Diffuse Axonal Injury and Oxidative Stress: A Comprehensive Review
Abstract
:1. Introduction
2. Mechanism of Injury
Experimental Models
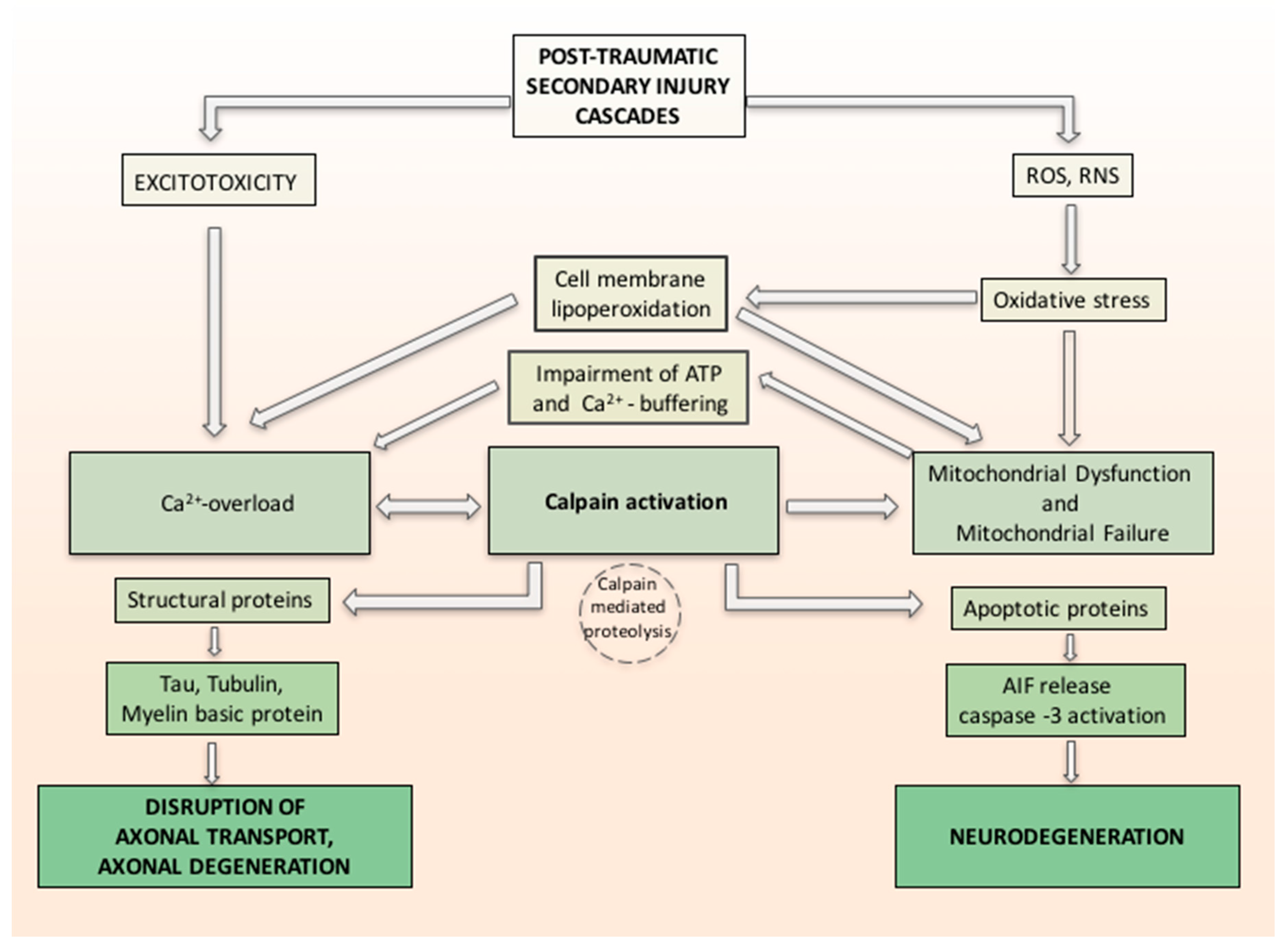
3. Physiopathology
4. TBI and Oxidative Stress
4.1. Role of Mitochondria and Calcium
4.2. Role of Mitochondrial Membrane Permeability
4.3. Oxidative Stress, Calcium Influx and Calpain Activation
5. Pathological Anatomy and Morphologic Findings
5.1. Macroscopic Findings
- Diffuse supratentorial damage to axons (grade I)
- A focal lesion in the corpus callosum (grade II)
- A focal lesion or lesions in the rostral brain stem (grade III).
5.2. Microscopic Findings
- Grade 1: there is scattered axonal retraction balls in the parasagittal white matter of the cerebral hemispheres, the corpus callosum, the brain stem and, less commonly, the cerebellum
- Grade 2: in addition to axonal damage in the white matter of the cerebral hemisphere, there is a focal lesion in the corpus callosum
- Grade 3: in addition to axonal damage in the white matter of the hemispheres, the focal lesions are present in the dorsolateral quadrant of the rostral brain stem and the corpus callosum.
