Role of Transforming Growth Factor β in Uterine Fibroid Biology
Abstract
:1. Introduction
1.1. Transforming Growth Factor β—Signaling Pathways, Proliferation and Fibrosis
1.2. Extracellular Matrix
1.3. Regulation by Steroids
1.4. Genetics
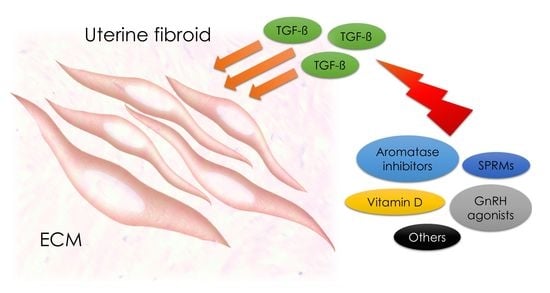
1.5. TGF-β and Implications for Therapy
1.6. Future Ideas
2. Discussion
2.1. Transforming Growth Factor β—Signaling Pathways, Proliferation and Fibrosis
2.2. Extracellular Matrix
2.3. Regulation by Steroids
2.4. Genetics
2.5. TGF-β and Implications for Therapy
2.6. Future Ideas
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AI | Aromatase inhibtor |
| Akt | Protein kinase B |
| ALK | TGFβ type I receptor kinase |
| BMP | Bone morphogenic protein |
| ECM | Extracellular matrix |
| ER | Estrogen receptor |
| ERK | Extracellular signal-regulated kinases |
| FAK | Focal adhesion kinase |
| GnRH | Gonadotropin releasing hormone |
| GnRHa | Gonadotropin releasing hormone agonist |
| JNK | c-Jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinase |
| MED12 | Mediator complex subunit 12 |
| MEK | MAPK/ERK kinase |
| MKK | Mitogen-activated protein kinase kinase |
| MMP | Matrix metalloproteinase |
| mRNA | messenger RNA |
| mTOR | mechanistic target of rapamycin |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| p38 | p38 mitogen-activated protein kinases |
| PI3K | Phosphoinositide 3-kinase |
| PR | Progesterone receptor |
| Raf | Raf protein |
| Ras | Ras protein |
| Smad | Smad protein |
| SPRM | Selective progesterone receptor modulator |
| TAK | Transforming growth factor β-activated kinase |
| TGF-β | Transforming growth factor β |
| TGF-βR | Transforming growth factor β receptor |
| TIMP | Tissue inhibitor of matrix metalloproteinase |
| UF | Uterine fibroid |
References
- Parker, W.H. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil. Steril. 2007, 87, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Prim. 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Margolis, M.K.; Castelli-Haley, J.; Fuldeore, M.J.; Owens, C.D.; Coyne, K.S. Impact of uterine fibroid symptoms on health-related quality of life of us women: Evidence from a cross-sectional survey. Curr. Med. Res. Opin. 2017, 33, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A. Clinical practice. Uterine fibroids. N. Engl. J. Med. 2015, 372, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.; Farquhar, C.M.; Li, T.C. Is another meta-analysis on the effects of intramural fibroids on reproductive outcomes needed? Reprod. Biomed. Online 2011, 23, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Zepiridis, L.I.; Grimbizis, G.F.; Tarlatzis, B.C. Infertility and uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Cook, H.; Ezzati, M.; Segars, J.H.; McCarthy, K. The impact of uterine leiomyomas on reproductive outcomes. Minerva Ginecol. 2010, 62, 225–236. [Google Scholar] [PubMed]
- Kjerulff, K.H.; Langenberg, P.; Seidman, J.D.; Stolley, P.D.; Guzinski, G.M. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J. Reprod. Med. 1996, 41, 483–490. [Google Scholar] [PubMed]
- Cardozo, E.R.; Clark, A.D.; Banks, N.K.; Henne, M.B.; Stegmann, B.J.; Segars, J.H. The estimated annual cost of uterine leiomyomata in the United States. Am. J. Obstet. Gynecol. 2012, 206, 211.e1–211.e9. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Ozminkowski, R.J.; Carls, G.S.; Wang, S.; Gibson, T.B.; Stewart, E.A. The direct and indirect cost burden of clinically significant and symptomatic uterine fibroids. J. Occup. Environ. Med. 2007, 49, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Yang, H.; Du, E.X.; Kelkar, S.S.; Winkel, C. The direct and indirect costs of uterine fibroid tumors: A systematic review of the literature between 2000 and 2013. Am. J. Obstet. Gynecol. 2015, 213, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N. Proinflammatory and profibrotic mediators: Principal effectors of leiomyoma development as a fibrotic disorder. Semin. Reprod. Med. 2010, 28, 180–203. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, P.; Islam, M.S.; Reis, F.M.; Gray, P.C.; Bloise, E.; Petraglia, F.; Vale, W.; Castellucci, M. Growth factors and myometrium: Biological effects in uterine fibroid and possible clinical implications. Hum. Reprod. Update 2011, 17, 772–790. [Google Scholar] [CrossRef] [PubMed]
