Angelica sinensis Polysaccharides Ameliorate Stress-Induced Premature Senescence of Hematopoietic Cell via Protecting Bone Marrow Stromal Cells from Oxidative Injuries Caused by 5-Fluorouracil
Abstract
:1. Introduction
2. Results
2.1. Tumor Suppressor 5-FU Had an Inhibitory Effect on BMSCs Growth
2.2. Angelica Sinensis Polysaccharides Alleviated the Inhibitory Effect of 5-FU on BMSCs Growth
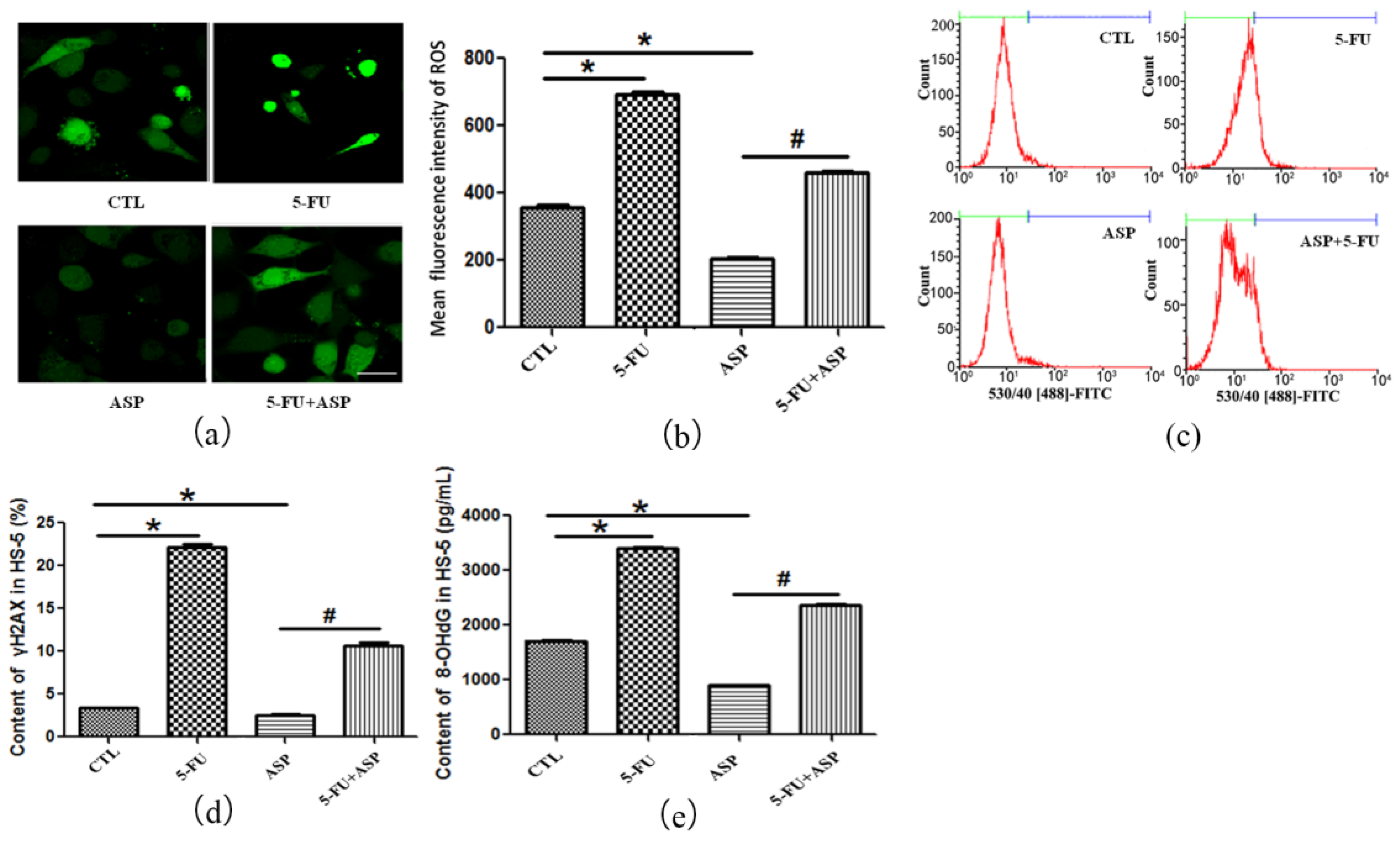
2.3. Angelica Sinensis Polysaccharide Alleviated Oxidative Stress in BMSCs Caused by 5-FU
2.4. Angelica Sinensis Polysaccharide Restored the Function of BMSCs after 5-FU Injury
2.5. ASP-Treated HS-5 Feeder Layer Protected Co-Cultured Hematopoietic Cells from Oxidative Stress-Induced Premature Senescence
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines and Culture
4.3. CCK-8 Cell Proliferation Assay
4.4. Cell Cycle Analysis
4.5. Fibroblast Colony Culture and Count
4.6. Apoptosis Detection
4.7. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
4.8. Detection of Oxidation-Associated Biological Indicators
4.9. Measurement of DNA Damage Markers
4.10. Determination of Cytokines
4.11. Analysis of Cellular Gap Junction Cx43
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| SP | Angelica sinensis polysaccharides |
| BMSC | Bone marrow stromal cell |
| Cx43 | Connexin 43 |
| CXCL12 | C-X-C motif chemokine 12 |
| CFU-F | Fibroblast-colony forming unit |
| DCF-DA | Dichlorodihydrofluorescein diacetate |
| DDR | DNA damage response |
| D-Gal | D-galactose |
| DMEM | Dulbecco’s Modified Eagle Medium |
| IMDM | Iscove’s Modified Dulbecco’s Medium |
| DSB | DNA double-strand break |
| FBS | Fetal bovine serum |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GJ | Gap Junction |
| GJIC | Gap junction of intercellular communication |
| GSH-Px | Glutathione peroxidase |
| HM | Hematopoietic microenvironment |
| HSC | Hematopoietic stem cell |
| HSPC | Hematopoietic stem/progenitor cell |
| hUCBD-MNC | Human umbilical cord blood-derived mononuclear cells |
| MSC | Mesenchymal stem cell |
| OD | Optical density |
| PI | Propidium iodide |
| RANTES | Regulated upon activation normal T cell expressed and secreted factor |
| ROS | Reactive oxygen species |
| SA-β-Gal | Senescence-associated β-galactosidase |
| SCF | Stem cell factor |
| SIPS | Stress-induced premature senescence |
| CTL | Control |
References
- Reya, T. Regulation of hematopoietic stem cell self-renewal. Recent Prog. Horm. Res. 2003, 58, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Smith, C. Hematopoietic stem cells and hematopoiesis. Cancer Control 2003, 10, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Weissman, I.L.; Anderson, D.J.; Gage, F. Stem and progenitor cells: Origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell Dev. Biol. 2001, 17, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Wickremasinghe, R.G.; Hoffbrand, A.V. Biochemical and genetic control of apoptosis: Relevance to normal hematopoiesis and hematological malignancies. Blood 1999, 93, 3587–3600. [Google Scholar] [PubMed]
- Dainiak, N. Hematologic consequences of exposure to ionizing radiation. Exp. Hematol. 2002, 30, 513–528. [Google Scholar] [CrossRef]
- Domen, J. The role of apoptosis in regulating hematopoiesis and hematopoietic stem cells. Immunol. Res. 2000, 22, 83–94. [Google Scholar] [CrossRef]
- Wang, Y.; Probin, V.; Zhou, D. Cancer therapy-induced residual bone marrow injury-mechanisms of induction and implication for therapy. Curr. Cancer Ther. Rev. 2006, 2, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Mauch, P.; Constine, L.; Greenberger, J.; Knospe, W.; Sullivan, J.; Liesveld, J.L.; Deeg, H.J. Hematopoietic stem cell compartment: Acute and late effects of radiation therapy and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1319–1339. [Google Scholar] [CrossRef]