5.3. Immunohistochemistry and Stainings
5.4. Biomarkers
6. Diagnosis and Radiology
6.1. CT Scans
6.2. MRI: Short-Term MRI and Follow-Up
7. Conclusions: What Is Currently Ascertained
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gerber, L.M.; Chiu, Y.L.; Carney, N.; Härtl, R.; Ghajar, J. Marked reduction in mortality in patients with severe traumatic brain injur. J. Neurosurg. 2013, 119, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Finfer, S.R.; Cohen, J. Severe traumatic brain injury. Resuscitation 2001, 48, 77–90. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Corso, P.S.; Miller, T.R. The Incidence and Economic Burden of Injuries in the United States; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Holbourn, A.H.S. Mechanics of Head Injury. Lancet 1943, 242, 438–441. [Google Scholar] [CrossRef]
- Holbourn, A.H.S. Mechanics of Brain Injuries. Br. Med. Bull. 1945, 3, 147–149. [Google Scholar] [CrossRef]
- Strich, S.J. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J. Neurol. Neurosurg. Psychiatry 1956, 19, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.H.; Graham, D.I.; Murray, L.S.; Scott, G. Diffuse axonal injury due to nonmissile head injury in humans: An analysis of 45 cases. Ann. Neurol. 1982, 12, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.H.; Doyle, D.; Graham, D.I.; Lawrence, A.E.; McLellan, D.R. Diffuse axonal injury in head injuries caused by a fall. Lancet 1984, 2, 1420–1422. [Google Scholar] [CrossRef]
- Moenninghoff, C.; Kraff, O.; Maderwald, S.; Umutlu, L.; Theysohn, J.M.; Ringelstein, A.; Wrede, K.H.; Deuschl, C.; Altmeppen, J.; Ladd, M.E.; et al. Diffuse axonal injury at ultra-high field MRI. PLoS ONE 2015, 10, e0122329. [Google Scholar] [CrossRef] [PubMed]
- Davceva, N.; Basheska, N.; Balazic, J. Diffuse Axonal Injury—A Distinct Clinicopathological Entity in Closed Head Injuries. Am. J. Forensic Med. Pathol. 2015, 36, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.; Johnson, V.E.; Smith, D.H.; Stewart, W. Chronic traumatic encephalopathy: The neuropathological legacy of traumatic brain injury. Ann. Rev. Pathol. 2016, 11, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Vile, A.R.; Atkinson, L. Chronic Traumatic Encephalopathy: The cellular sequela to repetitive brain injury. J. Clin. Neurosc. 2017, 41, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Niess, C.; Grauel, U.; Toennes, S.W.; Bratzke, H. Incidence of axonal injury in human brain tissue. Acta Neuropathol. 2002, 104, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Gennarelli, T.A.; Thibault, L.E.; Adams, J.H.; Graham, D.I.; Thompson, C.J.; Marcincin, R.P. Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 1982, 12, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Kleiven, S. Why most traumatic brain injuries are not caused by linear acceleration but skull fractures are. Front. Bioeng. Biotechnol. 2013, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.W. Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci. 2011, 13, 287–300. [Google Scholar] [PubMed]
- Thompson, H.J.; Lifshitz, J.; Marklund, N.; Grady, M.S.; Graham, D.I.; Hovda, D.A.; McIntosh, T.K. Lateral fluid percussion brain injury: A 15-year review and evaluation. J. Neurotrauma 2005, 22, 42–75. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.E.; Clifton, G.L.; Lighthall, J.W.; Yaghmai, A.A.; Hayes, R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 1991, 39, 253–262. [Google Scholar] [CrossRef]
- Onyszchuk, G.; Al-Hafez, B.; He, Y.Y.; Bilgen, M.; Berman, N.E.; Brooks, W.M. A mouse model of sensorimotor controlled cortical impact: Characterization using longitudinal magnetic resonance imaging, behavioral assessments and histology. J. Neurosci. Methods 2007, 160, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Namjoshi, D.R.; Good, C.; Cheng, W.H.; Panenka, W.; Richards, D.; Cripton, P.A.; Wellington, C.L. Towards clinical management of traumatic brain injury: A review of models and mechanisms from a biomechanical perspective. Dis. Models Mech. 2013, 6, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Namjoshi, D.R.; Cheng, W.H.; McInnes, K.A.; Martens, K.M.; Carr, M.; Wilkinson, A.; Fan, J.; Robert, J.; Hayat, A.; Cripton, P.A.; et al. Merging pathology with biomechanics using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): A novel, surgery-free model of traumatic brain injury. Mol. Neurodegener. 2014, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Tang-Schomer, M.D.; Johnson, V.E.; Baas, P.W.; Stewart, W.; Smith, D.H. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp. Neurol. 2012, 233, 364–372. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.J.; McLellan, D.R.; Gentleman, S.M.; Maxwell, W.L.; Gennarelli, T.A.; Graham, D.I. Is β-APP a marker of axonal damage in short-surviving head injury? Acta Neuropathol. 1996, 92, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.Y.; Feng, D.F.; Pan, D.C. Biomarkers associated with diffuse traumatic axonal injury: Exploring pathogenesis, early diagnosis, and prognosis. J. Trauma 2010, 69, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Song, Y.; Zhang, J.; Lin, W.; Dong, H. Calcium signaling is implicated in the diffuse axonal injury of brain stem. Int. J. Clin. Exp. Pathol. 2015, 8, 4388–4397. [Google Scholar] [PubMed]