- Borahay, M.A.; Al-Hendy, A.; Kilic, G.S.; Boehning, D. Signaling pathways in leiomyoma: Understanding pathobiology and implications for therapy. Mol. Med. 2015, 21, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Historical insights into cytokines. Eur. J. Immunol. 2007, 37, S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Protic, O.; Toti, P.; Islam, M.S.; Occhini, R.; Giannubilo, S.R.; Catherino, W.H.; Cinti, S.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016, 364, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Sozen, I.; Arici, A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil. Steril. 2002, 78, 1–12. [Google Scholar] [CrossRef]
- Malik, M.; Norian, J.; McCarthy-Keith, D.; Britten, J.; Catherino, W.H. Why leiomyomas are called fibroids: The central role of extracellular matrix in symptomatic women. Semin. Reprod. Med. 2010, 28, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Pohlers, D.; Brenmoehl, J.; Loffler, I.; Muller, C.K.; Leipner, C.; Schultze-Mosgau, S.; Stallmach, A.; Kinne, R.W.; Wolf, G. TGF-β and fibrosis in different organs—Molecular pathway imprints. Biochim. Biophys. Acta 2009, 1792, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. TGF-β signal transduction. Annu. Rev. Biochem. 1998, 67, 753–791. [Google Scholar] [CrossRef] [PubMed]
- Yen-Ping Ho, J.; Man, W.C.; Wen, Y.; Polan, M.L.; Shih-Chu Ho, E.; Chen, B. Transforming growth interacting factor expression in leiomyoma compared with myometrium. Fertil. Steril. 2010, 94, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. TGF-β signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Hocevar, B.A.; Howe, P.H. Analysis of TGF-β-mediated synthesis of extracellular matrix components. Methods Mol. Biol. 2000, 142, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Uterine fibroids. N. Engl. J. Med. 2013, 369, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Nowak, R.A. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-β3 (TGF-β3) and altered responses to the antiproliferative effects of TGF-β. J. Clin. Endocrinol. Metab. 2001, 86, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Arici, A.; Sozen, I. Expression, menstrual cycle-dependent activation, and bimodal mitogenic effect of transforming growth factor-β1 in human myometrium and leiomyoma. Am. J. Obstet. Gynecol. 2003, 188, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. 1,25-dihydroxyvitamin D3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol. Reprod. 2013, 89, 150. [Google Scholar] [CrossRef] [PubMed]
- Zeyneloglu, H.B.; Esinler, I.; Ozdemir, B.H.; Haydardedeoglu, B.; Oktem, M.; Batioglu, S. Immunohistochemical characteristics of intramural leiomyomata that enlarge during controlled ovarian hyperstimulation for in vitro fertilization. Gynecol. Obstet. Investig. 2008, 65, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.S.; Malik, M.; Nurudeen, S.; Catherino, W.H. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor β-3. Fertil. Steril. 2010, 93, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A. Uterine fibroids. Lancet 2001, 357, 293–298. [Google Scholar] [CrossRef]
- Dou, Q.; Zhao, Y.; Tarnuzzer, R.W.; Rong, H.; Williams, R.S.; Schultz, G.S.; Chegini, N. Suppression of transforming growth factor-β (TGF-β) and TGF-β receptor messenger ribonucleic acid and protein expression in leiomyomata in women receiving gonadotropin-releasing hormone agonist therapy. J. Clin. Endocrinol. Metab. 1996, 81, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Walker, C.L.; Stewart, E.A. Uterine fibroids: The elephant in the room. Science 2005, 308, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Norian, J.M.; Malik, M.; Parker, C.Y.; Joseph, D.; Leppert, P.C.; Segars, J.H.; Catherino, W.H. Transforming growth factor β3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod. Sci. 2009, 16, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C.; Baginski, T.; Prupas, C.; Catherino, W.H.; Pletcher, S.; Segars, J.H. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil. Steril. 2004, 82, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Ohara, N.; Wang, J.; Matsuo, H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum. Reprod. Update 2004, 10, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Borahay, M.A.; Asoglu, M.R.; Mas, A.; Adam, S.; Kilic, G.S.; Al-Hendy, A. Estrogen receptors and signaling in fibroids: Role in pathobiology and therapeutic implications. Reprod. Sci. 2017, 24, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Garrett, T.A.; Laughlin, S.K.; Davis, B.; Semelka, R.C.; Peddada, S.D. Short-term change in growth of uterine leiomyoma: Tumor growth spurts. Fertil. Steril. 2011, 95, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth-Carson, S.J.; Zaitseva, M.; Vollenhoven, B.J.; Rogers, P.A. Clonality of smooth muscle and fibroblast cell populations isolated from human fibroid and myometrial tissues. Mol. Hum. Reprod. 2014, 20, 250–259. [Google Scholar] [CrossRef] [PubMed]
