Secretion of IFN-γ Associated with Galectin-9 Production by Pleural Fluid Cells from a Patient with Extrapulmonary Tuberculosis
Abstract
:1. Introduction
2. Results
2.1. Immunological Profiling of Plasma and Pleural Fluid in Extrapulmonary Tuberculosis
2.2. Immunohistochemical Findings
2.3. Difference in Interferon-γ (IFN-γ)-Producing Cells between Peripheral Blood Mononuclear Cells (PBMCs) and Pleural Fluid Cells (PFCs)
2.4. Frequency of Gal-9 and Tim3 Positive T Cells in PFCs and PBMCs
2.5. Gal-9 Effects on PFCs Not Stimulated with TB Antigens
2.6. Gal-9 Effects on PFCs Stimulated with TB Antigens
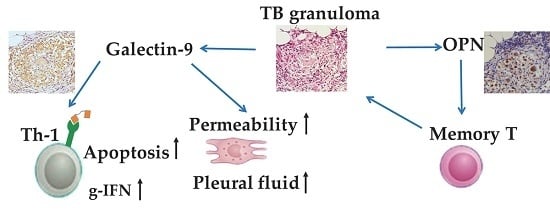
3. Discussion
4. Materials and Methods
4.1. Patient and Sample Collection
4.2. Enzyme-Linked Immunosorbent Assay and Luminex
4.3. Immunohistochemistry
4.4. Enzyme-Linked ImmunoSpot
4.5. Fluorescence-Activated Cell Sorting
4.6. Effect of Gal-9 on IFN-γ Production
4.7. Necrosis and Apoptosis
4.8. Data Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Hattori, T.; Kobayashi, N.; Nagai, H.; Chagan-Yasutan, H.; Telan, E.; Solante, M.B. Nationwide hiv-, mdr-tb survey in Japan and collaborative study in the philippines. Int. J. Mycobacteriol. 2016, 5, S18–S19. [Google Scholar] [CrossRef] [PubMed]
- Berger, H.W.; Mejia, E. Tuberculous pleurisy. Chest 1973, 63, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Wongtim, S.; Silachamroon, U.; Ruxrungtham, K.; Udompanich, V.; Limthongkul, S.; Charoenlap, P.; Nuchprayoon, C. Interferon gamma for diagnosing tuberculous pleural effusions. Thorax 1999, 54, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Sester, M.; Sotgiu, G.; Lange, C.; Giehl, C.; Girardi, E.; Migliori, G.B.; Bossink, A.; Dheda, K.; Diel, R.; Dominguez, J.; et al. Interferon-gamma release assays for the diagnosis of active tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2011, 37, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Psatha, A.; Makris, D.; Kerenidi, T.; Daniil, Z.; Kiropoulos, T.; Gourgoulianis, K. A potential role for vegf in the diagnostic approach of pleural effusions. J. Thorac. Dis. 2016, 8, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Giannou, A.D.; Marazioti, A.; Spella, M.; Kanellakis, N.I.; Apostolopoulou, H.; Psallidas, I.; Prijovich, Z.M.; Vreka, M.; Zazara, D.E.; Lilis, I.; et al. Mast cells mediate malignant pleural effusion formation. J. Clin. Investig. 2015, 125, 2317–2334. [Google Scholar] [CrossRef] [PubMed]
- Carlos, D.; de Souza Junior, D.A.; de Paula, L.; Jamur, M.C.; Oliver, C.; Ramos, S.G.; Silva, C.L.; Faccioli, L.H. Mast cells modulate pulmonary acute inflammation and host defense in a murine model of tuberculosis. J. Infect. Dis. 2007, 196, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Nieminen, J. Seeing strangers or announcing “danger”: Galectin-3 in two models of innate immunity. Glycoconj. J. 2004, 19, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Chagan-Yasutan, H.; Shiratori, B.; Siddiqi, U.R.; Saitoh, H.; Ashino, Y.; Arikawa, T.; Hirashima, M.; Hattori, T. The increase of plasma galectin-9 in a patient with insulin allergy: A case report. Clin. Mol. Allergy CMA 2010, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Chagan-Yasutan, H.; Ndhlovu, L.C.; Lacuesta, T.L.; Kubo, T.; Leano, P.S.; Niki, T.; Oguma, S.; Morita, K.; Chew, G.M.; Barbour, J.D.; et al. Galectin-9 plasma levels reflect adverse hematological and immunological features in acute dengue virus infection. J. Clin. Virol. 