Anti-Inflammatory and Antinociceptive Effects of Ethyl Acetate Fraction of an Edible Red Macroalgae Sarcodia ceylanica
Abstract
:1. Introduction
2. Results
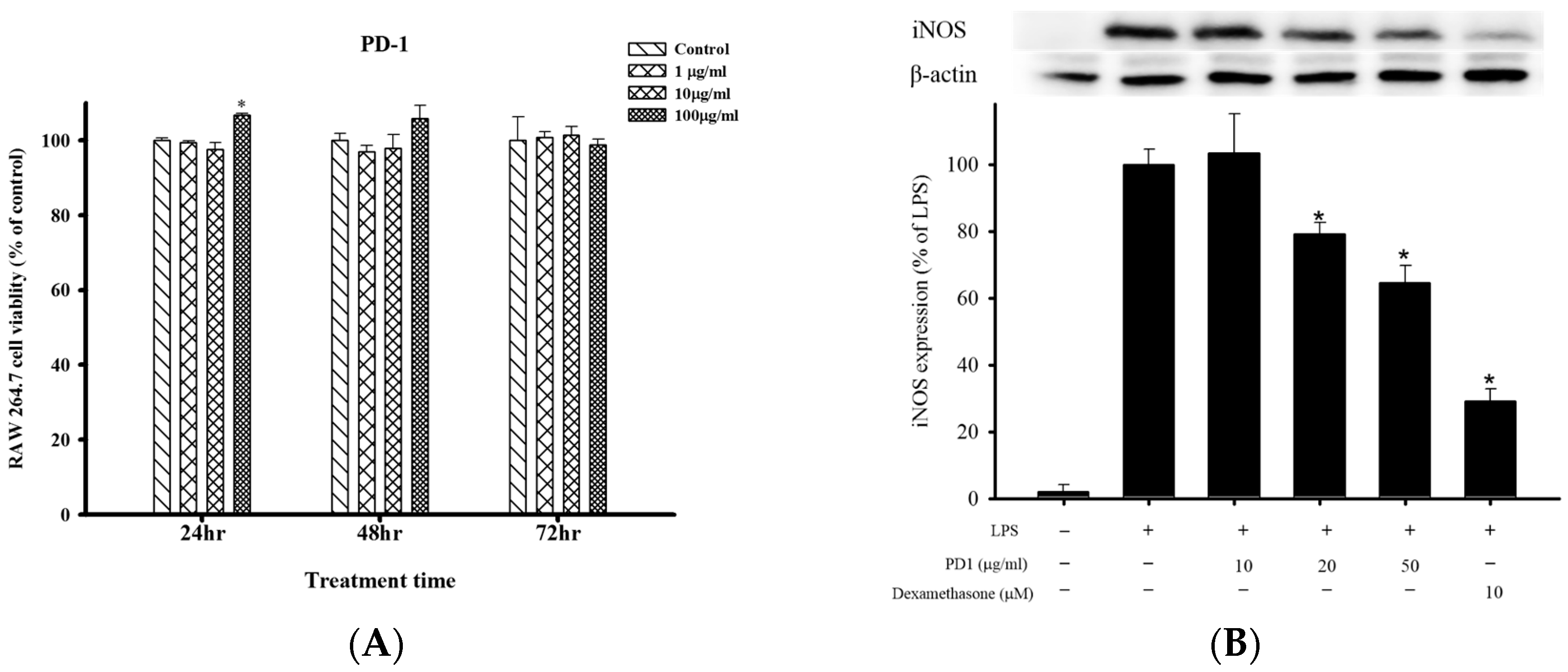
2.1. Cell Viability and Anti-Inflammatory Effects of PD1 with Respect to LPS-Induced iNOS and COX-2 in RAW 264.7 Cells
2.2. Effects of Oral PD1 on Carrageenan-Induced Rat Paw Edema
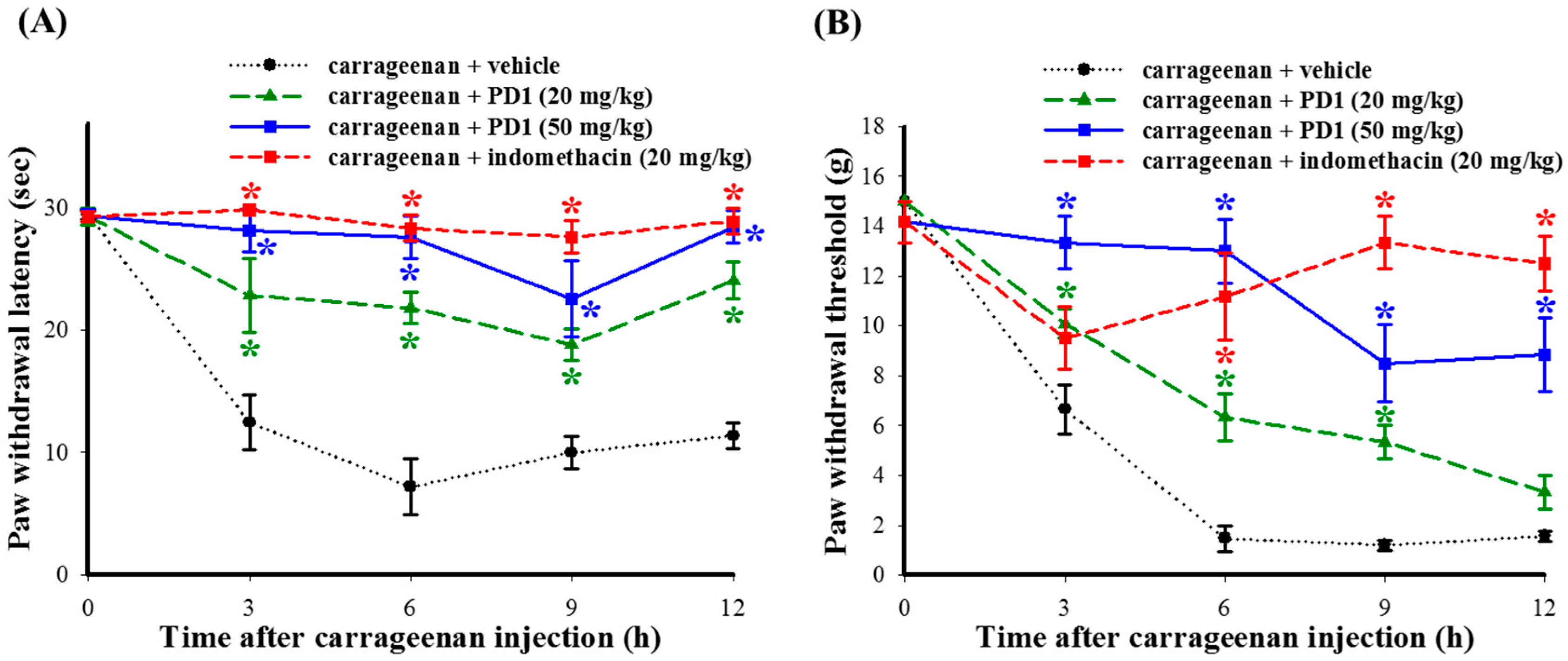
2.3. Effects of PD1 on Carrageenan-Induced Inflammatory Nociceptions
2.4. Histopathologic Analyses of the Effects of PD1 on Cell Infiltration in Carrageen-Injected Paws
2.5. Effects of PD1 on Inflammatory-Related Protein Expression in Rat Paw Tissues Induced by Carrageenan
2.6. Effects of PD1 on Expression of Carrageenan-Induced IL-1β and iNOS in Leukocytes in Rat Paw Tissues
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation Method for Crude Extract
4.3. The Preparation of Analytical Sample and HPLC Conditions
4.4. Cell Culture
4.4.1. Preparation of Cell
4.4.2. Cell Viability
4.5. In Vitro Anti-Inflammatory Assay
4.6. In Vivo Model for Carrageenan-Induced Rat Paw Inflammation
4.7. Analyses of the Edema and Pain Behavior of Rats
4.8. Histopathology and Immunohistochemistry of Paw Tissues
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Coura, C.O.; de Araujo, I.W.; Vanderlei, E.S.; Rodrigues, J.A.; Quindere, A.L.; Fontes, B.P.; de Queiroz, I.N.; de Menezes, D.B.; Bezerra, M.M.; e Silva, A.A.; et al. Antinociceptive and anti-inflammatory activities of sulphated polysaccharides from the red seaweed Gracilaria cornea. Basic Clin. Pharmacol. Toxicol. 2012, 110, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Liaw, E.T.; Ruan, Y.A.; Huang, C.C.J. Glycemic regulation and characterization of aqueous extract from Sarcodia ceylanica. In Proceedings of the 7th Taiwan-Thailand Bilateral Conference, Pingtung, Taiwan, 18–19 October 2012. [Google Scholar]
- Liao, W.R.; Lin, J.Y.; Shieh, W.Y.; Jeng, W.L.; Huang, R. Antibiotic activity of lectins from marine algae against marine vibrios. J. Ind. Microbiol. Biotechnol. 2003, 30, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lin, M.; Luo, A.; Chun, Z.; Luo, A. Characterization and antitumor activity of a polysaccharide from Sarcodia ceylonensis. Molecules 2014, 19, 10863–10876. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Feng, X.; Liu, Y.; Fan, Y. Polysaccharide fractions from Sarcodia ceylonensis and their antioxidant properties in vitro and in vivo. Pharmacologia 2014, 5, 235–240. [Google Scholar]