- El-Gharib, M.N.; Elsobky, E.S. Cytogenetic aberrations and the development of uterine leiomyomata. J. Obstet. Gynaecol. Res. 2010, 36, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Okolo, S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Makinen, N.; Mehine, M.; Tolvanen, J.; Kaasinen, E.; Li, Y.; Lehtonen, H.J.; Gentile, M.; Yan, J.; Enge, M.; Taipale, M.; et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011, 334, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Segars, J.H. The role of angiogenic factors in fibroid pathogenesis: Potential implications for future therapy. Hum. Reprod. Update 2014, 20, 194–216. [Google Scholar] [CrossRef] [PubMed]
- Sabry, M.; Al-Hendy, A. Medical treatment of uterine leiomyoma. Reprod. Sci. 2012, 19, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Donnez, O.; Courtoy, G.E.; Dolmans, M.M. The place of selective progesterone receptor modulators in myoma therapy. Minerva Ginecol. 2016, 68, 313–320. [Google Scholar] [PubMed]
- Vahdat, M.; Kashanian, M.; Ghaziani, N.; Sheikhansari, N. Evaluation of the effects of cabergoline (Dostinex) on women with symptomatic myomatous uterus: A randomized trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Roshdy, E.; Rajaratnam, V.; Maitra, S.; Sabry, M.; Allah, A.S.; Al-Hendy, A. Treatment of symptomatic uterine fibroids with green tea extract: A pilot randomized controlled clinical study. Int. J. Womens Health 2013, 5, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Brakta, S.; Diamond, J.S.; Al-Hendy, A.; Diamond, M.P.; Halder, S.K. Role of vitamin D in uterine fibroid biology. Fertil. Steril. 2015, 104, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Blauer, M.; Rovio, P.H.; Ylikomi, T.; Heinonen, P.K. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil. Steril. 2009, 91, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Sabry, M.; Al-Hendy, A. Innovative oral treatments of uterine leiomyoma. Obstet. Gynecol. Int. 2012, 2012, 943635. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massague, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Blobe, G.C.; Schiemann, W.P.; Lodish, H.F. Role of transforming growth factor β in human disease. N. Engl. J. Med. 2000, 342, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C.; Catherino, W.H.; Segars, J.H. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am. J. Obstet. Gynecol. 2006, 195, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.M.; Dou, Q.; Zhao, Y.; McLean, F.; Davis, J.; Chegini, N. The expression of transforming growth factor-βs and TGF-β receptor mrna and protein and the effect of TGF-βs on human myometrial smooth muscle cells in vitro. Mol. Hum. Reprod. 1997, 3, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Arici, A.; Sozen, I. Transforming growth factor-β3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil. Steril. 2000, 73, 1006–1011. [Google Scholar] [CrossRef]
- Massague, J. The transforming growth factor-β family. Annu. Rev. Cell Biol. 1990, 6, 597–641. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Levens, E.; Luo, X.; Ding, L.; Williams, R.S.; Chegini, N. Fibromodulin is expressed in leiomyoma and myometrium and regulated by gonadotropin-releasing hormone analogue therapy and TGF-β through Smad and MAPK-mediated signalling. Mol. Hum. Reprod. 2005, 11, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. How cells read TGF-β signals. Nat. Rev. Mol. Cell Biol. 2000, 1, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Shi-wen, X.; Parapuram, S.K.; Pala, D.; Chen, Y.; Carter, D.E.; Eastwood, M.; Denton, C.P.; Abraham, D.J.; Leask, A. Requirement of transforming growth factor β-activated kinase 1 for transforming growth factor β-induced α-smooth muscle actin expression and extracellular matrix contraction in fibroblasts. Arthritis Rheum. 