- Venkatesan, C.; Chrzaszcz, M.; Choi, N.; Wainwright, M.S. Chronic upregulation of activated microglia immunoreactive for galectin-3/Mac-2 and nerve growth factor following diffuse axonal injury. J. Neuroinflamm. 2010, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Oehmichen, M.; Theuerkauf, I.; Meissner, C. Is traumatic axonal injury (AI) associated with an early microglial activation? Application of a double-labeling technique for simultaneous detection of microglia and AI. Acta Neuropathol. 1999, 97, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.T.; Wang, Y.W.; Wo, Y.Y.; Yang, Y.L. Extracellular signal-regulated kinase-mediated IL-1-induced cortical neuron damage during traumatic brain injury. Neurosci. Lett. 2005, 386, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Hans, V.H.; Kossmann, T.; Lenzlinger, P.M.; Probstmeier, R.; Imhof, H.G.; Trentz, O.; Morganti-Kossmann, M.C. Experimental axonal injury triggers interleukin-6 mRNA, protein synthesis and release into cerebrospinal fluid. J. Cereb. Blood Flow Metab. 1999, 19, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Kita, T.; Tanaka, T.; Tanaka, N.; Kinoshita, Y. The role of tumor necrosis factor-alpha in diffuse axonal injury following fluid-percussive brain injury in rats. Int. J. Legal Med. 2000, 113, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Rancan, M.; Otto, V.I.; Hans, V.H.; Gerlach, I.; Jork, R.; Trentz, O.; Morganti-Kossmann, M.C. Upregulation of ICAM-1 and MCP-1 but not of MIP-2 and sensorimotor deficit in response to traumatic axonal injury in rats. J. Neurosci. Res. 2001, 63, 438–446. [Google Scholar] [CrossRef]
- Rhodes, J.K.; Sharkey, J.; Andrews, P.J. The temporal expression, cellular localization, and inhibition of the chemokines MIP-2 and MCP-1 after traumatic brain injury in the rat. J. Neurotrauma 2009, 26, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Meaney, D.F.; Xu, B.N.; Nonaka, M.; Mcintosh, T.K.; Wolf, J.A.; Saatman, K.E.; Smith, D.H. Evolution of neurofilament subtype accumulation in axons following diffuse brain injury in the pig. J. Neuropathol. Exp. Neurol. 1999, 58, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Uryu, K.; Saatman, K.E.; Trojanowski, J.Q.; Mcintosh, T.K. Protein accumulation in traumatic brain injury. Neuromol. Med. 2003, 4, 59–72. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Traumatic Brain Injury as a Trigger of Neurodegeneration. Adv. Neurobiol. 2017, 15, 383–400. [Google Scholar] [PubMed]
- Siedler, D.G.; Chuah, M.I.; Kirkcaldie, M.T.; Vickers, J.C.; King, A.E. Diffuse axonal injury in brain trauma: Insights from alterations in neurofilaments. Front. Cell Neurosci. 2014, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Farkas, O.; Lifshitz, J.; Povlishock, J.T. Mechanoporation induced by diffuse traumatic brain injury: An irreversible or reversible response to injury? J. Neurosci. 2006, 26, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, D.; Gallo, G.; Barbee, K.A. Mechanical membrane injury induces axonal beading through localized activation of calpain. Exp. Neurol. 2009, 219, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.; Crupi, R.; Calabrese, V.; Graziano, A.; Milone, P.; Pennisi, G.; Radak, Z.; Calabrese, E.J.; Cuzzocrea, S. Traumatic brain injury: Oxidative stress and neuroprotection. Antioxid. Redox Signal. 2013, 19, 836–853. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Vaishnav, R.A.; Mustafa, A.G. Antioxidant therapies for traumatic brain injury. Neurotherapeutics 2010, 7, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Staal, J.A. Cyclosporin-A treatment attenuates delayed cytoskeletal alterations and secondary axotomy following mild axonal stretch injury. Dev. Neurobiol. 2007, 67, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.P. Axoplasmic reticulum Ca2+ release causes secondary degeneration of spinal axons. Ann. Neurol. 2014, 75, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Jang, B.G.; Kim, J.H.; Lee, B.E.; Sohn, M.; Song, H.K.; Suh, S.W. Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res. 2012, 1481, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Büki, A.; Siman, R.; Trojanowski, J.Q.; Povlishock, J.T. The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. J. Neuropathol. Exp. Neurol. 1999, 58, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Büki, A.; Farkas, O.; Doczi, T.; Povlishock, J.T. Preinjury administration of the calpain inhibitor MDL-28170 attenuates traumatically induced axonal injury. J. Neurotrauma 2003, 20, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Gitler, D.; Spira, M.E. Real time imaging of calcium-induced localized proteolytic activity after axotomy and its relation to growth cone formation. Neuron 1998, 20, 1123–1135. [Google Scholar] [CrossRef]