- Marsh, J.C. The effects of cancer chemotherapeutic agents on normal hematopoietic precursor cells: A review. Cancer Res. 1976, 36, 1853–1882. [Google Scholar] [PubMed]
- Gardner, R.V.; Lerner, C.; Astle, C.M.; Harrison, D.E. Assessing permanent damage to primitive hematopoietic stem cells after chemotherapy using the competitive repopulation assay. Cancer Chemother. Pharmacol. 1993, 32, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Van Os, R.; Robinson, S.; Sheridan, T.; Mislow, J.M.; Dawes, D.; Mauch, P.M. Granulocyte colony-stimulating factor enhances bone marrow stem cell damage caused by repeated administration of cytotoxic agents. Blood 1998, 92, 1950–1956. [Google Scholar] [PubMed]
- Neben, S.; Hellman, S.; Montgomery, M.; Ferrara, J.; Mauch, P. Hematopoietic stem cell deficit of transplanted bone marrow previously exposed to cytotoxic agents. Exp. Hematol. 1993, 21, 156–162. [Google Scholar] [PubMed]
- Balderman, S.R.; Calvi, L.M. Biology of bm failure syndromes: Role of microenvironment and niches. Hematol. Am. Soc. Hematol. Educ. Program 2014, 2014, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Schmidmaier, R.; Baumann, P.; Emmerich, B.; Meinhardt, G. Evaluation of chemosensitivity of human bone marrow stromal cells--differences between common chemotherapeutic drugs. Anticancer Res. 2006, 26, 347–350. [Google Scholar] [PubMed]
- Strati, P.; Wierda, W.; Burger, J.; Ferrajoli, A.; Tam, C.; Lerner, S.; Keating, M.J.; O’Brien, S. Myelosuppression after frontline fludarabine, cyclophosphamide, and rituximab in patients with chronic lymphocytic leukemia: Analysis of persistent and new-onset cytopenia. Cancer 2013, 119, 3805–3811. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Wang, Y.; Chang, J.; Luo, Y.; Meng, A.; Zhou, D. Hematopoietic stem cell senescence and cancer therapy-induced long-term bone marrow injury. Transl. Cancer Res. 2013, 2, 397–411. [Google Scholar] [PubMed]
- Dan, T.D.; Eldredge-Hindy, H.B.; Hoffman-Censits, J.; Lin, J.; Kelly, W.K.; Gomella, L.G.; Lallas, C.D.; Trabulsi, E.J.; Hurwitz, M.D.; Dicker, A.P.; et al. Hematologic toxicity of concurrent administration of radium-223 and next-generation antiandrogen therapies. Am. J. Clin. Oncol. 2017, 40, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Ghosh, S.P.; Hauer-Jensen, M.; Kumar, K.S. Hematological targets of radiation damage. Curr. Drug Targets 2010, 11, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Himburg, H.A.; Doan, P.L.; Quarmyne, M.; Yan, X.; Sasine, J.; Zhao, L.; Hancock, G.V.; Kan, J.; Pohl, K.A.; Tran, E.; et al. Dickkopf-1 promotes hematopoietic regeneration via direct and niche-mediated mechanisms. Nat. Med. 2017, 23, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Woolthuis, C.M.; de Haan, G.; Huls, G. Aging of hematopoietic stem cells: Intrinsic changes or micro-environmental effects? Curr. Opin. Immunol. 2011, 23, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Vas, V.; Senger, K.; Dorr, K.; Niebel, A.; Geiger, H. Aging of the microenvironment influences clonality in hematopoiesis. PLoS ONE 2012, 7, e42080. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Horn, P.; Bork, S.; Ho, A.D. Aging of hematopoietic stem cells is regulated by the stem cell niche. Exp. Gerontol 2008, 43, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Wang, C.Q.; Lu, G.; Pan, X.Y.; Xu, K.L. Effects of bone marrow mesenchymal stem cells on hematopoietic recovery and acute graft-versus-host disease in murine allogeneic umbilical cord blood transplantation model. Cell Biochem. Biophys. 2014, 70, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Kim, S.H.; Lim, C.S.; Rubio, M. Cellular senescence, cancer and aging: The telomere connection. Exp. Gerontol 2001, 36, 1619–1637. [Google Scholar] [CrossRef]