2009, 60, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, A.U.; Meyer, A.; Jacobi, C.; Feige, J.N.; Glass, D.J. TAK-1/p38/nNFκB signaling inhibits myoblast differentiation by increasing levels of Activin A. Skelet. Muscle 2012, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R. Wnt signaling in disease and in development. Cell Res. 2005, 15, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Arango, N.A.; Szotek, P.P.; Manganaro, T.F.; Oliva, E.; Donahoe, P.K.; Teixeira, J. Conditional deletion of β-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev. Biol. 2005, 288, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.C.; Mastroyannis, A.; Taylor, H.S. Leiomyoma simultaneously impair endometrial BMP-2-mediated decidualization and anticoagulant expression through secretion of tgf-β3. J. Clin. Endocrinol. Metab. 2011, 96, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, P.S.; Lee, H.J.; Zhang, L.; Zukerberg, L.R.; Taketo, M.M.; Rueda, B.R.; Teixeira, J.M. Constitutive activation of β-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol. Reprod. 2009, 81, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Wlodarczyk, M.; Slabuszewska-Jozwiak, A.; Nowicka, G.; Jakiel, G. Influence of vitamin D and transforming growth factor β3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil. Steril. 2016, 106, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wen, Y.; Polan, M.L.; Qiao, J.; Chen, B.H. Increased expression of latent TGF-β binding protein-1 and fibrillin-1 in human uterine leiomyomata. Mol. Hum. Reprod. 2007, 13, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Doherty, L.F.; Taylor, H.S. Leiomyoma-derived transforming growth factor-β impairs bone morphogenetic protein-2-mediated endometrial receptivity. Fertil. Steril. 2015, 103, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Monsivais, D.; Clementi, C.; Peng, J.; Fullerton, P.T., Jr.; Prunskaite-Hyyrylainen, R.; Vainio, S.J.; Matzuk, M.M. BMP-7 induces uterine receptivity and blastocyst attachment. Endocrinology 2017, 158, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, B.; Bernabeu, C.; Kumar, S. Angiogenesis in breast cancer: The role of transforming growth factor β and CD105. Microsc. Res. Tech. 2001, 52, 437–449. [Google Scholar] [CrossRef]
- Wolanska, M.; Sobolewski, K.; Drozdzewicz, M.; Bankowski, E. Extracellular matrix components in uterine leiomyoma and their alteration during the tumour growth. Mol. Cell Biochem. 1998, 189, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Tarnuzzer, R.W.; Williams, R.S.; Schultz, G.S.; Chegini, N. Differential expression of matrix metalloproteinases and their tissue inhibitors in leiomyomata: A mechanism for gonadotrophin releasing hormone agonist-induced tumour regression. Mol. Hum. Reprod. 1997, 3, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Bogusiewicz, M.; Stryjecka-Zimmer, M.; Postawski, K.; Jakimiuk, A.J.; Rechberger, T. Activity of matrix metalloproteinase-2 and -9 and contents of their tissue inhibitors in uterine leiomyoma and corresponding myometrium. Gynecol. Endocrinol. 2007, 23, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Goodwin, J.S.; Al-Hendy, A. 1,25-dihydroxyvitamin D3 reduces TGF-β3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2011, 96, E754–E762. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chegini, N. Regulation of matrix metalloproteinases (MMPs) and their tissue inhibitors in human myometrial smooth muscle cells by TGF-β1. Mol. Hum. Reprod. 1999, 5, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.; He, H.; Haseman, J.K. Immunohistochemical localization of growth factors and their receptors in uterine leiomyomas and matched myometrium. Environ. Health Perspect. 2000, 108, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.; Ascher-Walsh, C. A comprehensive approach to the treatment of uterine leiomyomata. Mt. Sinai J. Med. 2009, 76, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Barbarisi, A.; Petillo, O.; Di Lieto, A.; Melone, M.A.; Margarucci, S.; Cannas, M.; Peluso, G. 17-β estradiol elicits an autocrine leiomyoma cell proliferation: Evidence for a stimulation of protein kinase-dependent pathway. J. Cell. Physiol. 2001, 186, 414–424. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Lu, Q.; Zhang, P.; Ren, M. Transforming growth factor-β signaling pathway cross-talking with ERα signaling pathway on regulating the growth of uterine leiomyoma activated by phenolic environmental estrogens in vitro. Tumour Biol. 2016, 37, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Liu, J.C.; Chen, J.J.; Lee, Y.M.; Hwang, J.L.; Tzeng, C.R. Combined differential gene expression profile and pathway enrichment analyses to elucidate the molecular mechanisms of uterine leiomyoma after gonadotropin-releasing hormone treatment. Fertil. Steril. 2008, 90, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Duhan, N.; Madaan, S.; Sen, J. Role of the aromatase inhibitor letrozole in the management of uterine leiomyomas in premenopausal women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 171, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.F. A mediator lost in the war on cancer. Cell 2012, 151, 927–929. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.M.; Yatsenko, A.; Hoffner, L.; Jones, M.; Surti, U.; Rajkovic, A. Whole exome sequencing in a random sample of North American women with leiomyomas identifies MED12 mutations in majority of uterine leiomyomas. PLoS ONE 2012, 7, e33251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makinen, N.; Vahteristo, P.; Butzow, R.; Sjoberg, J.; Aaltonen, L.A. Exomic landscape of MED12 mutation-negative and -positive uterine leiomyomas. Int. J. Cancer 2014, 134, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Laknaur, A.; Miller, J.; Layman, L.C.; Diamond, M.; Al-Hendy, A. Novel MED12 gene somatic mutations in women from the Southern United States with symptomatic uterine fibroids. Mol. Genet. Genom. 2015, 290, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Ravegnini, G.; Marino-Enriquez, A.; Slater, J.; Eilers, G.; Wang, Y.; Zhu, M.; Nucci, M.R.; George, S.; Angelini, S.; Raut, C.P.; et al. MED12 mutations in leiomyosarcoma and extrauterine leiomyoma. Mod. Pathol. 2013, 26, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Xu, X.; Hecht, A.; Boyer, T.G. Mediator is a transducer of Wnt/β-catenin signaling. J. Biol. Chem. 2006, 281, 14066–14075. [Google Scholar] [CrossRef] [PubMed]
- Markowski, D.N.; Bartnitzke, S.; Loning, T.; Drieschner, N.; Helmke, B.M.; Bullerdiek, J. MED12 mutations in uterine fibroids—Their relationship to cytogenetic subgroups. Int. J. Cancer 2012, 131, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Laknaur, A.; Diamond, M.P.; Ismail, N.; Boyer, T.G.; Halder, S.K. Silencing MED12 gene reduces proliferation of human leiomyoma cells mediated via Wnt/β-catenin signaling pathway. Endocrinology 2017, 158, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Holzel, M.; Knijnenburg, T.; Schlicker, A.; Roepman, P.; McDermott, U.; Garnett, M.; Grernrum, W.; Sun, C.; Prahallad, A.; et al. MED12 controls the response to multiple cancer drugs through regulation of tgf-β receptor signaling. Cell 2012, 151, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Attisano, L.; Wrana, J.L. Signal transduction by the TGF-β superfamily. Science 2002, 296, 1646–1647. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Shi, H.; Xu, Q.; Song, Q.; Xu, Y.; Huang, Y. Effects of TGF-β on uterine fibroids of women of childbearing age and uterine artery embolization. Minim. Invasive Ther. Allied Technol. 2017, 26, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N.; Ma, C.; Tang, X.M.; Williams, R.S. Effects of GnRH analogues, add-back’steroid therapy, antiestrogen and antiprogestins on leiomyoma and myometrial smooth muscle cell growth and transforming growth factor-β expression. Mol. Hum. Reprod. 2002, 8, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- De Falco, M.; Staibano, S.; D’Armiento, F.P.; Mascolo, M.; Salvatore, G.; Busiello, A.; Carbone, I.F.; Pollio, F.; Di Lieto, A. Preoperative treatment of uterine leiomyomas: Clinical findings and expression of transforming growth factor-β3 and connective tissue growth factor. J. Soc. Gynecol. Investig. 2006, 13, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N.; Luo, X.; Ding, L.; Ripley, D. The expression of smads and transforming growth factor β receptors in leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Mol. Cell Endocrinol. 2003, 209, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Committee opinion no. 663: Aromatase inhibitors in gynecologic practice. Obstet. Gynecol. 2016, 127, e170–e174. [CrossRef]
