Elevation of Serum APE1/Ref-1 in Experimental Murine Myocarditis
Abstract
:1. Introduction
2. Results
2.1. Changes of Serum Troponin I, NT-proBNP and APE1/Ref-1 over Time in Mice with Acute Viral Myocarditis
2.2. Changes of Heart Virus Titers over Time in Mice with Acute Viral Myocarditis
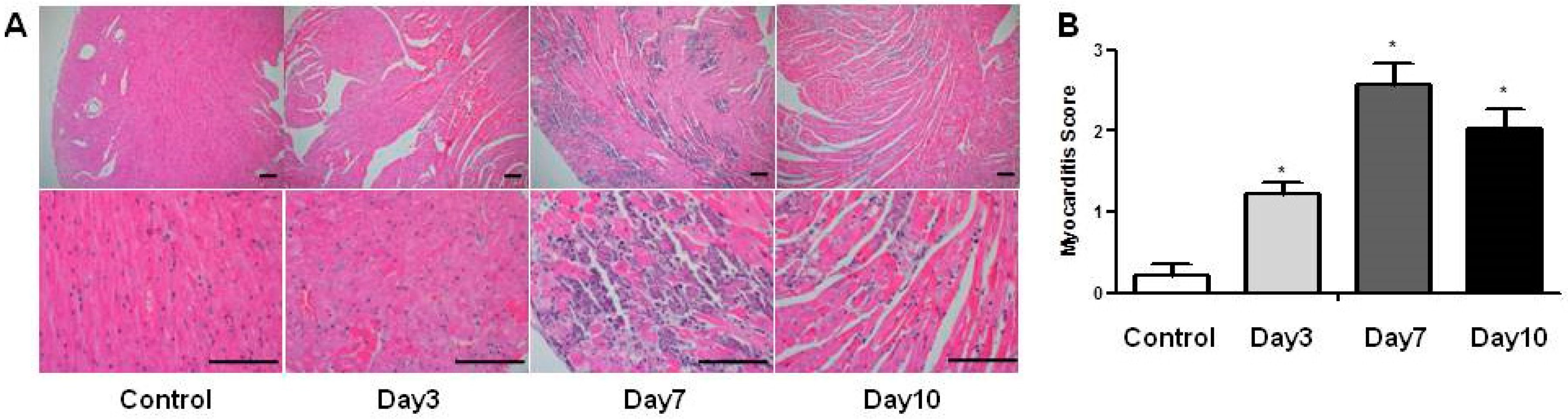
2.3. Changes of Histopathological Findings over Time in Mice with Acute Viral Myocarditis
2.4. Relationship of Serum Troponin I, NT-proBNP, APE1/Ref-1, Viral Titers and Myocardial Inflammation
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Viral Myocarditis Model
4.3. Enzyme-Linked Immunosorbent Assay (ELISA) for the Serum Levels of Troponin I, NT-proBNP and APE1/Ref-1
4.4. Organ Virus Titers
4.5. Histopathology
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APE1/Ref-1 | Apurinic/apyrimidinic endonuclease 1/redox effector factor-1 |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| EMB | Endomyocardial biopsy |
| CVB3 | Coxsackievirus B3 |
| PFU | Plaque-forming units |
| ELISA | Enzyme-Linked Immunosorbent Assay |
References
- Cooper, L.T., Jr. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Dennert, R.; Crijns, H.J.; Heymans, S. Acute viral myocarditis. Eur. Heart J. 2008, 29, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Magnani, J.W.; Dec, G.W. Myocarditis: Current trends in diagnosis and treatment. Circulation 2006, 113, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.Z.; Molkara, D.P.; Daniels, L.B.; Tremoulet, A.H.; Shimizu, C.; Kanegaye, J.T.; Best, B.M.; Snider, J.V.; Frazer, J.R.; Maisel, A.; et al. Cardiovascular biomarkers in acute Kawasaki disease. Int. J. Cardiol. 2013, 164, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Ukena, C.; Kindermann, M.; Mahfoud, F.; Geisel, J.; Lepper, P.M.; Kandolf, R.; Bohm, M.; Kindermann, I. Diagnostic and prognostic validity of different biomarkers in patients with suspected myocarditis. Clin. Res. Cardiol. 2014, 103, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Greaves, K.; Oxford, J.S.; Price, C.P.; Clarke, G.H.; Crake, T. The prevalence of myocarditis and skeletal muscle injury during acute viral infection in adults: Measurement of cardiac troponins I and T in 152 patients with acute influenza infection. Arch. Int. Med. 2003, 163, 165–168. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Helio, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C.; Ladenson, J.H.; Mason, J.W.; Jaffe, A.S. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997, 95, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Huber, S. Cellular autoimmunity in myocarditis. Heart Fail. Clin. 2005, 1, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.A.; Burek, C.L.; Baughman, K.L.; Rose, N.R.; Herskowitz, A. Circulating heart-reactive antibodies in patients with myocarditis or cardiomyopathy. J. Am. Coll. Cardiol. 1990, 16, 839–846. [Google Scholar] [CrossRef]