- Marcotte, R.; Wang, E. Replicative senescence revisited. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B257–B269. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Blasco, M.A. Putting the stress on senescence. Curr. Opin. Cell Biol. 2001, 13, 748–753. [Google Scholar] [CrossRef]
- Garinis, G.A.; van der Horst, G.T.; Vijg, J.; Hoeijmakers, J.H. DNA damage and ageing: New-age ideas for an age-old problem. Nat. Cell Biol. 2008, 10, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Lombard, D.B.; Chua, K.F.; Mostoslavsky, R.; Franco, S.; Gostissa, M.; Alt, F.W. DNA repair, genome stability, and aging. Cell 2005, 120, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, A.; Yamamoto, K. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Vilenchik, M.M.; Knudson, A.G. Endogenous DNA double-strand breaks: Production, fidelity of repair, and induction of cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 12871–12876. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C. DNA damage and repair. Nature 2003, 421, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.P.; Li, M.; Asaithamby, A. New insights into the roles of atm and DNA-PKcs in the cellular response to oxidative stress. Cancer Lett. 2012, 327, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, P.; Das, U.N.; Koratkar, R.; Suryaprabha, P. Increase in free radical generation and lipid peroxidation following chemotherapy in patients with cancer. Free Radic. Biol. Med. 1990, 8, 15–19. [Google Scholar] [CrossRef]
- Ladner, C.; Ehninger, G.; Gey, K.F.; Clemens, M.R. Effect of etoposide (vp16-213) on lipid peroxidation and antioxidant status in a high-dose radiochemotherapy regimen. Cancer Chemother. Pharmacol. 1989, 25, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Su, W.C.; Chang, S.L.; Chen, T.Y.; Chen, J.S.; Tsao, C.J. Comparison of in vitro growth-inhibitory activity of carboplatin and cisplatin on leukemic cells and hematopoietic progenitors: The myelosuppressive activity of carboplatin may be greater than its antileukemic effect. Jpn. J. Clin. Oncol. 2000, 30, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.M.; Adams, G.B.; Weibrecht, K.W.; Weber, J.M.; Olson, D.P.; Knight, M.C.; Martin, R.P.; Schipani, E.; Divieti, P.; Bringhurst, F.R.; et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003, 425, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Niu, C.; Ye, L.; Huang, H.; He, X.; Tong, W.G.; Ross, J.; Haug, J.; Johnson, T.; Feng, J.Q.; et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003, 425, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Arai, F.; Hirao, A. Hematopoietic stem cells and their niche. Trends Immunol. 2005, 26, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Urao, N.; Ushio-Fukai, M. Redox regulation of stem/progenitor cells and bone marrow niche. Free Radic. Biol. Med. 2013, 54, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Venza, I.; Visalli, M.; Cucinotta, M.; Teti, D.; Venza, M. Association between oxidative stress and macromolecular damage in elderly patients with age-related macular degeneration. Aging Clin. Exp. Res. 2012, 24, 21–27. [Google Scholar] [PubMed]