- Povlishock, J.T.; Buki, A.; Koiziumi, H.; Stone, J.; Okonkwo, D.O. Initiating mechanisms involved in the pathobiology of traumatically induced axonal injury and interventions targeted at blunting their progression. Acta Neurochir. Suppl. 1999, 73, 15–20. [Google Scholar] [PubMed]
- Maxwell, W.L.; Watt, C.; Pediani, J.D.; Graham, D.I.; Adams, J.H.; Gennarelli, T.A. Localisation of calcium ions and calcium-ATPase activity within myelinated nerve fibres of the adult guinea pig optic nerve. J. Anat. 1991, 176, 71–79. [Google Scholar] [PubMed]
- Maxwell, W.L.; McCreath, B.J.; Graham, D.I.; Gennarelli, T.A. Cytochemical evidence for redistribution of membrane pump calcium-ATPase and ecto-Ca-ATPase activity, and calcium influx in myelinated nerve fibres of the optic nerve after stretch injury. J. Neurocytol. 1995, 24, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, W.L.; Kosanlavit, R.; McCreath, B.J.; Reid, O.; Graham, D.I. Freeze-fracture and cytochemical evidence for structural and functional alteration in the axolemma and myelin 1019 sheath of adult guinea pig optic nerve fibers after stretch injury. J. Neurotrauma 1999, 16, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.A.; Stys, P.K.; Lusardi, T.; Meaney, D.; Smith, D.H. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J. Neurosci. 2001, 21, 1923–1930. [Google Scholar] [PubMed]
- Büki, A.; Okonkwo, D.O.; Wang, K.K.; Povlishock, J.T. Cytochrome c release and caspase activation in traumatic axonal injury. J. Neurosci. 2000, 20, 2825–2834. [Google Scholar] [PubMed]
- Finkel, E. The mitochondrion: Is it central to apoptosis? Science 2001, 292, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Hunot, S.; Flavell, R.A. Apoptosis: Death of a monopoly? Science 2001, 292, 865–866. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, J.; Friberg, H.; Neumar, R.W.; Raghupathi, R.; Welsh, F.A.; Janmey, P.; Saatman, K.E.; Wieloch, T.; Grady, M.S.; McIntosh, T.K. Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: Evidence for differentially sensitive populations in the cortex and hippocampus. J. Cereb. Blood Flow Metab. 2003, 23, 219–231. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Reynolds, I.J. Mitochondrial depolarization in glutamate-stimulated neurons: An early signal specific to excitotoxin exposure. J. Neurosci. 1996, 16, 5688–5697. [Google Scholar] [PubMed]
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Sullivan, P.G.; Sensi, S.L.; Steward, O.; Weiss, J.H. Zn(2+) induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J. Biol. Chem. 2001, 276, 47524–47529. [Google Scholar] [CrossRef] [PubMed]
- Barkhoudarian, G.; Hovda, D.A.; Giza, C.C. The molecular pathophysiology of concussive brain injury. Clin. Sports Med. 2011, 30, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Hosie, K.A.; King, A.E.; Blizzard, C.A.; Vickers, J.C.; Dickson, T.C. Chronic excitotoxin-induced axon degeneration in a compartmented neuronal culture model. ASN Neuro 2012, 4, e00076. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Southam, K.A.; Dittman, J.; Vickers, J.C. Excitotoxin-induced caspase-3 activation and microtubule disintegration in axons is inhibited by taxol. Acta Neuropathol. Commun. 2013, 1, 59. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Dickson, T.C.; Blizzard, C.A.; Foster, S.S.; Chung, R.S.; West, A.K.; Chuah, M.I.; Vickers, J.C. Excitotoxicity mediated by non-NMDA receptors causes distal axonopathy in long-term cultured spinal motor neurons. Eur. J. Neurosci. 2007, 26, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M. Axon degeneration mechanisms: Commonality amid diversity. Nat. Rev. Neurosci. 2005, 6, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Barsukova, A.G. Focal increases of axoplasmic Ca2+, aggregation of sodium–calcium exchanger, N-type Ca2+ channel, and actin define the sites of spheroids in axons undergoing oxidative stress. J. Neurosci. 2012, 32, 12028–12037. [Google Scholar] [CrossRef] [PubMed]
- Nikic, I.; Merkler, D.; Sorbara, C.; Brinkoetter, M.; Kreutzfeldt, M.; Bareyre, F.M.; Brück, W.; Bishop, D.; Misgeld, T.; Kerschensteiner, M. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 2011, 17, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Medress, Z.A.; Barres, B.A. Axon degeneration: Molecular mechanisms of a self-destruction pathway. J. Cell Biol. 2012, 196, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Barsukova, A.G.; Bourdette, D.; Forte, M. Mitochondrial calcium and its regulation in neurodegeneration induced by oxidative stress. Eur. J. Neurosci. 2011, 34, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Beirowski, B.; Nogradi, A.; Babetto, E.; Garcia-Alias, G.; Coleman, M.P. Mechanisms of axonal spheroid formation in central nervous system Wallerian degeneration. J. Neuropathol. Exp. Neurol. 