- Sangha, J.S.; Wally, O.; Banskota, A.H.; Stefanova, R.; Hafting, J.T.; Critchley, A.T.; Prithiviraj, B. A cultivated form of a red seaweed (Chondrus crispus), suppresses β-amyloid-induced paralysis in Caenorhabditis elegans. Mar. Drugs 2015, 13, 6407–6424. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Willoughby, D.A.; Gilroy, D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002, 2, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Abdel-Azeem, A.Z.; Al-Sanea, M.M.; Yoo, K.H.; Tae, J.S.; Lee, S.H. Staurosporine analogues from microbial and synthetic sources and their biological activities. Curr. Med. Chem. 2013, 20, 3872–3902. [Google Scholar] [CrossRef] [PubMed]
- Sweitzer, S.; Martin, D.; DeLeo, J.A. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience 2001, 103, 529–539. [Google Scholar] [CrossRef]
- Lobsiger, C.S.; Cleveland, D.W. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat. Neurosci. 2007, 10, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chen, J.S.; Wu, W.M.; Liao, T.N.; Chu, P.; Lin, S.H.; Chuang, C.H.; Lin, Y.F. Myeloperoxidase serves as a marker of oxidative stress during single haemodialysis session using two different biocompatible dialysis membranes. Nephrol. Dial. Transplant. 2005, 20, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.C.; Massey, K.; Nicolaou, A. Fish oil supplementation alters levels of lipid mediators of inflammation in microenvironment of acute human wounds. Wound Repair Regen. 2011, 19, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, K.; Yang, R.; Porter, T.F.; Agrawal, N.; Petasis, N.A.; Irimia, D.; Toner, M.; Serhan, C.N. Rapid appearance of resolvin precursors in inflammatory exudates: Novel mechanisms in resolution. J. Immunol. 2008, 181, 8677–8687. [Google Scholar] [CrossRef] [PubMed]
- Gaut, J.P.; Byun, J.; Tran, H.D.; Lauber, W.M.; Carroll, J.A.; Hotchkiss, R.S.; Belaaouaj, A.; Heinecke, J.W. Myeloperoxidase produces nitrating oxidants in vivo. J. Clin. Investig. 2002, 109, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Eiserich, J.P.; Hristova, M.; Cross, C.E.; Jones, A.D.; Freeman, B.A.; Halliwell, B.; van der Vliet, A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 1998, 391, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Ferrante, C.; Carradori, S.