in phosphorylation at ser269 potentiates plasma membrane retention of aquaporin-2. J. Biol. Chem. 2008, 283, 24617–24627. [Google Scholar] [CrossRef] [PubMed]
- Hoffert, J.D.; Nielsen, J.; Yu, M.J.; Pisitkun, T.; Schleicher, S.M.; Nielsen, S.; Knepper, M.A. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am. J. Physiol. Renal Physiol. 2007, 292, F691–F700. [Google Scholar] [CrossRef] [PubMed]
- Tamma, G.; Robben, J.H.; Trimpert, C.; Boone, M.; Deen, P.M. Regulation of AQP2 localization by S256 and S261 phosphorylation and ubiquitination. Am. J. Physiol. Cell Physiol. 2011, 300, C636–C646. [Google Scholar] [CrossRef] [PubMed]
- Kamsteeg, E.J.; Hendriks, G.; Boone, M.; Konings, I.B.; Oorschot, V.; van der Sluijs, P.; Klumperman, J.; Deen, P.M. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc. Natl. Acad. Sci. USA 2006, 103, 18344–18349. [Google Scholar] [CrossRef] [PubMed]
- Kirchhausen, T. Three ways to make a vesicle. Nat. Rev. Mol. Cell Biol. 2000, 1, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Derby, M.C.; Gleeson, P.A. New insights into membrane trafficking and protein sorting. Int. Rev. Cytol. 2007, 261, 47–116. [Google Scholar] [PubMed]
- Zwang, N.A.; Hoffert, J.D.; Pisitkun, T.; Moeller, H.B.; Fenton, R.A.; Knepper, M.A. Identification of phosphorylation-dependent binding partners of aquaporin-2 using protein mass spectrometry. J. Proteome Res. 2009, 8, 1540–1554. [Google Scholar] [CrossRef] [PubMed]
- Tamma, G.; Procino, G.; Mola, M.G.; Svelto, M.; Valenti, G. Functional involvement of Annexin-2 in cAMP induced AQP2 trafficking. Pflugers Arch. 2008, 456, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Van Balkom, B.W.; Boone, M.; Hendriks, G.; Kamsteeg, E.J.; Robben, J.H.; Stronks, H.C.; van der Voorde, A.; van Herp, F.; van der Sluijs, P.; Deen, P.M. LIP5 interacts with aquaporin 2 and facilitates its lysosomal degradation. J. Am. Soc. Nephrol. 2009, 20, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Horikawa, S.; Katayama, Y.; Sasaki, S. Identification of a multiprotein “motor” complex binding to water channel aquaporin-2. Biochem. Biophys. Res. Commun. 2005, 330, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Horsefield, R.; Norden, K.; Fellert, M.; Backmark, A.; Tornroth-Horsefield, S.; van Scheltinga, A.C.T.; Kvassman, J.; Kjellbom, P.; Johanson, U.; Neutze, R. High-resolution X-ray structure of human aquaporin 5. Proc. Natl. Acad. Sci. USA 2008, 105, 13327–13332. [Google Scholar] [CrossRef] [PubMed]
- Skalicky, J.J.; Arii, J.; Wenzel, D.M.; Stubblefield, W.M.; Katsuyama, A.; Uter, N.T.; Bajorek, M.; Myszka, D.G.; Sundquist, W.I. Interactions of the human LIP5 regulatory protein with endosomal sorting complexes required for transport. J. Biol. Chem. 2012, 287, 43910–43926. [Google Scholar] [CrossRef] [PubMed]
- Vild, C.J.; Li, Y.; Guo, E.Z.; Liu, Y.; Xu, Z. A novel mechanism of regulating the ATPase VPS4 by its cofactor LIP5 and the endosomal sorting complex required for transport (ESCRT)-III protein CHMP5. J. Biol. Chem. 2015, 290, 7291–7303. [Google Scholar] [CrossRef] [PubMed]
- Guo, E.Z.; Xu, Z. Distinct mechanisms of recognizing endosomal sorting complex required for transport III (ESCRT-III) protein IST1 by different microtubule interacting and trafficking (MIT) domains. J. Biol. Chem. 2015, 290, 8396–8408. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Oberg, F.; Sjohamn, J.; Hedfalk, K.; Bill, R.M.; Conner, A.C.; Conner, M.T.; Tornroth-Horsefield, S. Plasma membrane abundance of human aquaporin 5 is dynamically regulated by multiple pathways. PLoS ONE 2015, 10, e0143027. [Google Scholar] [CrossRef] [PubMed]








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roche, J.V.; Törnroth-Horsefield, S. Aquaporin Protein-Protein Interactions. Int. J. Mol. Sci. 2017, 18, 2255. https://doi.org/10.3390/ijms18112255
Roche JV, Törnroth-Horsefield S. Aquaporin Protein-Protein Interactions. International Journal of Molecular Sciences. 2017; 18(11):2255. https://doi.org/10.3390/ijms18112255
Chicago/Turabian StyleRoche, Jennifer Virginia, and Susanna Törnroth-Horsefield. 2017. "Aquaporin Protein-Protein Interactions" International Journal of Molecular Sciences 18, no. 11: 2255. https://doi.org/10.3390/ijms18112255
APA StyleRoche, J. V., & Törnroth-Horsefield, S. (2017). Aquaporin Protein-Protein Interactions. International Journal of Molecular Sciences, 18(11), 2255. https://doi.org/10.3390/ijms18112255