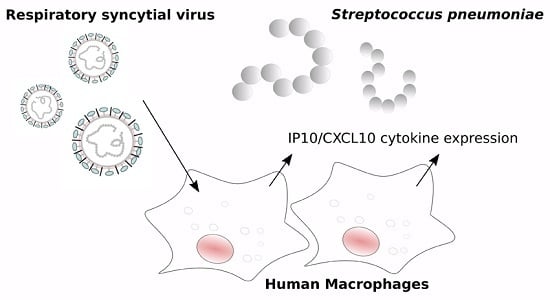
RSV Infection in Human Macrophages Promotes CXCL10/IP-10 Expression during Bacterial Co-Infection
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Cell Lines and Culture Conditions
3.2. Monocyte Isolation and Differentiation
3.3. Pathogen Preparation
3.4. Infection of Monocyte-Derived Macrophages (MDM) Cells
3.5. Multiplex Real-Time RT-qPCR
3.6. IP-10 mRNA Expression
3.7. Apoptosis Assay
3.8. Immunofluorescence Microscopy
3.9. Ethical Statement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RSV | Respiratory syncytial virus |
| Sp | Streptococcus pneumoniae |
| MDMs | Monocyte-derived macrophages |
| LRIs | Lower respiratory infections |
| IP-10 | Interferon-γ-induced protein 10 |
References
- Stupka, J.E.; Mortensen, E.M.; Anzueto, A.; Restrepo, M.I. Community-acquired pneumonia in elderly patients. Aging Health 2009, 5, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.; Evans, S.E. Bacterial Pneumonia in Patients with Cancer: Novel Risk Factors and Management. Clin. Chest Med. 2017, 38, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Vakil, E.; Evans, S.E. Viral Pneumonia in Patients with Hematologic Malignancy or Hematopoietic Stem Cell Transplantation. Clin. Chest Med. 2017, 38, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Johansson, N.; Kalin, M.; Tiveljung-Lindell, A.; Giske, C.G.; Hedlund, J. Etiology of community-acquired pneumonia: Increased microbiological yield with new diagnostic methods. Clin. Infect. Dis. 2010, 50, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Honkinen, M.; Lahti, E.; Osterback, R.; Ruuskanen, O.; Waris, M. Viruses and bacteria in sputum samples of children with community-acquired pneumonia. Clin. Microbiol. Infect. 2012, 18, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Machado, D.; Terrier, O.; Pouzol, S.; Messaoudi, M.; Basualdo, W.; Espínola, E.E.; Guillen, R.M.; Rosa-Calatrava, M.; Picot, V.; et al. Viral and bacterial co-infection in severe pneumonia triggers innate immune responses and specifically enhances IP-10: A translational study. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Morens David, M.; Taubenberger Jeffery, K.; Fauci Anthony, S. Predominant Role of Bacterial Pneumonia as a Cause of Death in Pandemic Influenza: Implications for Pandemic Influenza Preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.C.; Peltola, V.; Iverson, A.R.; McCullers, J.A. Contribution of Vaccine-Induced Immunity toward either the HA or the NA Component of Influenza Viruses Limits Secondary Bacterial Complications. J. Virol. 2010, 84, 4105–4108. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L.; Walters, M.I.; Sellman, J.; Quinlisk, P.; Regnery, H.; Schwartz, B.; Dowell, S.F. Severe pneumococcal pneumonia in previously healthy children: The role of preceding influenza infection. Clin. Infect. Dis. 2000, 30, 784–789. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A.; Bartmess, K.C. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 2003, 187, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Simell, B.; Auranen, K.; Käyhty, H.; Goldblatt, D.; Dagan, R.; O’Brien, K.L.; Pneumococcal Carriage Group. The fundamental link between pneumococcal carriage and disease. Expert Rev. Vaccines 2014, 11, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.M.; Klugman, K.P.; Steiner, C.A.; Simonsen, L.; Viboud, C. Association between respiratory syncytial virus activity and pneumococcal disease in infants: A time series analysis of US hospitalization data. PLoS Med. 2015, 12, e1001776. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A.; McAuley, J.L.; Browall, S.; Iverson, A.R.; Boyd, K.L.; Henriques Normark, B. Influenza Enhances Susceptibility to Natural Acquisition of and Disease due to Streptococcus pneumoniaein Ferrets. J. Infect. Dis. 2010, 202, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Davis, K.M.; Weiser, J.N. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J. Clin. Investig. 2011, 121, 3657–3665. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Reading, P.C.; Wang, N.; Diavatopoulos, D.A.; Wijburg, O.L. Increased Nasopharyngeal Bacterial Titers and Local Inflammation Facilitate Transmission of Streptococcus pneumoniae. mBio 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Yoshida, L.M.; Suzuki, M.; Nguyen, H.A.T.; Nguyen, C.D.; Nguyen, A.T.; Oishi, K.; Yamamoto, T.; Watanabe, K.; Vu, T.D. Association between Nasopharyngeal Load of Streptococcus pneumoniae, Viral Coinfection, and Radiologically Confirmed Pneumonia in Vietnamese Children. Pediatr. Infect. Dis. J. 2011, 30, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.; Bont, L.; Siezen, C.L.; Hodemaekers, H.M.; Ermers, M.J.; Doornbos, G.; van’t Slot, R.; Wijmenga, C.; Goeman, J.J.; Kimpen, J.L.; et al. Genetic Susceptibility to Respiratory Syncytial Virus Bronchiolitis Is Predominantly Associated with Innate Immune Genes. J. Infect. Dis. 2007, 196, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Duttweiler, L. Pulmonary and systemic bacterial co-infections in severe RSV bronchiolitis. Arch. Dis. Child. 2004, 89, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, K.; Harigopal, S.; Reddy, V.; Taylor, N.; van Saene, H.K. High incidence of pulmonary bacterial co-infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax 2006, 61, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Hishiki, H.; Ishiwada, N.; Fukasawa, C.; Abe, K.; Hoshino, T.; Aizawa, J.; Ishikawa, N.; Kohno, Y. Incidence of bacterial coinfection with respiratory syncytial virus bronchopulmonary infection in pediatric inpatients. J. Infect. Chemother. 2011, 17, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.M.; Stark, M.A.; Colasurdo, G.N.; LeVine, A.M. Decreased bacterial clearance from the lungs of mice following primary respiratory syncytial virus infection. J. Med. Virol. 2006, 78, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Stensballe Lone, G.; Hjuler, T.; Andersen, A.; Kaltoft, M.; Ravn, H.; Aaby, P.; Simoes, E.A. Hospitalization for Respiratory Syncytial Virus Infection and Invasive Pneumococcal Disease in Danish Children Aged <2 Years: A Population-Based Cohort Study. Clin. Infect. Dis. 2008, 46, 1165–1171. [Google Scholar]
- Madhi, S.A.; Klugman, K.P. The Vaccine Trialist G. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat. Med. 2004, 10, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, K.; Bender, J.; Sheng, X.; Korgenski, K.; Daly, J.; Pavia, A.T.; Byington, C.L. Seasonal invasive pneumococcal disease in children: Role of preceding respiratory viral infection. Pediatrics 2008, 122, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Haeberle, H.A.; Takizawa, R.; Casola, A.; Brasier, A.R.; Dieterich, H.J.; Van Rooijen, N.; Gatalica, Z.; Garofalo, R.P. Respiratory syncytial virus-induced activation of nuclear factor-κB in the lung involves alveolar macrophages and toll-like receptor 4-dependent pathways. J. Infect. Dis. 2002, 186, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.L.; Brewah, Y.A.; Delaney, T.; Welliver, T.; Burwell, T.; Burwell, T.; Benjamin, E.; Kuta, E.; Kozhich, A.; McKinney, L.; Suzich, J.; et al. Macrophage impairment underlies airway occlusion in primary respiratory syncytial virus bronchiolitis. J. Infect. Dis. 2008, 198, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Ravi, L.I.; Li, L.; Sutejo, R.; Chen, H.; Wong, P.S.; Tan, B.H.; Sugrue, R.J. A systems-based approach to analyse the host response in murine lung macrophages challenged with respiratory syncytial virus. BMC Genom. 2013, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Pribul, P.K.; Harker, J.; Wang, B.; Wang, H.; Tregoning, J.S.; Schwarze, J.; Openshaw, P.J. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol. 2008, 82, 4441–4448. [Google Scholar] [CrossRef] [PubMed]
- Benoit, A.; Huang, Y.; Proctor, J.; Rowden, G.; Anderson, R. Effects of alveolar macrophage depletion on liposomal vaccine protection against respiratory syncytial virus (RSV). Clin. Exp. Immunol. 2006, 145, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Franke-Ullmann, G.; Pfortner, C.; Walter, P.; Steinmuller, C.; Lohmann-Matthes, M.L.; Kobzik, L.; Freihorst, J. Alteration of pulmonary macrophage function by respiratory syncytial virus infection in vitro. J. Immunol. 1995, 154, 268–280. [Google Scholar] [PubMed]