- Faustino, F.; Martinho, M.; Reis, J.; Aguas, F. Update on medical treatment of uterine fibroids. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 216, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Donnez, O.; Dolmans, M.M. With the advent of selective progesterone receptor modulators, what is the place of myoma surgery in current practice? Fertil. Steril. 2014, 102, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Tomaszewski, J.; Vazquez, F.; Bouchard, P.; Lemieszczuk, B.; Baro, F.; Nouri, K.; Selvaggi, L.; Sodowski, K.; Bestel, E.; et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N. Engl. J. Med. 2012, 366, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Tatarchuk, T.F.; Bouchard, P.; Puscasiu, L.; Zakharenko, N.F.; Ivanova, T.; Ugocsai, G.; Mara, M.; Jilla, M.P.; Bestel, E.; et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N. Engl. J. Med. 2012, 366, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Murji, A.; Whitaker, L.; Chow, T.L.; Sobel, M.L. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane Database Syst. Rev. 2017, 4, CD010770. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Diamond, M.P.; El-Sohemy, A.; Halder, S.K. 1,25-dihydroxyvitamin D3 regulates expression of sex steroid receptors in human uterine fibroid cells. J. Clin. Endocrinol. Metab. 2015, 100, E572–E582. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Sharan, C.; Al-Hendy, A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol. Reprod. 2012, 86, 116. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Sharan, C.; Al-Hendy, O.; Al-Hendy, A. Paricalcitol, a vitamin D receptor activator, inhibits tumor formation in a murine model of uterine fibroids. Reprod. Sci. 2014, 21, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Grygielko, E.T.; Martin, W.M.; Tweed, C.; Thornton, P.; Harling, J.; Brooks, D.P.; Laping, N.J. Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-β type I receptor kinase in puromycin-induced nephritis. J. Pharmacol. Exp. Ther. 2005, 313, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Laping, N.J.; Everitt, J.I.; Frazier, K.S.; Burgert, M.; Portis, M.J.; Cadacio, C.; Gold, L.I.; Walker, C.L. Tumor-specific efficacy of transforming growth factor-βRI inhibition in Eker rats. Clin. Cancer. Res. 2007, 13, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Protic, O.; Giannubilo, S.R.; Toti, P.; Tranquilli, A.L.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Uterine leiomyoma: Available medical treatments and new possible therapeutic options. J. Clin. Endocrinol. Metab. 2013, 98, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Jee, B.C.; Kim, S.H.; Cho, Y.J.; Han, M. Cyclooxygenase-2 inhibitor, celecoxib, inhibits leiomyoma cell proliferation through the nuclear factor κB pathway. Reprod. Sci. 2014, 21, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Darakhshan, S.; Pour, A.B. Tranilast: A review of its therapeutic applications. Pharmacol. Res. 2015, 91, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.D.; Khorram, O. Tranilast inhibits genes functionally involved in cell proliferation, fibrosis, and epigenetic regulation and epigenetically induces miR-29c expression in leiomyoma cells. Reprod. Sci. 2017, 24, 1253–1263. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciebiera, M.; Włodarczyk, M.; Wrzosek, M.; Męczekalski, B.; Nowicka, G.; Łukaszuk, K.; Ciebiera, M.; Słabuszewska-Jóźwiak, A.; Jakiel, G. Role of Transforming Growth Factor β in Uterine Fibroid Biology. Int. J. Mol. Sci. 2017, 18, 2435. https://doi.org/10.3390/ijms18112435
Ciebiera M, Włodarczyk M, Wrzosek M, Męczekalski B, Nowicka G, Łukaszuk K, Ciebiera M, Słabuszewska-Jóźwiak A, Jakiel G. Role of Transforming Growth Factor β in Uterine Fibroid Biology. International Journal of Molecular Sciences. 2017; 18(11):2435. https://doi.org/10.3390/ijms18112435
Chicago/Turabian StyleCiebiera, Michał, Marta Włodarczyk, Małgorzata Wrzosek, Błażej Męczekalski, Grażyna Nowicka, Krzysztof Łukaszuk, Magdalena Ciebiera, Aneta Słabuszewska-Jóźwiak, and Grzegorz Jakiel. 2017. "Role of Transforming Growth Factor β in Uterine Fibroid Biology" International Journal of Molecular Sciences 18, no. 11: 2435. https://doi.org/10.3390/ijms18112435
APA StyleCiebiera, M., Włodarczyk, M., Wrzosek, M., Męczekalski, B., Nowicka, G., Łukaszuk, K., Ciebiera, M., Słabuszewska-Jóźwiak, A., & Jakiel, G. (2017). Role of Transforming Growth Factor β in Uterine Fibroid Biology. International Journal of Molecular Sciences, 18(11), 2435. https://doi.org/10.3390/ijms18112435