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Recinella, L.; Orlando, G.; Martinotti, S.; et al. Optimization of aqueous extraction and biological activity of Harpagophytum procumbens root on ex vivo rat colon inflammatory model. Phytother. Res. 2017, 31, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, L.; Mollica, M.; Re, A.T.; Wu, S.; Zuo, L. Nitric oxide in cancer metastasis. Cancer Lett. 2014, 353, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.M.; Lai, C.C.; Huang, L.J.; Kuo, T.C.; Chao, C.M.; Lin, W.W. The anti-inflammatory carbazole, LCY-2-CHO, inhibits lipopolysaccharide-induced inflammatory mediator expression through inhibition of the p38 mitogen-activated protein kinase signaling pathway in macrophages. Br. J. Pharmacol. 2004, 141, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Woo, M.S.; Kim, S.Y.; Kim, W.K.; Kim, H.S. Repression of interferon-gamma-induced inducible nitric oxide synthase (iNOS) gene expression in microglia by sodium butyrate is mediated through specific inhibition of ERK signaling pathways. J. Neuroimmunol. 2005, 168, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.H.; Chen, W.F.; Duh, C.Y.; Huang, S.Y.; Hsu, C.H.; Lin, C.S.; Sung, C.S.; Chen, I.M.; Wen, Z.H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Su, J.H.; Wen, Z.H. Bioactive cembrane-based diterpenoids from the soft coral Sinularia triangular. Mar. Drugs 2011, 9, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, F.; Mita, Y.; Kamanaka, Y.; Kawao, N.; Matsuya, H.; Taga, C.; Kawabata, A. The potent inducible nitric oxide synthase inhibitor ONO-1714 inhibits neuronal NOS and exerts antinociception in rats. Neurosci. Lett. 2004, 365, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Salvemini, D.; Wang, Z.Q.; Wyatt, P.S.; Bourdon, D.M.; Marino, M.H.; Manning, P.T.; Currie, M.G. Nitric oxide: A key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996, 118, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Chen, N.F.; Chen, W.F.; Hung, H.C.; Lee, H.P.; Lin, Y.Y.; Wang, H.M.; Sung, P.J.; Sheu, J.H.; Wen, Z.H. Sinularin from indigenous soft coral attenuates nociceptive responses and spinal neuroinflammation in carrageenan-induced inflammatory rat model. Mar. Drugs 2012, 10, 1899–1919. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Heeschen, C.; Meinertz, T.; Zeiher, A.M.; Eiserich, J.P.; Munzel, T.; Simoons, M.L.; Hamm, C.W.; Investigators, C. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation 2003, 108, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, B.; Hearty, S.; Finlay, W.J.; O’Kennedy, R. A high-affinity recombinant antibody permits rapid and sensitive direct detection of myeloperoxidase. Anal. Biochem. 2011, 410, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Vissers, M.C.; Kettle, A.J. Myeloperoxidase. Curr. Opin. Hematol. 2000, 7, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Renaissance of marine natural product drug discovery and development. J. Mar. Sci. Res. Dev. 2012, 2, e106. [Google Scholar] [CrossRef]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Preeprame, S.; Hayashi, K.; Lee, J.B.; Sankawa, U.; Hayashi, T. A novel antivirally active fucan sulfate derived from an edible brown alga, Sargassum horneri. Chem. Pharm. Bull. 2001, 49, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Toume, K.; Ishibashi, M.; Miyata, M. A study on chemical constituents of three red algae of the Corallinaceae from Boso Peninsula in Japan. Nat. Hist. Res. 2001, 6, 39–42. [Google Scholar]
- Maroon, J.C.; Bost, J.W.; Maroon, A. Natural anti-inflammatory agents for pain relief. Surg. Neurol. Int. 2010, 1, 80. [Google Scholar] [PubMed]
- Hwang, P.A.; Phan, N.N.; Lu, W.J.; Ngoc Hieu, B.T.; Lin, Y.C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [PubMed]
- Abhari, K.; Shekarforoush, S.S.; Hosseinzadeh, S.; Nazifi, S.; Sajedianfard, J.; Eskandari, M.H. The effects of orally administered Bacillus coagulans and inulin on prevention and progression of rheumatoid arthritis in rats. Food Nutr. Res. 2016, 60, 30876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.K.; Tao, S.S.; Li, T.T.; Li, Y.S.; Li, X.J.; Tang, H.B.; Cong, R.H.; Ma, F.L.; Wan, C.J. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr. Res. 2016, 60, 30849. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.Z.; Chu, P.Y.; Jou, I.M. Enteral sesame oil therapeutically relieves disease severity in rat experimental osteoarthritis. Food Nutr. Res. 2016, 60, 29807. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Lin, Y.Y.; Jean, Y.H.; Lu, Y.; Chen, W.F.; Yang, S.N.; Wang, H.M.; Jang, I.Y.; Chen, I.M.; Su, J.H.; et al. Anti-inflammatory and analgesic effects of the marine-derived compound comaparvin isolated from the crinoid Comanthus bennetti. Molecules 2014, 19, 14667–14686. [Google Scholar] [CrossRef] [PubMed]
