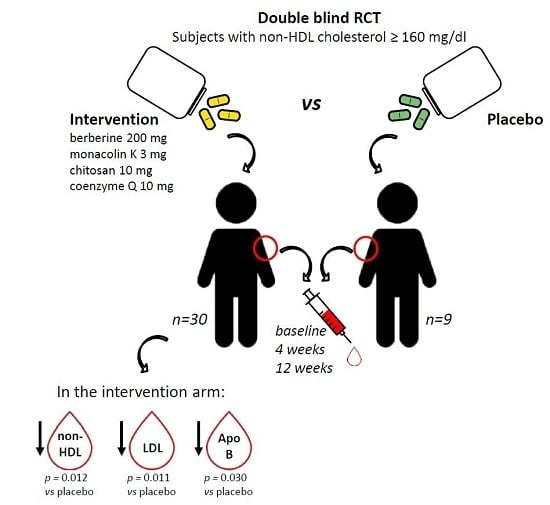
Effects of a New Nutraceutical Formulation (Berberine, Red Yeast Rice and Chitosan) on Non-HDL Cholesterol Levels in Individuals with Dyslipidemia: Results from a Randomized, Double Blind, Placebo-Controlled Study
Abstract
:1. Introduction
2. Results
2.1. Study Subjects
2.2. Effect of Treatment on Primary Endpoint
2.3. Effect of Treatment on Secondary Endpoints
2.3.1. Effect on Metabolic Parameters
2.3.2. Effect on Inflammatory Markers and Hormone Profile
2.3.3. Effects on PCSK9
2.3.4. Effects on EPC Number
2.3.5. Adverse Events and Safety Assessment
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Study Design
4.3. Coleosoma Technology
4.4. Hormones and Inflammatory Marker Assessment
4.5. PCSK9 Assessment
4.6. Quantification of Circulating Endothelial Progenitor Cells
4.7. Study Endpoints
4.8. Safety Assessments-Adverse Events
4.9. Sample Size Determination
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| APC | Allophycocyanin |
| APO | Apolipoprotein |
| BC | Berberine chloride |
| BMI | Body mass index |
| CH | Chitosan |
| CPK | Creatine phosphokinase |
| CV | Cardiovascular |
| CVD | Cardiovascular disease |
| EPC | Endothelial progenitor cell |
| FITC | Fluorescein isothiocyanate |
| FPG | Fasting plasma glucose |
| GFR | Glomerular filtration rate |
| GIP | Gastric inhibitory peptide |
| GLM | General linear model |
| GLP-1 | Glucagon-like peptide-1 |
| HbA1C | Glycated haemoglobin |
| HMG-CoA | 3-Hydroxy-3-methyl-glutaryl-CoA |
| hsCRP | High-sensitivity C-reactive protein |
| IL | Interleukin |
| IQR | Interquartile range |
| ITT | Intention to treat |
| KDR | Kinase insert domain receptor |
| LDL-C | Low-density lipoprotein cholesterol |
| MC | Monacolin |
| Non-HDL-C | Non-High Density Lipoprotein cholesterol |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| PE | Phycoerythrin |
| PS | Phosphoserine |
| RRYE | Red rice yeast extract |
| SAE | Serious adverse events |
| SD | Standard deviation |
| TNF | Tumor necrosis factor |
References
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, SL.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Wongcharoen, W.; Sutthiwutthichai, S.; Gunaparn, S.; Phrommintikul, A. Is non-HDL-cholesterol a better predictor of long-term outcome in patients after acute myocardial infarction compared to LDL-cholesterol? A retrospective study. BMC Cardiovasc. Disord. 2017, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Harari, G.; Green, M.S.; Magid, A.; Zelber-Sagi, S. Usefulness of non-high-density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men in 22-year follow-up. Am. J. Cardiol. 2017, 119, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Blumenthal, R.S.; Flaws, J.A.; Whiteman, M.K.; Langenberg, P.; Bachorik, P.S.; Bush, T.L. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch. Intern. Med. 2001, 161, 1413–1439. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sempos, C.T.; Donahue, R.P.; Dorn, J.; Trevisan, M.; Grundy, S.M. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am. J. Cardiol. 2006, 98, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, K.; Takahashi, E. Non-HDL cholesterol is a more superior predictor of small-dense LDL cholesterol than LDL cholesterol in Japanese subjects with TG levels <400 mg/dL. J. Atheroscler. Thromb. 