2013, 58, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, B.; Zhao, J.; Okumura, M.; Chagan-Yasutan, H.; Yanai, H.; Mizuno, K.; Yoshiyama, T.; Idei, T.; Ashino, Y.; Nakajima, C.; et al. Immunological roles of elevated plasma levels of matricellular proteins in japanese patients with pulmonary tuberculosis. Int. J. Mol. Sci. 2016, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.J.L.; Poirier, F.; Baum, L.G.; Griffioen, A.W. Galectins in the tumor endothelium: Opportunities for combined cancer therapy. Blood 2007, 110, 2819–2827. [Google Scholar] [CrossRef] [PubMed]
- Grigorian, A.; Torossian, S.; Demetriou, M. T-cell growth, cell surface organization, and the galectin-glycoprotein lattice. Immunol. Rev. 2009, 230, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.B.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates t helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.J.M.; Wiersma, V.R.; Samplonius, D.F.; Gerssen, J.; van Ginkel, R.J.; Nijman, H.W.; Hirashima, M.; Niki, T.; Eggleton, P.; Helfrich, W.; et al. Galectin-9 activates and expands human T-helper 1 cells. PLoS ONE 2013, 8, e65616. [Google Scholar] [CrossRef] [PubMed]
- Sada-Ovalle, I.; Chavez-Galan, L.; Torre-Bouscoulet, L.; Nava-Gamino, L.; Barrera, L.; Jayaraman, P.; Torres-Rojas, M.; Salazar-Lezama, M.A.; Behar, S.M. The Tim3-galectin 9 pathway induces antibacterial activity in human macrophages infected with mycobacterium tuberculosis. J. Immunol. 2012, 189, 5896–5902. [Google Scholar] [CrossRef] [PubMed]
- Gleason, M.K.; Lenvik, T.R.; McCullar, V.; Felices, M.; O’Brien, M.S.; Cooley, S.A.; Verneris, M.R.; Cichocki, F.; Holman, C.J.; Panoskaltsis-Mortari, A.; et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 2012, 119, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Jean, M.D.; Brignole, F.; Feldmann, G.; Goguel, A.; Baudouin, C. Interferon-gamma induces apoptosis and expression of inflammation-related proteins in chang conjunctival cells. Investig. Ophth. Vis. Sci. 1999, 40, 2199–2212. [Google Scholar]
- Inagaki, Y.; Yamagishi, S.; Amano, S.; Okamoto, T.; Koga, K.; Makita, Z. Interferon-γ-induced apoptosis and activation of THP-1 macrophages. Life Sci. 2002, 71, 2499–2508. [Google Scholar] [CrossRef]
- Gieseke, F.; Kruchen, A.; Tzaribachev, N.; Bentzien, F.; Dominici, M.; Muller, I. Proinflammatory stimuli induce galectin-9 in human mesenchymal stromal cells to suppress T-cell proliferation. Eur. J. Immunol. 2013, 43, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, C.; Quade-Lyssy, P.; Radeke, H.H.; Henschler, R.; Konigs, C.; Kohl, U.; Seifried, E.; Schuttrumpf, J. Galectin-9 is a suppressor of t and b cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells Dev. 2014, 23, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, L.M.; Antonangelo, L.; Vargas, F.S.; Zerbini, M.C.; Sales, M.M.; Uip, D.E.; Saldiva, P.H. Malignant and tuberculous pleural effusions: Immunophenotypic