- Geiger, H.; de Haan, G.; Florian, M.C. The ageing haematopoietic stem cell compartment. Nat. Rev. Immunol. 2013, 13, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Van Zant, G.; Szilvassy, S.J. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 2005, 106, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Li, T.S.; Kurazumi, H.; Takemoto, Y.; Ohshima, M.; Murata, T.; Katsura, S.; Morikage, N.; Furutani, A.; Hamano, K. Hypoxic preconditioning enhances angiogenic potential of bone marrow cells with aging-related functional impairment. Circ. J. 2012, 76, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Ludin, A.; Gur-Cohen, S.; Golan, K.; Kaufmann, K.B.; Itkin, T.; Medaglia, C.; Lu, X.J.; Ledergor, G.; Kollet, O.; Lapidot, T. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid. Redox Signal. 2014, 21, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi Ishikawa, E.; Gonzalez-Nieto, D.; Ghiaur, G.; Dunn, S.K.; Ficker, A.M.; Murali, B.; Madhu, M.; Gutstein, D.E.; Fishman, G.I.; Barrio, L.C.; et al. Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proc. Natl. Acad. Sci. USA 2012, 109, 9071–9076. [Google Scholar] [CrossRef] [PubMed]
- Durig, J.; Rosenthal, C.; Halfmeyer, K.; Wiemann, M.; Novotny, J.; Bingmann, D.; Duhrsen, U.; Schirrmacher, K. Intercellular communication between bone marrow stromal cells and CD34+ haematopoietic progenitor cells is mediated by connexin 43-type gap junctions. Br. J. Haematol. 2000, 111, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Nieto, D.; Li, L.; Kohler, A.; Ghiaur, G.; Ishikawa, E.; Sengupta, A.; Madhu, M.; Arnett, J.L.; Santho, R.A.; Dunn, S.K.; et al. Connexin-43 in the osteogenic bm niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors. Blood 2012, 119, 5144–5154. [Google Scholar] [CrossRef] [PubMed]
- Cancelas, J.A.; Koevoet, W.L.; de Koning, A.E.; Mayen, A.E.; Rombouts, E.J.; Ploemacher, R.E. Connexin-43 gap junctions are involved in multiconnexin-expressing stromal support of hemopoietic progenitors and stem cells. Blood 2000, 96, 498–505. [Google Scholar] [PubMed]
- Harris, A.L. Connexin channel permeability to cytoplasmic molecules. Prog. Biophys. Mol. Biol. 2007, 94, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Civitelli, R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch. Biochem. Biophys. 2008, 473, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, E.C.; Prohaska, S.S.; Katzman, S.; Heffner, G.C.; Stuart, J.M.; Weissman, I.L. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005, 1, e28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ploemacher, R.E.; Mayen, A.E.; De Koning, A.E.; Krenacs, T.; Rosendaal, M. Hematopoiesis: Gap junction intercellular communication is likely to be involved in regulation of stroma-dependent proliferation of hemopoietic stem cells. Hematology 2000, 5, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hirao, A.; Arai, F.; Takubo, K.; Matsuoka, S.; Miyamoto, K.; Ohmura, M.; Naka, K.; Hosokawa, K.; Ikeda, Y.; et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006, 12, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Saunders, T.L.; Enikolopov, G.; Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012, 481, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by cxcl12-cxcr4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.S.; Li, H.; Kang, Y.L.; Chen, W.C.; Cheng, W.C.; Lai, D.M. Loss of CXCL12/SDF-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood 2011, 117, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Chudziak, D.; Spohn, G.; Karpova, D.; Dauber, K.; Wiercinska, E.; Miettinen, J.A.; Papayannopoulou, T.; Bonig, H. Functional consequences of perturbed CXCL12 signal processing: Analyses of immature hematopoiesis in GRK6-deficient mice. Stem Cells Dev. 2015, 24, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.; Lorthioir, O.; Valenzuela, A.; Magerus, A.; Thelen, M.; Montes, M.; Virelizier, J.L.; Delepierre, M.; Baleux, F.; Lortat-Jacob, H.; et al. Stromal cell-derived factor-1α associates with heparan sulfates through the first β-strand of the chemokine. J. Biol. Chem. 1999, 274, 23916–23925. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Han, Y.C.; Zou, Y.R. Cxcr4 is required for the quiescence of primitive hematopoietic cells. J. Exp. Med. 2008, 205, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Petit, I. Current understanding of stem cell mobilization: The roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 2002, 30, 973–981. [Google Scholar] [CrossRef]
- Petit, I.; Szyper-Kravitz, M.; Nagler, A.; Lahav, M.; Peled, A.; Habler, L.; Ponomaryov, T.; Taichman, R.S.; Arenzana-Seisdedos, F.; Fujii, N.; et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating cxcr4. Nat. Immunol. 2002, 3, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Shaw, C.A.; Gatza, C.; Fisk, C.J.; Donehower, L.A.; Goodell, M.A. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007, 5, e201. [Google Scholar] [CrossRef] [PubMed]
- Mirantes, C.; Passegue, E.; Pietras, E.M. Pro-inflammatory cytokines: Emerging players regulating HSC function in normal and diseased hematopoiesis. Exp. Cell Res. 2014, 329, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sejas, D.P.; Qiu, Y.; Williams, D.A.; Pang, Q. Inflammatory ros promote and cooperate with the fanconi anemia mutation for hematopoietic senescence. J. Cell Sci. 2007, 120, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, M.T.; King, K.Y.; Goodell, M.A. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011, 32, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ergen, A.V.; Boles, N.C.; Goodell, M.A. Rantes/ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood 2012, 119, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.R.; Cunniff, P.J.; Pereira, B.J.; Jaber, B.L. Hematopoietic effect of radix angelicae sinensis in a hemodialysis patient. Am. J. Kidney Dis. 1999, 34, 349–354. [Google Scholar] [CrossRef]
- Cao, W.; Li, X.Q.; Wang, X.; Li, T.; Chen, X.; Liu, S.B.; Mei, Q.B. Characterizations and anti-tumor activities of three acidic polysaccharides from angelica sinensis (oliv.) diels. Int. J. Biol. Macromol. 2010, 46, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Tsai, N.M.; Lin, S.Z.; Lee, C.C.; Chen, S.P.; Su, H.C.; Chang, W.L.; Harn, H.J. The antitumor effects of angelica sinensis on malignant brain tumors in vitro and in vivo. Clin. Cancer Res. 2005, 11, 3475–3484. [Google Scholar] [CrossRef] [PubMed]
- Bunel, V.; Antoine, M.H.; Nortier, J.; Duez, P.; Stevigny, C. Potential nephroprotective effects of the chinese herb Angelica sinensis against cisplatin tubulotoxicity. Pharm. Biol. 2015, 53, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Duan, J.A.; Qian, D.; Guo, J.; Song, B.; Yang, M. Assessment and comparison of immunoregulatory activity of four hydrosoluble fractions of angelica sinensisin vitro on the peritoneal macrophages in icr mice. Int. Immunopharmacol. 2010, 10, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.R.; Chiu, H.F.; Chen, S.L.; Tsai, J.H.; Lee, M.Y.; Lee, H.S.; Shen, Y.C.; Yan, Y.Y.; Shane, G.T.; Wang, C.K. Effects of a chinese medical herbs complex on cellular immunity and toxicity-related conditions of breast cancer patients. Br. J. Nutr. 2012, 107, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhang, Y.; Li, J.; Xia, J.; Chen, X.; Jing, P.; Song, X.; Wang, L.; Wang, Y. Angelica sinensis polysaccharide prevents hematopoietic stem cells senescence in d-galactose-induced aging mouse model. Stem Cells Int. 2017, 2017, 3508907. [Google Scholar] [CrossRef] [PubMed]