2016, 23, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.Y. PCSK9 as a therapeutic target for cardiovascular disease. Exp. Ther. Med. 2017, 13, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.S.; Fong, L.G.; Young, S.G. PCSK9 function and physiology. J. Lipid Res. 2008, 49, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Simental-Mendía, L.E.; Guerrero-Romero, F.; Golledge, J.; Watts, G.F. Effect of statin therapy on plasma proprotein convertase subtilisin kexin 9 (PCSK9) concentrations: A systematic review and meta-analysis of clinical trials. Diabetes Obes. Metab. 2015, 17, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Jiao, R.; Ma, K.Y. Cholesterol-lowering nutraceuticals and functional foods. J. Agric. Food Chem. 2008, 56, 8761–8773. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.B.; Grundy, S.M.; Jones, P.; Law, M.; Miettinen, T.; Paoletti, R. Stresa Workshop Participants. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin. Proc. 2003, 78, 965–978. [Google Scholar] [CrossRef]
- Klimek, M.; Wang, S.; Ogunkanmi, A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. Pharm. Ther. 2009, 34, 313–327. [Google Scholar]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Momtazi, A.A.; Banach, M.; Pirro, M.; Katsiki, N.; Sahebkar, A. Regulation of PCSK9 by nutraceuticals. Pharmacol. Res. 2017, 120, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Fratter, A. New chitosan salt in gastroresistant oral formulation could interfere with enteric bile salts emulsification of diet fats: Preliminary laboratory observations and physiologic rationale. J. Med. Food 2014, 17, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Vasa, M.; Fichtlscherer, S.; Aicher, A.; Adler, K.; Urbich, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001, 89, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Rigato, M.; Avogaro, A.; Fadini, G.P. Levels of circulating progenitor cells, cardiovascular outcomes and death: A meta-analysis of prospective observational studies. Circ. Res. 2016, 118, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Reiner, Z.; Catapano, A.L.; de Backer, G.; Graham, I.; Taskinen, M.R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P.; et al. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Nissen, S.E.; Shao, M.; Elshazly, M.B.; Kataoka, Y.; Kapadia, S.R.; Tuzcu, E.M.; Nicholls, S.J. Non-HDL cholesterol and triglycerides: Implications for coronary atheroma progression and clinical events. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, N.Q.; Li, S.; Zhu, C.G.; Guo, Y.L.; Qing, P.; Gao, Y.; Li, X.L.; Liu, G.; Dong, Q.; et al. Non-HDL-C is a better predictor for the severity of coronary atherosclerosis compared with LDL-C. Heart Lung Circ. 2016, 25, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Boekholdt, S.M.; Arsenault, B.J.; Mora, S.; Pedersen, T.R.; LaRosa, J.C.; Nestel, P.J.; Simes, R.; Durrington, P.; Hitman, G.A.; Welch, K.M.; et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: A meta-analysis. JAMA 2012, 307, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- McQueen, M.J.; Hawken, S.; Wang, X.; Ounpuu, S.; Sniderman, A.; Probstfield, J.; Steyn, K.; Sanderson, J.E.; Hasani, M.; Volkova, E.; et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): A case-control study. Lancet 2008, 372, 224–233. [Google Scholar] [CrossRef]