2010, 69, 455. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br. J. Pharmacol. 2012, 167, 699–719. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. What is the mitochondrial permeability transition pore? J. Mol. Cell Cardiol. 2009, 46, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, A.T. The role of mitochondrial transition pore, and its modulation, in traumatic brain injury and delayed neurodegeneration after TBI. Exp. Neurol. 2009, 218, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Büki, A.; Okonkwo, D.O.; Povlishock, J.T. Postinjury cyclosporin a administration limits axonal damage and disconnection in traumatic brain injury. J. Neurotrauma 1999, 16, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, D.O.; Povlishock, J.T. An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J. Cereb. Blood Flow Metab. 1999, 19, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, D.O.; Buki, A.; Siman, R.; Povlishock, J.T. Cyclosporin A limits calcium-induced axonal damage following traumatic brain injury. Neuroreport 1999, 10, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.A. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J. Neurosci. 2011, 31, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Staal, J.A. Initial calcium release from intracellular stores followed by calcium dysregulation is linked to secondary axotomy following transient axonal stretch injury. J. Neurochem. 2010, 112, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Gold, B.G.; Marracci, G.; Chaudhary, P.; Basso, E.; Johnsen, D.; Yu, X.; Fowlkes, J.; Rahder, M.; Stem, K.; et al. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2007, 104, 7558–7563. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, E.; Povlishock, J.T. Exacerbation of traumatically induced axonal injury by rapid posthypothermic rewarming and attenuation of axonal change by cyclosporin A. J. Neurosurg. 2001, 94, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.H.; Kozlowski, D.A.; Seidl, S.E.; Lance, S.; Wieschhaus, A.J.; Sundivakkam, P.; Tiruppathi, C.; Chishti, I.; Herman, I.M.; Kuchay, S.M.; et al. Targeted gene inactivation of calpain-1 suppresses cortical degeneration due to traumatic brain injury and neuronal apoptosis induced by oxidative stress. J. Biol. Chem. 2012, 287, 13182–13193. [Google Scholar] [CrossRef] [PubMed]
- Saatman, K.E.; Creed, J.; Raghupathi, R. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics 2010, 7, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.H.; Doyle, D.; Ford, I.; Gennarelli, T.A.; Graham, D.I.; McLellan, D.R. Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology 1989, 15, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.H.; Doyle, D.; Graham, D.I.; Lawrence, A.E.; McLellan, D.R. Microscopic diffuse axonal injury in cases of head injury. Med. Sci. Law 1985, 25, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.S.; Coleman, M.P.; Menon, D.K. Traumatic Axonal Injury: Mechanisms and Translational Opportunities. Trends Neurosci. 2016, 39, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Geddes, J.F.; Vowles, G.H.; Beer, T.W.; Ellison, D.W. The diagnosis of diffuse axonal injury: Implications for forensic practice. Neuropathol. Appl. Neurobiol. 1997, 23, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Lambri, M.; Djurovic, V.; Kibble, M.; Cairns, N.; Al-Sarraj, S. Specificity and sensitivity of beta APP in head injury. Clin. Neuropathol. 2001, 20, 263–271. [Google Scholar] [PubMed]
- Reichard, R.R.; Smith, C.; Graham, D.I. The significance of beta-APP immunoreactivity in forensic practice. Neuropathol. Appl. Neurobiol. 2005, 31, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Rutty, G.N.; Timperley, W.R. The possible role of hypoxia in the formation of axonal bulbs. J. Clin. Pathol. 1999, 52, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Ago, K.; Ago, M.; Ogata, M. Two patterns of beta-amyloid precursor protein (APP) immunoreactivity in cases of blunt head injury. Leg. Med. (Tokyo) 2009, 11 (Suppl. 1), S171–S173. [Google Scholar] [CrossRef] [PubMed]
- Oehmichen, M.; Meissner, C.; Schmidt, V.; Pedal, I.; König, H.G.; Saternus, K.S. Axonal injury—A diagnostic tool in forensic neuropathology? A review. Forensic Sci. Int. 1998, 95, 67–83. [Google Scholar] [CrossRef]
- Graham, D.I.; Smith, C.; Reichard, R.; Leclercq, P.D.; Gentleman, S.M. Trials and tribulations of using beta-amyloid precursor protein immunohistochemistry to evaluate traumatic brain injury in adults. Forensic Sci. Int. 2004, 146, 89–96. [Google Scholar] [CrossRef]
- Farkas, O.; Polgár, B.; Szekeres-Barthó, J.; Dóczi, T.; Povlishock, J.T.; Büki, A. Spectrin breakdown products in the cerebrospinal fluid in severe head injury—Preliminary observations. Acta Neurochir. (Wien) 2005, 147, 855–861. [Google Scholar] [PubMed]