- Deveci, H.A.; Naziroglu, M.; Nur, G. 5-fluorouracil-induced mitochondrial oxidative cytotoxicity and apoptosis are increased in mcf-7 human breast cancer cells by trpv1 channel activation but not hypericum perforatum treatment. Mol. Cell. Biochem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nazim, U.M.; Rasheduzzaman, M.; Lee, Y.J.; Seol, D.W.; Park, S.Y. Enhancement of trail-induced apoptosis by 5-fluorouracil requires activating bax and p53 pathways in trail-resistant lung cancers. Oncotarget 2017, 8, 18095–18105. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, B.; Bracht, E.V.; Reijnders, D.; Safarikova, B.; Jelinkova, I.; Grandien, A.; Vaculova, A.H.; Zhivotovsky, B.; Olsson, M. Correction: 5-fluorouracil-induced rna stress engages a trail-disc-dependent apoptosis axis facilitated by p53. Oncotarget 2016, 7, 72380. [Google Scholar] [CrossRef] [PubMed]
- Can, G.; Akpinar, B.; Baran, Y.; Zhivotovsky, B.; Olsson, M. 5-fluorouracil signaling through a calcium-calmodulin-dependent pathway is required for p53 activation and apoptosis in colon carcinoma cells. Oncogene 2013, 32, 4529–4538. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; El-Deiry, W.S. Inducible silencing of killer/dr5 in vivo promotes bioluminescent colon tumor xenograft growth and confers resistance to chemotherapeutic agent 5-fluorouracil. Cancer Res. 2004, 64, 6666–6672. [Google Scholar] [CrossRef] [PubMed]
- Rani, I.; Sharma, B.; Kumar, S.; Kaur, S.; Agnihotri, N. Apoptosis mediated chemosensitization of tumor cells to 5-fluorouracil on supplementation of fish oil in experimental colon carcinoma. Tumour Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Shi, H.; Yang, L.; Qiu, C.; Lin, S.; Qi, Y.; Li, J.; Zhao, A.; Liu, J. Inhibition of polypyrimidine tract-binding protein 3 induces apoptosis and cell cycle arrest, and enhances the cytotoxicity of 5- fluorouracil in gastric cancer cells. Br. J. Cancer 2017, 116, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Buttiglieri, S.; Ruella, M.; Risso, A.; Spatola, T.; Silengo, L.; Avvedimento, E.V.; Tarella, C. The aging effect of chemotherapy on cultured human mesenchymal stem cells. Exp. Hematol. 2011, 39, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Atale, N.; Gupta, S.; Yadav, U.C.; Rani, V. Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques. J. Microsc. 2014, 255, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Altieri, P.; Murialdo, R.; Barisione, C.; Lazzarini, E.; Garibaldi, S.; Fabbi, P.; Ruggeri, C.; Borile, S.; Carbone, F.; Armirotti, A.; et al. 5-fluorouracil causes endothelial cell senescence: Potential protective role of glucagon-like peptide 1. Br. J. Pharmacol. 2017, 174, 3713–3726. [Google Scholar] [CrossRef] [PubMed]
- Flach, J.; Bakker, S.T.; Mohrin, M.; Conroy, P.C.; Pietras, E.M.; Reynaud, D.; Alvarez, S.; Diolaiti, M.E.; Ugarte, F.; Forsberg, E.C.; et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature 2014, 512, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Sperka, T.; Wang, J.; Rudolph, K.L. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012, 13, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Robinson, K.A.; Gabbita, S.P.; Salsman, S.; Floyd, R.A. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef]