cellular characterization. Clinics 2008, 63, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiao, D.; Li, Q.; Zhang, X.; Lao, S.; Wu, C. Distinct polyfunctional CD4+ T cell responses to BCG, ESAT-6 and CFP-10 in tuberculous pleurisy. Tuberculosis 2012, 92, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yang, B.; Lao, S.; Fan, Y.; Wu, C. Human memory-like NK cells migrating to tuberculous pleural fluid via IP-10/CXCR3 and SDF-1/CXCR4 axis produce ifn-gamma in response to bacille calmette guerin. Clin. Immunol. 2013, 148, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yu, S.; Yang, B.; Lao, S.; Li, B.; Wu, C. Memory-like antigen-specific human NK cells from TB pleural fluids produced IL-22 in response to IL-15 or mycobacterium tuberculosis antigens. PLoS ONE 2016, 11, e0151721. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, S.; Schaible, U.E. The granuloma in tuberculosis: Dynamics of a host-pathogen collusion. Front. Immunol. 2012, 3, 411. [Google Scholar] [CrossRef] [PubMed]
- Vega-Carrascal, I.; Reeves, E.P.; Niki, T.; Arikawa, T.; McNally, P.; O’Neill, S.J.; Hirashima, M.; McElvaney, N.G. Dysregulation of Tim-3-galectin-9 pathway in the cystic fibrosis airways. J. Immunol. 2011, 186, 2897–2909. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef] [PubMed]
- Hasibuan, F.M.; Shiratori, B.; Senoputra, M.A.; Chagan-Yasutan, H.; Koesoemadinata, R.C.; Apriani, L.; Takahashi, Y.; Niki, T.; Alisjahbana, B.; Hattori, T. Evaluation of matricellular proteins in systemic and local immune response to mycobacterium tuberculosis infection. Microbiol. Immunol. 2015, 59, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 2007, 5, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Deveci, F.; Akbulut, H.H.; Turgut, T.; Muz, M.H. Changes in serum cytokine levels in active tuberculosis with treatment. Med. Inflamm. 2005, 2005, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Mundt, F.; Johansson, H.J.; Forshed, J.; Arslan, S.; Metintas, M.; Dobra, K.; Lehtio, J.; Hjerpe, A. Proteome screening of pleural effusions identifies galectin 1 as a diagnostic biomarker and highlights several prognostic biomarkers for malignant mesothelioma. Mol. Cell. Proteom. 2014, 13, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Blanquart, C.; Gueugnon, F.; Nguyen, J.M.; Roulois, D.; Cellerin, L.; Sagan, C.; Perigaud, C.; Scherpereel, A.; Gregoire, M. CCL2, galectin-3, and SMRP combination improves the diagnosis of mesothelioma in pleural effusions. J. Thorac. Oncol. 2012, 7, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Sack, U.; Hoffmann, M.; Zhao, X.J.; Chan, K.S.; Hui, D.S.; Gosse, H.; Engelmann, L.; Schauer, J.; Emmrich, F.; Hoheisel, G. Vascular endothelial growth factor in pleural effusions of different origin. Eur. Respir. J. 2005, 25, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.W.; Dutta, A.; Chang, L.Y.; Mahalingam, J.; Lin, Y.C.; Chiang, J.M.; Hsu, C.Y.; Huang, C.T.; Su, W.T.; Chu, Y.Y.; et al. Apoptosis of tumor infiltrating effector Tim-3+CD8+ T cells in colon cancer. Sci. Rep. 2015, 5, 15659. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Kumagai, M.; Sasaki, N.; Kurotaki, H.; Mori, F.; Seki, M.; Nishi, N.; Fujimoto, K.; Tanji, K.; Shibata, T.; et al. Interferon-gamma stimulates the expression of galectin-9 in cultured human endothelial cells. J. Leukoc. Biol. 2002, 72, 486–491. [Google Scholar] [PubMed]