- Jeon, B.H.; Gupta, G.; Park, Y.C.; Qi, B.; Haile, A.; Khanday, F.A.; Liu, Y.X.; Kim, J.M.; Ozaki, M.; White, A.R.; et al. Apurinic/apyrimidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ. Res. 2004, 95, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.A.; Seo, H.J.; Kim, S.K.; Lee, Y.R.; Choi, S.; Ahn, K.T.; Kim, J.H.; Park, J.H.; Lee, J.H.; Choi, S.W.; et al. Elevation of the Serum Apurinic/Apyrimidinic Endonuclease 1/Redox Factor-1 in Coronary Artery Disease. Korean Circ. J. 2015, 45, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, E.O.; Lee, Y.R.; Joo, H.K.; Park, M.S.; Kim, C.S.; Choi, S.; Jeong, J.O.; Jeon, B.H. APE1/Ref-1 Inhibits Phosphate-Induced Calcification and Osteoblastic Phenotype Changes in Vascular Smooth Muscle Cells. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, T.; Nakayama, T.; Sato, N.; Fu, Z.; Soma, M.; Yamaguchi, M.; Shimodaira, M.; Aoi, N.; Usami, R. Haplotype-based case-control study on human apurinic/apyrimidinic endonuclease 1/redox effector factor-1 gene and essential hypertension. Am. J. Hypertens. 2010, 23, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Sarkar, B.; Cholia, R.P.; Gautam, N.; Dhiman, M.; Mantha, A.K. APE1/Ref-1 as an emerging therapeutic target for various human diseases: Phytochemical modulation of its functions. Exp. Mol. Med. 2014, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Xiang, D.; Wang, D.; Xin, X. Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE1/Ref-1) in human ovarian cancer and indentification of the therapeutic potential of APE1/Ref-1 inhibitor. Int. J. Oncol. 2009, 35, 1069–1079. [Google Scholar] [PubMed]
- Choi, S.; Joo, H.K.; Jeon, B.H. Dynamic Regulation of APE1/Ref-1 as a Therapeutic Target Protein. Chonnam Med. J. 2016, 52, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Cao, X.J.; Li, M.X.; Qing, Y.; Liao, L.; Lu, X.F.; Zhang, S.H.; Li, Z.; Yang, Y.X.; Wang, D. Serum APE1 autoantibodies: A novel potential tumor marker and predictor of chemotherapeutic efficacy in non-small cell lung cancer. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Choi, S.; Lee, Y.R.; Park, M.S.; Na, Y.G.; Irani, K.; Lee, S.D.; Park, J.B.; Kim, J.M.; Lim, J.S.; et al. APE1/Ref-1 as a Serological Biomarker for the Detection of Bladder Cancer. Cancer Res. Treat. 2015, 47, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Tell, G.; Quadrifoglio, F.; Tiribelli, C.; Kelley, M.R. The many functions of APE1/Ref-1: Not only a DNA repair enzyme. Antioxid. Redox Signal. 2009, 11, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Martinet, W.; Knaapen, M.W.; de Meyer, G.R.; Herman, A.G.; Kockx, M.M. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation 2002, 106, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, Y.R.; Park, M.S.; Joo, H.K.; Cho, E.J.; Kim, H.S.; Kim, C.S.; Park, J.B.; Irani, K.; Jeon, B.H. Histone deacetylases inhibitor trichostatin A modulates the extracellular release of APE1/Ref-1. Biochem. Biophys. Res. Commun. 2013, 435, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Lee, Y.R.; Choi, S.; Joo, H.K.; Cho, E.J.; Kim, C.S.; Park, J.B.; Jo, E.K.; Jeon, B.H. Identification of plasma APE1/Ref-1 in lipopolysaccharide-induced endotoxemic rats: Implication of serological biomarker for an endotoxemia. Biochem. Biophys. Res. Commun. 