- Brophy, G.M.; Pineda, J.A.; Papa, L.; Lewis, S.B.; Valadka, A.B.; Hannay, H.J.; Heaton, S.C.; Demery, J.A.; Liu, M.C.; Tepas, J.J., 3rd; et al. αII-Spectrin Breakdown Product Cerebrospinal Fluid Exposure Metrics Suggest Differences in Cellular Injury Mechanisms after Severe Traumatic Brain Injury. J. Neurotrauma 2009, 26, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Pineda, J.A.; Lewis, S.B.; Valadka, A.B.; Papa, L.; Hannay, H.J.; Heaton, S.C.; Demery, J.A.; Liu, M.C.; Aikman, J.M.; Akle, V.; et al. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J. Neurotrauma 2007, 24, 354–366. [Google Scholar] [CrossRef] [PubMed]
- McCracken, E.; Hunter, A.J.; Patel, S.; Graham, D.I.; Dewar, D. Calpain activation and cytoskeletal protein breakdown in the corpus callosum of head-injured patients. J. Neurotrauma 1999, 16, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Cardali, S.; Maugeri, R. Detection of alphaII-spectrin and breakdown products in humans after severe traumatic brain injury. J. Neurosurg. Sci. 2006, 50, 25–31. [Google Scholar] [PubMed]
- Gatson, J.W.; Barillas, J.; Hynan, L.S.; Diaz-Arrastia, R.; Wolf, S.E.; Minei, J.P. Detection of neurofilament-H in serum as a diagnostic tool to predict injury severity in patients who have suffered mild traumatic brain injury. J. Neurosurg. 2014, 121, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Shibahashi, K.; Doi, T.; Tanaka, S.; Hoda, H.; Chikuda, H.; Sawada, Y.; Takasu, Y.; Chiba, K.; Nozaki, T.; Hamabe, Y.; et al. The Serum Phosphorylated Neurofilament Heavy Subunit as a Predictive Marker for Outcome in Adult Patients after Traumatic Brain Injury. J. Neurotrauma 2016, 33, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Ljungqvist, J.; Zetterberg, H.; Mitsis, M.; Blennow, K.; Skoglund, T. Serum Neurofilament Light Protein as a Marker for Diffuse Axonal Injury: Results from a Case Series Study. J. Neurotrauma 2017, 34, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Nylén, K.; Ost, M.; Csajbok, L.Z.; Nilsson, I.; Blennow, K.; Nellgård, B.; Rosengren, L. Increased serum-GFAP in patients with severe traumatic brain injury is related to outcome. J. Neurol. Sci. 2006, 240, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zemlan, F.P.; Jauch, E.C.; Mulchahey, J.J.; Gabbita, S.P.; Rosenberg, W.S.; Speciale, S.G.; Zuccarello, M. C-tau biomarker of neuronal damage in severe brain injured patients: Association with elevated intracranial pressure and clinical outcome. Brain Res. 2002, 947, 131–139. [Google Scholar] [CrossRef]
- Zemlan, F.P.; Rosenberg, W.S.; Luebbe, P.A.; Campbell, T.A.; Dean, G.E.; Weiner, N.E.; Cohen, J.A.; Rudick, R.A.; Woo, D. Quantification of axonal damage in traumatic brain injury: Affinity purification and characterization of cerebrospinal fluid tau proteins. J. Neurochem. 1999, 72, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.W.; Gentleman, S.M.; Lynch, A.; Murray, L.; Landon, M.; Graham, D.I. Beta amyloid protein deposition in the brain after severe head injury: Implications for the pathogenesis of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Chabok, S.Y.; Moghadam, A.D.; Saneei, Z.; Amlashi, F.G.; Leili, E.K.; Amiri, Z.M. Neuron-specific enolase and S100BB as outcome predictors in severe diffuse axonal injury. J. Trauma Acute Care Surg. 2012, 72, 1654–1657. [Google Scholar] [CrossRef] [PubMed]
- Bogoslovsky, T.; Wilson, D.; Chen, Y.; Hanlon, D.; Gill, J.; Jeromin, A.; Song, L.; Moore, C.; Gong, Y.; Kenney, K.; et al. Increases of Plasma Levels of Glial Fibrillary Acidic Protein, Tau, and Amyloid ß up to 90 Days after Traumatic Brain Injury. J. Neurotrauma 2017, 34, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jeter, C.B.; Hergenroeder, G.W.; Hylin, M.J.; Redell, J.B.; Moore, A.N.; Dash, P.K. Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J. Neurotrauma 2013, 30, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Raabe, A.; Grolms, C.; Sorge, O.; Zimmermann, M.; Seifert, V. Serum S-100B protein in severe head injury. Neurosurgery 1999, 45, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Romner, B.; Ingebrigtsen, T.; Kongstad, P.; Børgesen, S.E. Traumatic brain damage: Serum S-100 protein measurements related to neuroradiological findings. J. Neurotrauma 2000, 17, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, T.; Romner, B. Biochemical serum markers for brain damage: A short review with emphasis on clinical utility in mild head injury. Restor. Neurol. Neurosci. 2003, 21, 171–176. [Google Scholar] [PubMed]
- Kleindienst, A.; Schmidt, C.; Parsch, H.; Emtmann, I.; Xu, Y.; Buchfelder, M. The passage of S100B from brain to blood is not specifically related to the blood-brain barrier integrity. Cardiovasc. Psychiatry Neurol. 2010, 2010, 801295. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.E.; Hansson, L.O.; Nilsson, O.; Dijlai-Merzoug, R.; Settergren, G. High serum S100B levels for trauma patients without head injuries. Neurosurgery 2001, 48, 1255–1258. [Google Scholar] [PubMed]