- Brooks, A.K.; Lawson, M.A.; Rytych, J.L.; Yu, K.C.; Janda, T.M.; Steelman, A.J.; McCusker, R.H. Immunomodulatory factors galectin-9 and interferon-gamma synergize to induce expression of rate-limiting enzymes of the kynurenine pathway in the mouse hippocampus. Front. Immunol. 2016, 7, 422. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; McKinstry, K.K.; Swain, S.L.; Dalton, D.K. Ifn-gamma acts directly on activated CD4+ T cells during mycobacterial infection to promote apoptosis by inducing components of the intracellular apoptosis machinery and by inducing extracellular proapoptotic signals. J. Immunol. 2007, 179, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Todryk, S.M.; Pathan, A.A.; Keating, S.; Porter, D.W.; Berthoud, T.; Thompson, F.; Klenerman, P.; Hill, A.V. The relationship between human effector and memory T cells measured by ex vivo and cultured elispot following recent and distal priming. Immunology 2009, 128, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Y.; Lao, S.; Yang, B.; Yu, S.; Zhang, Y.; Wu, C. Mycobacterium tuberculosis-specific IL-21+IFN-γ+CD4+ T cells are regulated by IL-12. PLoS ONE 2016, 11, e0147356. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-γ during innate and adaptive immune responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar] [PubMed]
- Li, H.; Wu, K.; Tao, K.; Chen, L.; Zheng, Q.; Lu, X.; Liu, J.; Shi, L.; Liu, C.; Wang, G.; et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis b virus-associated hepatocellular carcinoma. Hepatology 2012, 56, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Hastings, W.D.; Anderson, D.E.; Kassam, N.; Koguchi, K.; Greenfield, E.A.; Kent, S.C.; Zheng, X.X.; Strom, T.B.; Hafler, D.A.; Kuchroo, V.K. Tim-3 is expressed on activated human CD4+ T cells and regulates TH1 and TH17 cytokines. Eur. J. Immunol. 2009, 39, 2492–2501. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, B.; Usami, O.; Hattori, T.; Ashino, Y. A man from south asia presenting with abdominal pain. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, Z.; Zhang, X.; Suzuki, Y.; Chagan-Yasutan, H.; Chen, H.; Wan, Y.; Xu, J.; Ashino, Y.; Hattori, T. Evaluation of anti-TBGL antibody in the diagnosis of tuberculosis patients in china. J. Immunol. Res. 2015, 2015, 834749. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Sakata, K.M.; Oomizu, S.; Arikawa, T.; Sakata, A.; Ueno, M.; Nobumoto, A.; Niki, T.; Saita, N.; Ito, K.; et al. Beneficial effect of galectin 9 on rheumatoid arthritis by induction of apoptosis of synovial fibroblasts. Arthr. Rheum. 2007, 56, 3968–3976. [Google Scholar] [CrossRef] [PubMed]
- Chagan-Yasutan, H.; Tsukasaki, K.; Takahashi, Y.; Oguma, S.; Harigae, H.; Ishii, N.; Zhang, J.; Fukumoto, M.; Hattori, T. Involvement of osteopontin and its signaling molecule CD44 in clinicopathological features of adult T cell leukemia. Leuk. Res. 2011, 35, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Holdenrieder, S.; Stieber, P.; Bodenmuller, H.; Busch, M.; Fertig, G.; Furst, H.; Schalhorn, A.; Schmeller, N.; Untch, M.; Seidel, D. Nucleosomes in serum of patients with benign and malignant diseases. Int. J. Cancer 2001, 95, 114–120. [Google Scholar] [CrossRef]