- Kolli, D.; Gupta, M.R.; Sbrana, E.; Velayutham, T.S.; Chao, H.; Casola, A.; Garofalo, R.P. Alveolar macrophages contribute to the pathogenesis of human metapneumovirus infection while protecting against respiratory syncytial virus infection. Am. J. Respir. Cell Mol. Biol. 2014, 51, 502–515. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.S.; Flanagan, B.F.; Hart, C.A.; Smyth, R.L. Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J. Infect. Dis. 2005, 191, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Panuska, J.R.; Cirino, N.M.; Midulla, F.; Despot, J.E.; McFadden, E.R., Jr.; Huang, Y.T. Productive infection of isolated human alveolar macrophages by respiratory syncytial virus. J. Clin. Investig. 1990, 86, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Midulla, F.; Villani, A.; Panuska, J.R.; Dab, I.; Kolls, J.K.; Merolla, R.; Ronchetti, R. Respiratory syncytial virus lung infection in infants: Immunoregulatory role of infected alveolar macrophages. J. Infect. Dis. 1993, 168, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Quay, J.; Soukup, J. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J. Immunol. 1991, 147, 4307–4312. [Google Scholar] [PubMed]
- Becker, S.; Soukup, J.; Yankaskas, J.R. Respiratory syncytial virus infection of human primary nasal and bronchial epithelial cell cultures and bronchoalveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1992, 6, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Cirino, N.M.; Panuska, J.R.; Villani, A.; Taraf, H.; Rebert, N.A.; Merolla, R.; Tsivitse, P.; Gilbert, I.A. Restricted replication of respiratory syncytial virus in human alveolar macrophages. J. Gen. Virol. 1993, 74 Pt 8, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Zlateva, K.T.; Vijgen, L.; Dekeersmaeker, N.; Naranjo, C.; Van Ranst, M. Subgroup prevalence and genotype circulation patterns of human respiratory syncytial virus in Belgium during ten successive epidemic seasons. J. Clin. Microbiol. 2007, 45, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Terrier, O.; Carron, C.; De Chassey, B.; Dubois, J.; Traversier, A.; Julien, T.; Cartet, G.; Proust, A.; Hacot, S.; Ressnikoff, D.; et al. Nucleolin interacts with influenza A nucleoprotein and contributes to viral ribonucleoprotein complexes nuclear trafficking and efficient influenza viral replication. Sci. Rep. 2016, 6, 29006. [Google Scholar] [CrossRef] [PubMed]
- Levitz, R.; Gao, Y.; Dozmorov, I.; Song, R.; Wakeland, E.K.; Kahn, J.S. Distinct patterns of innate immune activation by clinical isolates of respiratory syncytial virus. PLoS ONE 2017, 12, e0184318. [Google Scholar] [CrossRef] [PubMed]
- Lindell, D.M.; Lane, T.E.; Lukacs, N.W. CXCL10/CXCR3-mediated responses promote immunity to respiratory syncytial virus infection by augmenting dendritic cell and CD8(+) T cell efficacy. Eur. J. Immunol. 2008, 38, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- De Steenhuijsen Piters, W.A.; Heinonen, S.; Hasrat, R.; Bunsow, E.; Smith, B.; Suarez-Arrabal, M.C.; Chaussabel, D.; Cohen, D.M.; Sanders, E.A.; Ramilo, O.; et al. Nasopharyngeal Microbiota, Host Transcriptome, and Disease Severity in Children with Respiratory Syncytial Virus Infection. Am. J. Respir. Crit. Care Med. 2016, 194, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.; Schreurs, I.; Jans, J.; Heldens, J.; de Groot, R.; de Jonge, M.I.; Ferwerda, G. Antibodies enhance CXCL10 production during RSV infection of infant and adult immune cells. Cytokine 2015, 76, 458–464. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, D.; Hoffmann, J.; Moroso, M.; Rosa-Calatrava, M.; Endtz, H.; Terrier, O.; Paranhos-Baccalà, G. RSV Infection in Human Macrophages Promotes CXCL10/IP-10 Expression during Bacterial Co-Infection. Int. J. Mol. Sci. 2017, 18, 2654. https://doi.org/10.3390/ijms18122654
Machado D, Hoffmann J, Moroso M, Rosa-Calatrava M, Endtz H, Terrier O, Paranhos-Baccalà G. RSV Infection in Human Macrophages Promotes CXCL10/IP-10 Expression during Bacterial Co-Infection. International Journal of Molecular Sciences. 2017; 18(12):2654. https://doi.org/10.3390/ijms18122654
Chicago/Turabian StyleMachado, Daniela, Jonathan Hoffmann, Marie Moroso, Manuel Rosa-Calatrava, Hubert Endtz, Olivier Terrier, and Glaucia Paranhos-Baccalà. 2017. "RSV Infection in Human Macrophages Promotes CXCL10/IP-10 Expression during Bacterial Co-Infection" International Journal of Molecular Sciences 18, no. 12: 2654. https://doi.org/10.3390/ijms18122654
APA StyleMachado, D., Hoffmann, J., Moroso, M., Rosa-Calatrava, M., Endtz, H., Terrier, O., & Paranhos-Baccalà, G. (2017). RSV Infection in Human Macrophages Promotes CXCL10/IP-10 Expression during Bacterial Co-Infection. International Journal of Molecular Sciences, 18(12), 2654. https://doi.org/10.3390/ijms18122654