- Thelin, E.P.; Jeppsson, E.; Frostell, A.; Svensson, M.; Mondello, S.; Bellander, B.M.; Nelson, D.W. Utility of neuron-specific enolase in traumatic brain injury; relations to S100B levels, outcome, and extracranial injury severity. Crit Care 2016, 20, 285. [Google Scholar] [CrossRef] [PubMed]
- Pelinka, L.E.; Kroepfl, A.; Leixnering, M.; Buchinger, W.; Raabe, A.; Redl, H. GFAP versus S100B in serum after traumatic brain injury: Relationship to brain damage and outcome. J. Neurotrauma 2004, 21, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Kövesdi, E.; Lückl, J.; Bukovics, P.; Farkas, O.; Pál, J.; Czeiter, E.; Szellár, D.; Dóczi, T.; Komoly, S.; Büki, A. Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and paediatrics. Acta Neurochir. (Wien) 2010, 152, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.P.; Beers, S.R.; Richichi, R.; Wiesman, D.; Adelson, P.D. Serum biomarker concentrations and outcome after pediatric traumatic brain injury. J. Neurotrauma 2007, 24, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.F.; Marshall, S.B.; Klauber, M.R.; Van Berkum Clark, M.; Eisenberg, H.M.; Jane, J.A.; Luerssen, T.G.; Marmarou, A.; Foulkes, M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991, 75, S14–S20. [Google Scholar]
- Parizel, P.M.; Ozsarlak; Van Goethem, J.W.; van den Hauwe, L.; Dillen, C.; Verlooy, J.; Cosyns, P.; De Schepper, A.M. Imaging findings in diffuse axonal injury after closed head trauma. Eur. Radiol. 1998, 8, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.; Hukkelhoven, C.W.; Marshall, L.F.; Steyerberg, E.W. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: A comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 2005, 57, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, H.; Shinoda, M.; Fujii, M.; Takahashi, O.; Murakata, A.; Yamamoto, D.; Sumiyoshi, S.; Ishikawa, R. Intraventricular hemorrhage on computed tomography and corpus callosum injury on magnetic resonance imaging in patients with isolated blunt traumatic brain injury. J. Neurosurg. 2012, 117, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Mata-Mbemba, D.; Mugikura, S.; Nakagawa, A.; Murata, T.; Kato, Y.; Tatewaki, Y.; Li, L.; Takase, K.; Ishii, K.; Kushimoto, S.; et al. Intraventricular hemorrhage on initial computed tomography as marker of diffuse axonal injury after traumatic brain injury. J. Neurotrauma 2015, 32, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Currie, S.; Saleem, N.; Straiton, J.A.; Macmullen-Price, J.; Warren, D.J.; Craven, I.J. Imaging assessment of traumatic brain injury. Postgrad. Med. J. 2016, 92, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Thelin, E.P.; Nelson, D.W.; Vehviläinen, J.; Nyström, H.; Kivisaari, R.; Siironen, J.; Svensson, M.; Skrifvars, M.B.; Bellander, B.M.; Raj, R. Evaluation of novel computerized tomography scoring systems in human traumatic brain injury: An observational, multicenter study. PLoS Med. 2017, 14, e1002368. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, M.A.; Siösteen, B.; Hartman, M.; Raininko, R. MR detectability and appearance of small experimental intracranial hematomas at 1.5 T and 0.5 T. A 6–7-month follow-up study. Acta Radiol. 2003, 44, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Hur, J.W.; Kwon, K.Y.; Rhee, J.J.; Lee, J.W.; Lee, H.K. Time to recover consciousness in patients with diffuse axonal injury: Assessment with reference to magnetic resonance grading. J. Korean Neurosurg. Soc. 2009, 46, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Scheid, R.; Ott, D.V.; Roth, H.; Schroeter, M.L.; von Cramon, D.Y. Comparative magnetic resonance imaging at 1.5 and 3 Tesla for the evaluation of traumatic microbleeds. J. Neurotrauma 2007, 24, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Scheid, R.; Preul, C.; Gruber, O.; Wiggins, C.; von Cramon, D.Y. Diffuse axonal injury associated with chronic traumatic brain injury: Evidence from T2*-weighted gradient-echo imaging at 3 T. AJNR Am. J. Neuroradiol. 2003, 24, 1049–1056. [Google Scholar] [PubMed]
- Yanagawa, Y.; Tsushima, Y.; Tokumaru, A.; Un-no, Y.; Sakamoto, T.; Okada, Y.; Nawashiro, H.; Shima, K. A quantitative analysis of head injury using T2*-weighted gradient-echo imaging. J. Trauma 2000, 49, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Feng, D.F. Diffuse axonal injury: Novel insights into detection and treatment. J. Clin. Neurosci. 2009, 16, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Rasmussen, I.A.; Lagopoulos, J.; Håberg, A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J. Neurotrauma 2007, 24, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Jang, S.H. Corpus callosum injury in patients with diffuse axonal injury: A diffusion tensor imaging study. NeuroRehabilitation 2010, 26, 339–345. [Google Scholar] [PubMed]
- Kirov, I.I.; Tal, A.; Babb, J.S.; Reaume, J.; Bushnik, T.; Ashman, T.A.; Flanagan, S.; Grossman, R.I.; Gonen, O. Proton MR spectroscopy correlates diffuse axonal abnormalities with post-concussive symptoms in mild traumatic brain injury. J. Neurotrauma 2013, 30, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Huisman, T.A.; Sorensen, A.G.; Hergan, K.; Gonzalez, R.G.; Schaefer, P.W. Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head injury. J. Comput. Assist. Tomogr. 2003, 27, 5–11. [Google Scholar] [CrossRef] [PubMed]