| Parameters | Pleural Fluid | Plasma |
|---|---|---|
| OPN (ng/mL) | 95 | 37 |
| Gal-9 (pg/mL) | 936 | 3 |
| IFN-γ (pg/mL) | 55.5 | 1.6 |
| IL-10 (pg/mL) | 179.9 | 4.2 |
| IL-12 (pg/mL) | 4.0 | 0 |
| VEGF (pg/mL) | 55.0 | 11.3 |
| CRP (mg/dL) | n.a | 4.3 |
| Amylase (IU/L) | 48 | 70 |
| SAA (μg/mL) | n.a | 18.7 |
| Total protein (mg/dL) | 5.5 | 7.7 |
| Glucose (mg/dL) | 83 | 110 |
| LDH (U/L) | 153 | 217 |
| ADA (U/L) | 46.9 | n.a. |
| Cell count per μL | 2450 | n.a. |
| Lymphocytes (%) | 85.7 | 18 |
| Anti-TBGL-IgG (U/mL) | 6.1 | 3.9 |
| Anti-TBGL-IgA (U/mL) | 0.4 | 0.3 |
| Antigen | Label | Sequence |
|---|---|---|
| ESAT-6 | 1–20 | H-MTEQQWNFAGIEAAASAIQG-EEEKKK-OH |
| 11–30 | H-IEAAASAIQGNVTSIHSLLD-EEEKKK-OH | |
| 21–40 | H-NVTSIHSLLDEGKQSLTKLA-EEEKKK-OH | |
| 31–50 | H-EGKQSLTKLAAAWGGSGSEA-EEEKKK-OH | |
| 41–60 | H-AAWGGSGSEAYQGVQQKWDA-EEEKKK-OH | |
| 51–70 | H-YQGVQQKWDATATELNNALQ-EEEKKK-OH | |
| 61–80 | H-TATELNNALQNLARTISEAG-EEEKKK-OH | |
| 71–90 | H-NLARTISEAGQAMASTEGNV-EEEKKK-OH | |
| 76–95 | H-ISEAGQAMASTEGNVTGMFA-EEEKKK-OH | |
| CFP-10 | 1–20 | H-MAEMKTDAATLAQEAGNFER-EEEKKK-OH |
| 11–30 | H-LAQEAGNFERISGDLKTQID-EEEKKK-OH | |
| 21–40 | H-ISGDLKTQIDQVESTAGSLQ-EEEKKK-OH | |
| 31–50 | H-QVESTAGSLQGQWRGAAGTA-EEEKKK-OH | |
| 41–60 | H-GQWRGAAGTAAQAAVVRFQE-EEEKKK-OH | |
| 51–70 | H-AQAAVVRFQEAANKQKQELD-EEEKKK-OH | |
| 61–80 | H-AANKQKQELDEISTNIRQAG-EEEKKK-OH | |
| 71–90 | H-EISTNIRQAGVQYSRADEEQ-EEEKKK-OH | |
| 81–100 | H-VQYSRADEEQQQALSSQMGF-EEEKKK-OH |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Shiratori, B.; Chagan-Yasutan, H.; Matsumoto, M.; Niki, T.; Tanaka, M.; Takahashi, Y.; Usami, O.; Ashino, Y.; Hattori, T. Secretion of IFN-γ Associated with Galectin-9 Production by Pleural Fluid Cells from a Patient with Extrapulmonary Tuberculosis. Int. J. Mol. Sci. 2017, 18, 1382. https://doi.org/10.3390/ijms18071382
Zhao J, Shiratori B, Chagan-Yasutan H, Matsumoto M, Niki T, Tanaka M, Takahashi Y, Usami O, Ashino Y, Hattori T. Secretion of IFN-γ Associated with Galectin-9 Production by Pleural Fluid Cells from a Patient with Extrapulmonary Tuberculosis. International Journal of Molecular Sciences. 2017; 18(7):1382. https://doi.org/10.3390/ijms18071382
Chicago/Turabian StyleZhao, Jingge, Beata Shiratori, Haorile Chagan-Yasutan, Makoto Matsumoto, Toshiro Niki, Michinori Tanaka, Yayoi Takahashi, Osumu Usami, Yugo Ashino, and Toshio Hattori. 2017. "Secretion of IFN-γ Associated with Galectin-9 Production by Pleural Fluid Cells from a Patient with Extrapulmonary Tuberculosis" International Journal of Molecular Sciences 18, no. 7: 1382. https://doi.org/10.3390/ijms18071382
APA StyleZhao, J., Shiratori, B., Chagan-Yasutan, H., Matsumoto, M., Niki, T., Tanaka, M., Takahashi, Y., Usami, O., Ashino, Y., & Hattori, T. (2017). Secretion of IFN-γ Associated with Galectin-9 Production by Pleural Fluid Cells from a Patient with Extrapulmonary Tuberculosis. International Journal of Molecular Sciences, 18(7), 1382. https://doi.org/10.3390/ijms18071382