- Parish, S.; Peto, R.; Palmer, A.; Clarke, R.; Lewington, S.; Offer, A.; Whitlock, G.; Clark, S.; Youngman, L.; Sleight, P.; et al. The joint effects of apolipoprotein B, apolipoprotein A1, LDL cholesterol, and HDL cholesterol on risk: 3510 cases of acute myocardial infarction and 9805 controls. Eur. Heart J. 2009, 30, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Walldius, G.; Aastveit, A.H.; Jungner, I. Stroke mortality and the ApoB/ApoA-I ratio: Results of the AMORIS prospective study. J. Intern. Med. 2006, 259, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.C.; Yang, Y.H.; Lin, S.J.; Tai, S.H. A systematic review and meta-analysis on the therapeutic equivalence of statins. J. Clin. Pharm. Ther. 2010, 35, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Affuso, F.; Ruvolo, A.; Micillo, F.; Saccà, L.; Fazio, S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Pirro, M.; Mannarino, M.; Bianconi, V.; Simental-Mendía, L.E.; Bagaglia, F.; Mannarino, E.; Sahebkar, A. The effects of a nutraceutical combination on plasma lipids and glucose: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 110, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Adorni, M.P.; Cipollari, E.; Favari, E.; Zanotti, I.; Zimetti, F.; Corsini, A.; Ricci, C.; Bernini, F.; Ferri, N. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis 2017, 256, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Ferri., N.; Fogacci, F.; Rosticci, M.; Botta, M.; Marchiano, S.; Magni, P.; D’Addato, S.; Giovannini, M.; Borghi, C.; et al. Circulating levels of proprotein convertase subtilisin/kexin type 9 and arterial stiffness in a large population sample: Data from the brisighella heart study. J. Am. Heart Assoc. 2017, 6, E005764. [Google Scholar] [CrossRef] [PubMed]
- Solà, R.; Valls, R.M.; Puzo, J.; Calabuig, J.R.; Brea, A.; Pedret, A.; Moriña, D.; Villar, J.; Millán, J.; Anguera, A. Effects of poly-bioactive compounds on lipid profile and body weight in a moderately hypercholesterolemic population with low cardiovascular disease risk: A multicenter randomized trial. PLoS ONE 2014, 9, e101978. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Gomaraschi, M.; Mombelli, G.; Macchi, C.; Bosisio, R.; Pazzucconi, F.; Pavanello, C.; Calabresi, L.; Arnoldi, A.; Sirtori, C.R.; et al. Nutraceutical approach to moderate cardiometabolic risk: Results of a randomized, double-blind and crossover study with Armolipid Plus. J. Clin. Lipidol. 2014, 8, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Izzo, R.; de Simone, G.; Giudice, R.; Chinali, M.; Trimarco, V.; de Luca, N.; Trimarco, B. Effects of nutraceuticals on prevalence of metabolic syndrome and on calculated Framingham Risk Score in individuals with dyslipidemia. J. Hypertens. 2010, 28, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Willerson, J.T.; Ridker, P.M. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J. Am. Coll. Cardiol. 2006, 47, C19–C31. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Ras, R.T.; Gagliardi, A.C.; Mangili, L.C.; Trautwein, E.A.; Santos, R.D. Effects of phytosterols on markers of inflammation: A systematic review and meta-analysis. Atherosclerosis 2016, 248, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Romano, D.; Maffioli, P. Effects of a combination of berberis aristata, silybum marianum and monacolin on lipid profile in subjects at low cardiovascular risk; a double-blind, randomized, placebo-controlled trial. Int. J. Mol. Sci. 2017, 18, E343. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.G.; Wang, J.M.; Chen, L.; Wang, Y.; Yang, Z.; Tao, J. Berberine-induced upregulation of circulating endothelial progenitor cells is related to nitric oxide production in healthy subjects. Cardiology 2009, 112, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.J.; Morrissette, H.; Gagné, J.M.; Bergeron, J.; Gagné, C.; Couture, P. Validation of the Friedewald formula for the determination of low-density lipoprotein cholesterol compared with β-quantification in a large population. Clin. Biochem. 2004, 37, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Aldo, P.; Marusov, G.; Svancara, D.; David, J.; Mor, G. Simple Plex™: A novel multi-analyte, automated microfluidic immunoassay platform for the detection of human and mouse cytokines and chemokines. Am. J. Reprod. Immunol. 2016, 75, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, A.; Spigoni, V.; Cito, M.; Aldigeri, R.; Ridolfi, V.; Marchesi, E.; Marina, M.; Derlindati, E.; Aloe, R.; Bonadonna, R.C.; et al. Vildagliptin, but not glibenclamide, increases circulating endothelial progenitor cell number: A 12-month randomized controlled trial in patients with type 2 diabetes. Cardiovasc. Diabetol. 2017, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Spigoni, V.; Lombardi, C.; Cito, M.; Picconi, A.; Ridolfi, V.; Andreoli, R.; Anelli, N.; Gnudi, L.; Goldoni, M.; Zavaroni, I.; et al. N-3 PUFA increase bioavailability and function of endothelial progenitor cells. Food Funct. 2014, 5, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, A.; Spigoni, V.; Ardigò, D.; Pedrazzi, G.; Franzini, L.; Derlindati, E.; Urbani, S.; Monti, L.; Gnudi, L.; Zavaroni, I. Reduced circulating endothelial progenitor cell number in healthy young adult hyperinsulinemic men. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, A.; Spigoni, V.; Franzini, L.; Preti, M.; Ardigò, D.; Derlindati, E.; Metra, M.; Monti, L.D.; Dell’Era, P.; Gnudi, L. Lower endothelial progenitor cell number, family history of cardiovascular disease and reduced HDL-cholesterol levels are associated with shorter leukocyte telomere length in healthy young adults. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Akhter, J. The American Diabetes Association’s Clinical Practice Recommendations and the developing world. Diabetes Care 1997, 20, 1044–1445. [Google Scholar] [CrossRef] [PubMed]
- Simon, R. Optimal two-stage designs for phase II clinical trials. Control Clin. Trials 1989, 10, 1–10. [Google Scholar] [CrossRef]


| Variables | Total (n = 39) | Placebo (n = 9) | Nutraceutical Intervention (n = 30) | p Value |
|---|---|---|---|---|
| Age (years) | 52 ± 11 | 52 ± 6 | 52 ± 12 | 0.9 |