- Rothkamm, K.; Horn, S. γ-H2AX as protein biomarker for radiation exposure. Ann. Ist. Super. Sanita 2009, 45, 265–271. [Google Scholar] [PubMed]
- Krenacs, T.; Rosendaal, M. Connexin43 gap junctions in normal, regenerating, and cultured mouse bone marrow and in human leukemias: Their possible involvement in blood formation. Am. J. Pathol. 1998, 152, 993–1004. [Google Scholar] [PubMed]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Wang, Y.; Li, C.; Li, D.; Lu, L.; Wang, X.; et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med. 2013, 54, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kellner, J.; Liu, L.; Zhou, D. Inhibition of p38 mitogen-activated protein kinase promotes ex vivo hematopoietic stem cell expansion. Stem Cells Dev. 2011, 20, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, Y.Y.; Zhang, J.L.; Li, D.G.; Meng, A.M. P38 MAPK inhibitor insufficiently attenuates HSC senescence administered long-term after 6 Gy total body irradiation in mice. Int. J. Mol. Sci. 2016, 905. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, F.R.; Gundacker, H.; Head, D.R.; Slovak, M.L.; Willman, C.L.; Godwin, J.E.; Anderson, J.E.; Petersdorf, S.H. Age and acute myeloid leukemia. Blood 2006, 107, 3481–3485. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Ema, H.; Morita, Y.; Nakauchi, H. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 2000, 192, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Vas, V.; Wandhoff, C.; Dorr, K.; Niebel, A.; Geiger, H. Contribution of an aged microenvironment to aging-associated myeloproliferative disease. PLoS ONE 2012, 7, e31523. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J.; Casas-Selves, M.; Kim, J.; Zaberezhnyy, V.; Aghili, L.; Daniel, A.E.; Jimenez, L.; Azam, T.; McNamee, E.N.; Clambey, E.T.; et al. Aging-associated inflammation promotes selection for adaptive oncogenic events in b cell progenitors. J. Clin. Investig. 2015, 125, 4666–4680. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Lu, W.; Liu, K. Mitochondrial reactive oxygen species regulate adipocyte differentiation of mesenchymal stem cells in hematopoietic stress induced by arabinosylcytosine. PLoS ONE 2015, 10, e0120629. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.J.; Wu, M.Q.; Li, Z.J.; Zhang, Y.; Liu, K.Y. Hematopoietic recovery following chemotherapy is improved by badge-induced inhibition of adipogenesis. Int. J. Hematol. 2013, 97, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Khatri, R.; Krishnan, S.; Roy, S.; Chattopadhyay, S.; Kumar, V.; Mukhopadhyay, A. Reactive oxygen species limit the ability of bone marrow stromal cells to support hematopoietic reconstitution in aging mice. Stem Cells Dev. 2016, 25, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, E.T.; Cancelas, J.A. Lack of communication rusts and ages stem cells. Cell Cycle 2012, 11, 3149–3150. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.J.; Dudakov, J.A.; Takahashi, K.; Shieh, J.H.; Velardi, E.; Holland, A.M.; Singer, N.V.; West, M.L.; Smith, O.M.; Young, L.F.; et al. Nrf2 regulates haematopoietic stem cell function. Nat. Cell Biol. 2013, 15, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Xiaowei, C.; Jia, M.; Xiaowei, W.; Yina, Z. Overexpression of CXCL12 chemokine up-regulates connexin and integrin expression in mesenchymal stem cells through Pi3k/Akt pathway. Cell Commun. Adhes. 2013, 20, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Schajnovitz, A.; Itkin, T.; D’Uva, G.; Kalinkovich, A.; Golan, K.; Ludin, A.; Cohen, D.; Shulman, Z.; Avigdor, A.; Nagler, A.; et al. CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat. Immunol. 