- Altemeier, W.A.; Zhu, X.; Berrington, W.R.; Harlan, J.M.; Liles, W.C. Fas (CD95) induces macrophage proinflammatory chemokine production via a MyD88-dependent, caspase-independent pathway. J. Leukoc. Biol. 2007, 82, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.W.; Hung, H.C.; Huang, S.Y.; Chen, C.H.; Chen, Y.R.; Chen, C.Y.; Yang, S.N.; Wang, H.M.; Sung, P.J.; Sheu, J.H.; et al. Neuroprotective effect of the marine-derived compound 11-dehydrosinulariolide through DJ-1-related pathway in in vitro and in vivo models of Parkinson’s disease. Mar. Drugs 2016, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Jean, Y.H.; Lee, H.P.; Lin, S.C.; Pan, C.Y.; Chen, W.F.; Wu, S.F.; Su, J.H.; Tsui, K.H.; Sheu, J.H.; et al. Excavatolide B attenuates rheumatoid arthritis through the Inhibition of osteoclastogenesis. Mar. Drugs 2017, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, N.F.; Feng, C.W.; Cheng, S.Y.; Hung, H.C.; Tsui, K.H.; Hsu, C.H.; Sung, P.J.; Chen, W.F.; Wen, Z.H. A coral-derived compound improves functional recovery after spinal cord injury through its antiapoptotic and anti-inflammatory effects. Mar. Drugs 2016, 14, 160. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, C.-C.; Hwang, H.-R.; Chang, C.-I.; Su, H.-M.; Chen, P.-C.; Kuo, H.-M.; Li, P.-J.; Wang, H.-M.D.; Tsui, K.-H.; Lin, Y.-C.; et al. Anti-Inflammatory and Antinociceptive Effects of Ethyl Acetate Fraction of an Edible Red Macroalgae Sarcodia ceylanica. Int. J. Mol. Sci. 2017, 18, 2437. https://doi.org/10.3390/ijms18112437
Shih C-C, Hwang H-R, Chang C-I, Su H-M, Chen P-C, Kuo H-M, Li P-J, Wang H-MD, Tsui K-H, Lin Y-C, et al. Anti-Inflammatory and Antinociceptive Effects of Ethyl Acetate Fraction of an Edible Red Macroalgae Sarcodia ceylanica. International Journal of Molecular Sciences. 2017; 18(11):2437. https://doi.org/10.3390/ijms18112437
Chicago/Turabian StyleShih, Chieh-Chih, Hwong-Ru Hwang, Chi-I Chang, Huei-Meei Su, Pei-Chin Chen, Hsiao-Mei Kuo, Pei-Jyuan Li, Hui-Min David Wang, Kuan-Hao Tsui, Yu-Chi Lin, and et al. 2017. "Anti-Inflammatory and Antinociceptive Effects of Ethyl Acetate Fraction of an Edible Red Macroalgae Sarcodia ceylanica" International Journal of Molecular Sciences 18, no. 11: 2437. https://doi.org/10.3390/ijms18112437