| Gender n (%) | 18 M (46) 21 F (54) | 3 M (33) 6 F (67) | 15 M (50) 15 F (50) | 0.38 |
| BMI (kg/m2) | 27 ± 4 | 25.8 ± 5.3 | 27.3 ± 4.5 | 0.38 |
| Waist circumference (cm) | 90 ± 11 | 88 ± 8 | 91 ± 12 | 0.4 |
| Systolic blood pressure (mmHg) | 130 ± 14 | 132 ± 14 | 130 ± 14 | 0.65 |
| Diastolic blood pressure (mmHg) | 84 ± 8 | 84 ± 7 | 84 ± 8 | 0.84 |
| Heart rate (bpm) | 72 ± 9 | 74 ± 8 | 71 ± 9 | 0.44 |
| HbA1c (mmol/mol) | 33.9 ± 3.5 | 33.8 ± 3.9 | 34.0 ± 3.4 | 0.89 |
| Fasting plasma glucose (mg/dL) | 84 ± 10 | 82 ± 9 | 85 ± 11 | 0.51 |
| Insulin (µU/mL) | 8.5 ± 5.1 | 7.5± 3.5 | 8.7 ± 5.5 | 0.78 |
| Total cholesterol (mg/dL) | 259 ± 28 | 267 ± 25 | 257 ± 28 | 0.32 |
| HDL cholesterol (mg/dL) | 62 ± 13 | 62 ± 15 | 62 ± 12 | 0.96 |
| Non-HDL cholesterol (mg/dL) | 197 ± 26 | 205 ± 21 | 195 ± 27 | 0.29 |
| LDL cholesterol (mg/dL) | 170 ± 24 | 179 ± 20 | 168 ± 25 | 0.21 |
| Triglycerides (mg/dL) | 132 (91–151) | 136 (109–151) | 131 (91–152) | 0.61 |
| Apolipoprotein A (mg/dL) | 159.2 ± 19.7 | 158.9 ± 20.7 | 159.3 ± 19.7 | 0.87 |
| Apolipoprotein B (mg/dL) | 132.5 ± 15.8 | 137.1 ± 12.1 | 131.2 ± 16.7 | 0.23 |
| Creatinine (mg/dL) | 0.70 (0.70–0.90) | 0.70 (0.65–0.80) | 0.80 (0.68–0.90) | 0.28 |
| AST (U/L) | 24 (21–28) | 24 (22–26) | 24 (21–29) | 0.67 |
| ALT (U/L) | 25 (14–32) | 26 (24–33) | 23 (14–33) | 0.38 |
| CPK (U/L) | 108 (77–188) | 120 (87–169) | 107 (70–204) | 0.84 |
| hsCRP (mg/L) | 1.06 (0.58–2.08) | 1.37 (0.61–2.74) | 0.98 (0.55–1.96) | 0.51 |
| Concomitant Medications | Total (n = 39) | Placebo (n = 9) | Nutraceutical Intervention (n = 30) | p Value |
| Anti-hypertensive n (%) | 9 (23) | 1 (11) | 8 (27) | 0.33 |
| Thyroxine n (%) | 9 (23) | 2 (22) | 7 (23) | 0.88 |
| Variables | Placebo | Nutraceutical Intervention | Treatment Effect (GLM) |
|---|---|---|---|
| HbA1C (mmol/mol) | p = 0.89 | ||
| Baseline | 33.8 ± 3.9 | 34.1 ± 3.0 | |
| 4 weeks | 32.6 ± 2.0 | 32.6 ± 3.0 | |
| 12 weeks | 32.3 ± 3.9 | 32.4 ± 3.4 | |
| Plasma Glucose (mg/dL) | p = 0.89 | ||
| Baseline | 82.0 ± 9.5 | 84.8 ± 11.2 | |
| 4 weeks | 89.0 ± 9.3 | 86.6 ± 9.6 | |
| 12 weeks | 84.7 ± 8.8 | 85.5 ± 11.4 | |
| BMI (kg/m2) | p = 0.20 | ||
| Baseline | 25.8 ± 3.5 | 27.7 ± 4.5 | |
| 4 weeks | 25.6 ± 3.47 | 27.7 ± 4.3 | |
| 12 weeks | 25.5 ± 3.3 | 27.7 ± 4.3 | |
| Waist circumference (cm) | p = 0.35 | ||
| Baseline | 88 ± 8 | 91 ± 12 | |
| 4 weeks | 87 ± 7 | 91 ± 11 | |
| 12 weeks | 87 ± 6 | 90 ± 11 | |
| Non-HDL cholesterol (mg/dL) | p = 0.008 | ||
| Baseline | 205 ± 21 | 195 ± 27 | |
| 4 weeks | 190 ± 30 | 166 ± 32 | |
| 12 weeks | 207 ± 26 | 165 ± 25 | |
| LDL cholesterol (mg/dL) | p = 0.008 | ||
| Baseline | 179 ± 20 | 168 ± 25 | |
| 4 weeks | 163 ± 31 | 138 ± 31 | |
| 12 weeks | 179 ± 25 | 136 ± 28 | |
| Apo B (mg/dL) | p = 0.02 | ||
| Baseline | 137 ± 12 | 131 ± 17 | |
| 4 weeks | 121 ± 21 | 113 ± 18 | |
| 12 weeks | 142 ± 15 | 116 ± 18 | |
| HDL cholesterol (mg/dL) | p = 0.98 | ||
| Baseline | 61.8 ± 15.4 | 62.5 ± 12.3 | |
| 4 weeks | 61.5 ± 12.6 | 62.6 ± 13.5 | |
| 12 weeks | 60.3 ± 12.0 | 58.9 ± 13.5 | |
| Triglycerides (mg/dL) | p = 0.75 | ||
| Baseline | 136(109–151) | 131(91–152) | |
| 4 weeks | 121(100–160) | 104(87–143) | |
| 12 weeks | 145(81–179) | 119(93–154) | |