2011, 12, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.K.; Bliss, S.A.; Patel, S.A.; Taborga, M.; Dave, M.A.; Gregory, L.A.; Greco, S.J.; Bryan, M.; Patel, P.S.; Rameshwar, P. Gap junction-mediated import of microrna from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011, 71, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhao, K.; Huang, Q.; Xu, C.; Shang, P. Isolation, structure and bioactivities of the polysaccharides from angelica sinensis (oliv.) diels: A review. Carbohydr. Polym. 2012, 89, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Hsieh, W.T.; Chen, S.U.; Chiang, B.H. Hematopoietic and myeloprotective activities of an acidic angelica sinensis polysaccharide on human CD34+ stem cells. J. Ethnopharmacol. 2012, 139, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Wei, Y.; Xue, W.; Hua, Y.; Zhang, M.; Sun, H.; Song, Z.; Zhang, L.; Li, J.; Zhao, H.; et al. Characterization and antioxidative activities of polysaccharide in chinese angelica and its processed products. Int. J. Biol. Macromol. 2014, 67, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; Fan, X.; Fan, L.; Sun, Q.; Liu, Y.; Tao, X.; Dai, K. Extraction and chemical characterization of Angelica sinensis polysaccharides and its antioxidant activity. Carbohydr. Polym. 2013, 94, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef] [PubMed]





| Group | ROS (Flow Cytometry) | T-SOD (U/Mgprot) | GSH-Px (U/Mgprot) |
|---|---|---|---|
| Control | 599.33 ± 10.21 | 103.20 ± 1.00 | 59.61 ± 0.85 |
| 5-FU | 947.33 ± 6.66 * | 8.60 ± 1.11 * | 5.61 ± 0.38 * |
| ASP | 92.00 ± 6.25 * | 152.27 ± 1.00 * | 85.41 ± 1.27 * |
| ASP + 5-FU | 825.33 ± 24.54 # | 108.50 ± 0.90 # | 34.24 ± 1.13 # |
| Group | T-SOD (U/Mgprot) | GSH-Px (U/Mgprot) |
|---|---|---|
| Control | 237.24 ± 0.91 | 270.55 ± 0.91 |
| ASP | 283.86 ± 0.86 * | 367.21 ± 0.95 * |
| 5-FU | 174.68 ± 0.85 * | 6.45 ± 0.41 * |
| ASP + 5-FU | 202.44 ± 0.98 # | 156.53 ± 0.98 # |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Xiong, L.; Song, X.; Jin, P.; Chen, L.; Chen, X.; Yao, H.; Wang, Y.; Wang, L. Angelica sinensis Polysaccharides Ameliorate Stress-Induced Premature Senescence of Hematopoietic Cell via Protecting Bone Marrow Stromal Cells from Oxidative Injuries Caused by 5-Fluorouracil. Int. J. Mol. Sci. 2017, 18, 2265. https://doi.org/10.3390/ijms18112265
Xiao H, Xiong L, Song X, Jin P, Chen L, Chen X, Yao H, Wang Y, Wang L. Angelica sinensis Polysaccharides Ameliorate Stress-Induced Premature Senescence of Hematopoietic Cell via Protecting Bone Marrow Stromal Cells from Oxidative Injuries Caused by 5-Fluorouracil. International Journal of Molecular Sciences. 2017; 18(11):2265. https://doi.org/10.3390/ijms18112265
Chicago/Turabian StyleXiao, Hanxianzhi, Lirong Xiong, Xiaoying Song, Pengwei Jin, Linbo Chen, Xiongbin Chen, Hui Yao, Yaping Wang, and Lu Wang. 2017. "Angelica sinensis Polysaccharides Ameliorate Stress-Induced Premature Senescence of Hematopoietic Cell via Protecting Bone Marrow Stromal Cells from Oxidative Injuries Caused by 5-Fluorouracil" International Journal of Molecular Sciences 18, no. 11: 2265. https://doi.org/10.3390/ijms18112265
APA StyleXiao, H., Xiong, L., Song, X., Jin, P., Chen, L., Chen, X., Yao, H., Wang, Y., & Wang, L. (2017). Angelica sinensis Polysaccharides Ameliorate Stress-Induced Premature Senescence of Hematopoietic Cell via Protecting Bone Marrow Stromal Cells from Oxidative Injuries Caused by 5-Fluorouracil. International Journal of Molecular Sciences, 18(11), 2265. https://doi.org/10.3390/ijms18112265