2013, 435, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Lim, C.S.; Byun, H.S.; Cho, H.S.; Lee, Y.R.; Shin, Y.S.; Kim, H.W.; Jeon, B.H.; Kim, D.W.; Hong, J.; et al. The anti-inflammatory role of extranuclear apurinic/apyrimidinic endonuclease 1/redox effector factor-1 in reactive astrocytes. Mol. Brain 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Choi, S.; Lee, Y.R.; Joo, H.K.; Kang, G.; Kim, C.S.; Kim, S.J.; Lee, S.D.; Jeon, B.H. Secreted APE1/Ref-1 inhibits TNF-alpha-stimulated endothelial inflammation via thiol-disulfide exchange in TNF receptor. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Heymans, S. Myocarditis and heart failure: Need for better diagnostic, predictive, and therapeutic tools. Eur. Heart J. 2007, 28, 1279–1280. [Google Scholar] [CrossRef] [PubMed]
- Kindermann, I.; Barth, C.; Mahfoud, F.; Ukena, C.; Lenski, M.; Yilmaz, A.; Klingel, K.; Kandolf, R.; Sechtem, U.; Cooper, L.T.; et al. Update on myocarditis. J. Am. Coll. Cardiol. 2012, 59, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Henke, A.; Huber, S.; Stelzner, A.; Whitton, J.L. The role of CD8+T lymphocytes in coxsackievirus B3-induced myocarditis. J. Virol. 1995, 69, 6720–6728. [Google Scholar] [PubMed]
- Leipner, C.; Borchers, M.; Merkle, I.; Stelzner, A. Coxsackievirus B3-induced myocarditis in MHC class II-deficient mice. J. Hum. Virol. 1999, 2, 102–114. [Google Scholar] [PubMed]
- Shioi, T.; Matsumori, A.; Sasayama, S. Persistent expression of cytokine in the chronic stage of viral myocarditis in mice. Circulation 1996, 94, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.K.; Choe, S.C.; Shin, J.O.; Ho, S.H.; Kim, J.M.; Yu, S.S.; Kim, S.; Jeon, E.S. Local expression of interleukin-1 receptor antagonist by plasmid DNA improves mortality and decreases myocardial inflammation in experimental coxsackieviral myocarditis. Circulation 2002, 105, 1278–1281. [Google Scholar] [PubMed]
- Yun, S.H.; Lee, W.G.; Kim, Y.C.; Ju, E.S.; Lim, B.K.; Choi, J.O.; Kim, D.K.; Jeon, E.S. Antiviral activity of coxsackievirus B3 3C protease inhibitor in experimental murine myocarditis. J. Infect. Dis. 2012, 205, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, K.U.; Jeon, E.S.; Berkley, N.; Wessely, R.; Huber, S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J. Virol. 1996, 70, 7811–7818. [Google Scholar] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.-A.; Lim, B.-K.; Seo, H.J.; Kim, S.K.; Ahn, K.T.; Jeon, B.H.; Jeong, J.-O. Elevation of Serum APE1/Ref-1 in Experimental Murine Myocarditis. Int. J. Mol. Sci. 2017, 18, 2664. https://doi.org/10.3390/ijms18122664
Jin S-A, Lim B-K, Seo HJ, Kim SK, Ahn KT, Jeon BH, Jeong J-O. Elevation of Serum APE1/Ref-1 in Experimental Murine Myocarditis. International Journal of Molecular Sciences. 2017; 18(12):2664. https://doi.org/10.3390/ijms18122664
Chicago/Turabian StyleJin, Seon-Ah, Byung-Kwan Lim, Hee Jung Seo, Sun Kyeong Kim, Kye Taek Ahn, Byeong Hwa Jeon, and Jin-Ok Jeong. 2017. "Elevation of Serum APE1/Ref-1 in Experimental Murine Myocarditis" International Journal of Molecular Sciences 18, no. 12: 2664. https://doi.org/10.3390/ijms18122664
APA StyleJin, S.-A., Lim, B.-K., Seo, H. J., Kim, S. K., Ahn, K. T., Jeon, B. H., & Jeong, J.-O. (2017). Elevation of Serum APE1/Ref-1 in Experimental Murine Myocarditis. International Journal of Molecular Sciences, 18(12), 2664. https://doi.org/10.3390/ijms18122664