| Apo A/Apo B | p = 0.07 | ||
| Baseline | 0.88 ± 0.15 | 0.84 ± 0.17 | |
| 4 weeks | 0.82 ± 0.17 | 0.71 ± 0.16 | |
| 12 weeks | 0.89 ± 0.15 | 0.71 ± 0.16 | |
| PCSK9 (ng/mL) | p = 0.24 | ||
| Baseline | 250 ± 101 | 235 ± 87 | |
| 4 weeks | 326 ± 146 | 257 ± 107 | |
| 12 weeks | 293 ± 112 | 256 ± 108 |
| Variables | Placebo | Nutraceutical Intervention | Treatment Effect (GLM) |
|---|---|---|---|
| AST (U/L) | p = 0.69 | ||
| Baseline | 24 (22–26) | 24 (21–29) | |
| 4 weeks | 27 (21–29) | 27 (22–30) | |
| 12 weeks | 23 (21–29) | 26 (22–31) | |
| ALT (U/L) | p = 0.76 | ||
| Baseline | 26 (24–33) | 23 (14–33) | |
| 4 weeks | 25 (20–29) | 25 (17–35) | |
| 12 weeks | 22 (17–32) | 24 (17–34) | |
| CPK (U/L) | p = 0.92 | ||
| Baseline | 120 (87–169) | 107 (70–204) | |
| 4 weeks | 113 (86–150) | 113 (83–216) | |
| 12 weeks | 112 (100–175) | 136 (98–207) | |
| Creatinine (mg/dL) | p = 0.24 | ||
| Baseline | 0.70 (0.65–0.80) | 0.80 (0.68–0.90) | |
| 4 weeks | 0.70 (0.55–0.80) | 0.80 (0.70–0.90) | |
| 12 weeks | 0.70 (0.70–0.78) | 0.80 (0.70–0.93) | |
| GFR (mL/min/1.73 m2) | p = 0.34 | ||
| Baseline | 101.62 ± 16.71 | 98.59 ± 21.23 | |
| 4 weeks | 108.22 ± 23.64 | 96.28 ± 17.97 | |
| 12 weeks | 102.38 ± 24.15 | 96.48 ± 20.88 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spigoni, V.; Aldigeri, R.; Antonini, M.; Micheli, M.M.; Fantuzzi, F.; Fratter, A.; Pellizzato, M.; Derlindati, E.; Zavaroni, I.; Bonadonna, R.C.; et al. Effects of a New Nutraceutical Formulation (Berberine, Red Yeast Rice and Chitosan) on Non-HDL Cholesterol Levels in Individuals with Dyslipidemia: Results from a Randomized, Double Blind, Placebo-Controlled Study. Int. J. Mol. Sci. 2017, 18, 1498. https://doi.org/10.3390/ijms18071498
Spigoni V, Aldigeri R, Antonini M, Micheli MM, Fantuzzi F, Fratter A, Pellizzato M, Derlindati E, Zavaroni I, Bonadonna RC, et al. Effects of a New Nutraceutical Formulation (Berberine, Red Yeast Rice and Chitosan) on Non-HDL Cholesterol Levels in Individuals with Dyslipidemia: Results from a Randomized, Double Blind, Placebo-Controlled Study. International Journal of Molecular Sciences. 2017; 18(7):1498. https://doi.org/10.3390/ijms18071498
Chicago/Turabian StyleSpigoni, Valentina, Raffaella Aldigeri, Monica Antonini, Maria Maddalena Micheli, Federica Fantuzzi, Andrea Fratter, Marzia Pellizzato, Eleonora Derlindati, Ivana Zavaroni, Riccardo C. Bonadonna, and et al. 2017. "Effects of a New Nutraceutical Formulation (Berberine, Red Yeast Rice and Chitosan) on Non-HDL Cholesterol Levels in Individuals with Dyslipidemia: Results from a Randomized, Double Blind, Placebo-Controlled Study" International Journal of Molecular Sciences 18, no. 7: 1498. https://doi.org/10.3390/ijms18071498
APA StyleSpigoni, V., Aldigeri, R., Antonini, M., Micheli, M. M., Fantuzzi, F., Fratter, A., Pellizzato, M., Derlindati, E., Zavaroni, I., Bonadonna, R. C., & Dei Cas, A. (2017). Effects of a New Nutraceutical Formulation (Berberine, Red Yeast Rice and Chitosan) on Non-HDL Cholesterol Levels in Individuals with Dyslipidemia: Results from a Randomized, Double Blind, Placebo-Controlled Study. International Journal of Molecular Sciences, 18(7), 1498. https://doi.org/10.3390/ijms18071498