- D’souza, M.M.; Trivedi, R.; Singh, K.; Grover, H.; Choudhury, A.; Kaur, P.; Kumar, P.; Tripathi, R.P. Traumatic brain injury and the post-concussion syndrome: A diffusion tensor tractography study. Indian J. Radiol. Imaging 2015, 25, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Basser, P.J.; Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B 1996, 111, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Scheid, R.; von Cramon, D.Y. Clinical findings in the chronic phase of traumatic brain injury: Data from 12 years’ experience in the Cognitive Neurology Outpatient Clinic at the University of Leipzig. Dtsch. Arztebl. Int. 2010, 107, 199–205. [Google Scholar] [PubMed]
- Wardlaw, J.M.; Statham, P.F. How often is haemosiderin not visible on routine MRI following traumatic intracerebral haemorrhage? Neuroradiology 2000, 42, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Moen, K.G.; Skandsen, T.; Folvik, M.; Brezova, V.; Kvistad, K.A.; Rydland, J.; Manley, G.T.; Vik, A. A longitudinal MRI study of traumatic axonal injury in patients with moderate and severe traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Skandsen, T.; Kvistad, K.A.; Solheim, O.; Lydersen, S.; Strand, I.H.; Vik, A. Prognostic value of magnetic resonance imaging in moderate and severe head injury: A prospective study of early MRI findings and one-year outcome. J. Neurotrauma 2011, 28, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Marquez de la Plata, C.; Wang, J.Y.; Mumphrey, M.; Moore, C.; Harper, C.; Madden, C.J.; McColl, R.; Whittemore, A.; Devous, M.D.; et al. Cerebral atrophy after traumatic white matter injury: Correlation with acute neuroimaging and outcome. J. Neurotrauma 2008, 25, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Parizel, P.M.; Van Goethem, J.W.; Ozsarlak, O.; Maes, M.; Phillips, C.D. New developments in the neuroradiological diagnosis of craniocerebral trauma. Eur. Radiol. 2005, 15, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Sahuquillo, J.; Arikan, F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst. Rev. 2006, CD003983. [Google Scholar]
- Vahedi, K.; Hofmeijer, J.; Juettler, E.; Vicaut, E.; George, B.; Algra, A.; Amelink, G.J.; Schmiedeck, P.; Schwab, S.; Rothwell, P.M.; et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007, 6, 215–222. [Google Scholar] [CrossRef]
- Hofmeijer, J.; Kappelle, L.J.; Algra, A.; Amelink, G.J.; van Gijn, J.; van der Worp, H.B.; HAMLET Investigators. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy after Middle Cerebral Artery infarction with Life-threatening Edema Trial (HAMLET)): A multicentre, open, randomised trial. Lancet Neurol. 2009, 8, 326–333. [Google Scholar] [CrossRef]


| Precursor | Proteolytic System | Subunits | Potential Biomarkers |
|---|---|---|---|
| αII spectrin | Caspase-3 and calpain | Spectrin breakdown products (SBDP) | SBDP145, SBDP150, SBDP120 |
| Neurofilament protein (NF) | Caspase and calpain | Light (NFL), medium (NFM), and heavy (NFH) neurofilament subunit protein | pNF-H |
| Glial fibrillary acidic protein (GFAP) | Calpain | GFAP breakdown products (GFAP-BDP) | GFAP-BDP38, GFAP-BDP44 |
| Microtubule-associated protein tau (MAP-tau) | Caspase-3 and calpain | Tau breakdown products (TauBDP) | TauBDP45 TauBDP35 |
| β-amyloid precursor protein (β-APP) | Caspase-3 | Amyloid beta peptides | Amyloid β peptide42 (Aβ42) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frati, A.; Cerretani, D.; Fiaschi, A.I.; Frati, P.; Gatto, V.; La Russa, R.; Pesce, A.; Pinchi, E.; Santurro, A.; Fraschetti, F.; et al. Diffuse Axonal Injury and Oxidative Stress: A Comprehensive Review. Int. J. Mol. Sci. 2017, 18, 2600. https://doi.org/10.3390/ijms18122600
Frati A, Cerretani D, Fiaschi AI, Frati P, Gatto V, La Russa R, Pesce A, Pinchi E, Santurro A, Fraschetti F, et al. Diffuse Axonal Injury and Oxidative Stress: A Comprehensive Review. International Journal of Molecular Sciences. 2017; 18(12):2600. https://doi.org/10.3390/ijms18122600
Chicago/Turabian StyleFrati, Alessandro, Daniela Cerretani, Anna Ida Fiaschi, Paola Frati, Vittorio Gatto, Raffaele La Russa, Alessandro Pesce, Enrica Pinchi, Alessandro Santurro, Flavia Fraschetti, and et al. 2017. "Diffuse Axonal Injury and Oxidative Stress: A Comprehensive Review" International Journal of Molecular Sciences 18, no. 12: 2600. https://doi.org/10.3390/ijms18122600
APA StyleFrati, A., Cerretani, D., Fiaschi, A. I., Frati, P., Gatto, V., La Russa, R., Pesce, A., Pinchi, E., Santurro, A., Fraschetti, F., & Fineschi, V. (2017). Diffuse Axonal Injury and Oxidative Stress: A Comprehensive Review. International Journal of Molecular Sciences, 18(12), 2600. https://doi.org/10.3390/ijms18122600







